Abstract
Because dendritic cells (DC) are critically involved in both initiating primary and boosting secondary host immune responses, attention has focused on the use of DC in vaccine strategies to enhance reactivity to tumor-associated antigens. We have reported previously the induction of major histocompatibility complex class II-specific T-cell responses after stimulation with tumor antigen-pulsed DC in vitro. The identification of in vitro conditions that would generate large numbers of DC with more potent antigen-presenting cell (APC) capacity would be an important step in the further development of clinical cancer vaccine approaches in humans. We have focused attention on identifying certain exogenous cytokines added to DC cultures that would lead to augmented human DC number and function. DC progenitors from peripheral blood mononuclear cells (PBMC) were enriched by adherence to plastic, and the adherent cells were then cultured in serum-free XVIVO-15 medium (SFM) for 7 days with added granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). At day 7, cultures contained cells that displayed the typical phenotypic and morphologic characteristics of DC. Importantly, we have found that the further addition of tumor necrosis factor α (TNFα) at day 7 resulted in a twofold higher yield of DC compared with non–TNFα-containing DC cultures at day 14. Moreover, 14-day cultured DC generated in the presence of TNFα (when added at day 7) demonstrated marked enhancement in their capacity to stimulate a primary allogeneic mixed leukocyte reaction (8-fold increase in stimulation index [SI]) as well as to present soluble tetanus toxoid and candida albicans (10- to 100-fold increases in SI) to purified CD4+ T cells. These defined conditions allowed for significantly fewer DC and lower concentrations of soluble antigen to be used for the pulsing of DC to efficiently trigger specific T-cell proliferative responses in vitro. When compared with non–TNFα-supplemented cultures, these DC also displayed an increased surface expression of CD83 as well as the costimulatory molecules, CD80 and CD86. Removal of TNFα from the DC cultures after 2 or 4 days reduced its enhancing effect on DC yield, phenotype, and function. Thus, the continuous presence of TNFα over a 7-day period was necessary to achieve the maximum enhancing effect observed. Collectively, our findings point out the importance of exogenous TNFα added to cultures of cytokine-driven human DC under serum-free conditions, which resulted in an enhanced number and function of these APC. On the basis of these results, we plan to initiate clinical vaccine trials in patients that use tumor-pulsed DC generated under these defined conditions.
DENDRITIC CELLS (DC) are the most potent antigen-presenting cells (APC) distributed in many tissues of the body in humans and other species.1,2 DC can stimulate the primary activation of T cells due to their enhanced capacity of presenting immunogenic peptides in association with self-major histocompatibility complex (MHC) class I1-4and class II molecules,5-8 expression of coreceptor molecules such as CD40, CD80, CD86,9 and ICAM-3,10 as well as production of cytokines such as interleukin-12 (IL-12).11,12 DC can also process both exogenous protein13 and intracellular protein derived from DNA transfection14 for presentation to T cells. Recently, it was reported that DC can directly modulate B-cell growth and differentiation via CD40 ligation15 and through the production of soluble mediators such as IL-1, IL-6, and tumor necrosis factor α (TNFα), which have been shown to be produced by DC or DC-related cell lines.16 17
We and others have shown that DC pulsed with tumor-associated antigen(s) in the form of whole cell lysates,5-7peptides,1-4,18,19 proteins,20RNA,21 or DNA14,22 could initiate primary MHC class I- or class II-restricted T-cell responses that resulted in antitumor effects in vitro and in vivo. On the basis of these studies, attention has focused on the use of DC to enhance the host immune response to tumor-associated antigens in clinical vaccine strategies in humans with cancer.23 24 Thus, the identification of approaches that would generate large numbers of DC with more potent antigen-presenting capacity would be an important step in the further refinement of vaccine approaches based on DC.
There is general agreement that DC can be generated from bone marrow- and cord blood-derived CD34+ hematopoietic cell progenitors with cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, and TNFα.25,26 In addition, DC have been derived from precursors in unfractionated27,28 and CD34+ cell-depleted29 peripheral blood mononuclear cells (PBMC) as well as from CD14+ blood monocytes.17 30
Various recombinant cytokines have been used for the in vitro generation of DC derived from several tissue sources (denoted cytokine-driven DC).1,2 In particular, the activity of TNFα in combination with GM-CSF with or without IL-4 has been studied. For example, the addition of TNFα to cultures has been shown to inhibit spontaneous apoptosis of DC,31 to generate CD83+ DC from CD14+ blood monocytes,17 to cause phenotypic and functional maturation of DC,1,2,32,33 to increase DC mobility by rearrangement of microfilaments and microtubules,34 and to augment the capacity of DC to mediate delayed-type hypersensitivity (DTH) responses in vivo.35 In these studies and those of others,36 37 the level and specific type(s) (positive or negative) of TNFα effects on DC phenotype and function have appeared to be dependent on the timing and duration of exposure of the DC cultures to this particular exogenous cytokine.
We have recently initiated phase 1 clinical trials of autologous tumor lysate-pulsed dendritic cells as a vaccine in adult and pediatric patients with advanced solid tumors. In the current study, efforts were focused on strategies to improve human DC function and overall recovery. We have now investigated the effects of recombinant TNFα on the generation of human DC from PBMC under serum-free conditions in the presence of GM-CSF and IL-4. Moreover, to increase the overall recovery of DC, we have included DC precursors of both the lymphoid38,39 and myeloid1 2 lineages known to be present in PBMC by excluding the depletion of CD2+ cells before culture. Collectively, our data show that TNFα added for extended time periods can mediate improved function and yield of human cytokine-driven DC derived from PBMC.
MATERIALS AND METHODS
Media and reagents.
The medium used throughout the studies was XVIVO-15 (BioWhittaker, Gaithersburg, MD). Tetanus toxoid (TT) was purchased as a sterile liquid in vials from Connaught Laboratories, Ltd (North York, Ontario, Canada). The activity of TT was 2250 Lf/12.2 mg/mL (lot no. TAS 319 R8), which was diluted further in XVIVO-15 to achieve 225 Lf/mL and stored at 4°C before use. Candida albicans (CAD) was purchased from Greer Laboratories, Inc (Lenoir, NC). Sterile, lyophilized vials of CAD (lot no. XPLM73-7-X7-NV) were reconstituted to 100 μg/mL with phosphate-buffered saline (PBS) and stored at −20°C before use.
Recombinant human IL-4 was kindly provided by Schering-Plough Research Institute (Kenilworth, NJ). The specific activity was determined to be 6.35 × 107 IU/mg (lot no. 3-ENP-803). Vials were diluted with XVIVO-15 to 50 μg/mL and stored at −80°C before use.
Recombinant human GM-CSF was kindly provided by Dr C. Reynolds (BRMP, NCI, NIH, Frederick, MD). This GM-CSF (manufactured by Immunex Corp, Seattle, WA) had a specific activity of 1.4 × 106 IU/250 μg. Lyophilized GM-CSF was reconstituted with sterile water, diluted to 100 μg/mL with XVIVO-15, and stored at −80°C before use.
Recombinant human TNFα was a gift from Dr D. Fraker (Department of Surgery, University of Pennsylvania, Philadelphia, PA). This TNFα (manufactured by Knoll AG, Ludwigshafen, Germany) had a specific activity of 8.2 × 106 U/mg protein as measured in the L929 cytotoxicity assay without adding actinomycin D. Sterile, lyophilized TNFα (0.79 mg/vial) was first reconstituted with 1 mL sterile water before further dilutions in culture medium.
Generation of DC from peripheral blood.
PBMC were obtained from leukapheresis specimens of normal donors after Ficoll-hypaque density gradient separation. PBMC were washed twice in Hank's balanced salt solution (HBSS; GIBCO BRL, Life Technologies, Inc, Gaithersburg, MD) and were resuspended in XVIVO-15 medium at a concentration of 1.6 × 106 cells/mL. Three milliliters of this cell suspension (5 × 106 PBMC) were plated in 6-well tissue culture plates (Costar Corp, Cambridge, MA) and were incubated at 37°C, 5% CO2 for 2 hours. The nonadherent cells were gently removed by pipetting, and 3 mL/well of XVIVO-15 medium containing GM-CSF (100 ng/mL) and IL-4 (50 ng/mL) was added. All cultures were maintained at 37°C, 5% CO2 for 7 days. At day 7, culture medium was exchanged with fresh XVIVO-15 medium containing GM-CSF/IL-4 with or without TNFα (10 ng/mL). The cultures were maintained for another 7 days. In some experiments, TNFα was removed 2 or 4 days later by harvesting the cultures and washing the cells three times in medium. The washed cells were then replated in fresh XVIVO-15 medium containing GM-CSF and IL-4 for an additional 5 or 3 days, respectively.
Enrichment of DC.
At day 14, the DC were harvested from culture and loaded onto hypertonic 14.5% metrizamide columns and centrifuged for 10 minutes at 650g. The DC-enriched interface was collected and washed consecutively in 40 mmol/L and 25 mmol/L NaCl medium solution by centrifugation. The interface contained an average 75% DC as defined by typical morphology (veiled appearance) and surface phenotype by fluorescence-activated cell sorting (FACS; coexpression of high level CD86 and HLA-DR, but CD14−). In some experiments, DC were stained with fluorescein isothiocyanate (FITC)-conjugated HLA-DR and sorted by a Coulter EPICS-C Cell Sorter instrument (Coulter Corp, Miami, FL) gated according to large cells (by side and forward light scatter) that were positive for HLA-DR expression.
Isolation of CD4+ T cells.
Human CD4+ T cells were purified from PBMC using the MACS magnetic cell sorting system according to the manufacturer's recommendations (Miltenyi Biotec, Sunnyvale, CA). Briefly, the PBMC were resuspended in 80 μL MACS buffer for each 107 cells. Anti-CD4 magnetic beads were added at 20 μL per 107cells. The mixture was incubated at 4°C for 15 minutes, washed twice with MACS buffer, and then placed on an MACS column in the magnetic field; the CD4− cells were first eluted. The column was removed from the magnetic field and the CD4+ cells were collected. The purity of CD4+ cells was greater than 90% by FACS phenotypic analysis.
Phagocytosis assay.
Two hundred thousand DC in 500 μL XVIVO-15 were incubated at 4°C or 37°C with 50 μL of a 10 mg/mL stock solution of different molecular weight fluorescein-labeled dextran particles (Molecular Probes, Inc, Eugene, OR). After 30 minutes to 3 hours of incubation, samples were washed four times with PBS plus 2% fetal calf serum (FCS), fixed with 5% paraformaldehyde in PBS at 4°C, and examined for phagocytic uptake with a FACScan (Becton Dickinson, Mountain View, CA). Geographical mean fluorescence was calculated for all samples.
Allogeneic mixed leukocyte reaction assay.
One hundred thousand responding T cells from PBMC of allogeneic adult donors were cultured in 96-well U-bottom microplates (Costar Corp, Cambridge, MA) with different numbers of DC irradiated with 2,000 rad of 137Cs generated gamma radiation (Gamma Cell 1000; Nordion Corp, Kanata, Ontario, Canada). Cellular proliferation was measured on day 5 by an 18-hour pulse with [3H]thymidine at 1 μCi/well (3H-TdR, 6.7 Ci/mmol/L; DuPont-NEN, Boston, MA).
Antigen presentation assay.
To measure the efficiency of DC presentation of soluble antigen, 5 × 104 CD4+ T cells were cultured with 5 × 103 cultured DC (irradiated with 2,000 rad) in the presence of different concentrations of TT or CAD in 200 μL XVIVO-15 medium in 96-well U-bottom microplates. Cultures were pulsed with 1 μCi/well3H-TdR (6.7 Ci/mmol/L) on day 5. Cellular proliferation was measured by 3H-TdR incorporation 18 hours later with a liquid scintillation counter (Wallac 1205 Betaplate; Wallac, Gaithersburg, MD). In some experiments, 1 × 105CD4+ T cells were cultured with different numbers of irradiated DC in the presence of a fixed concentration of TT (12.2 μg/mL) or CAD (10 μg/mL).
FACS analysis.
Cell surface staining used direct immunofluorescence (FACScan; Becton Dickinson), and the samples were analyzed using Cell Quest software (Becton Dickinson). Staining was performed with the following FITC- and phycoerythrin (PE)-labeled monoclonal antibodies: PE-CD1a, FITC-CD3, PE-CD11c, FITC-CD32, PE-CD33, FITC-CD80, FITC-CD86, FITC-mouse IgG1 (all from Pharmingen, San Diego, CA); FITC-CD14, FITC-HLA-DR, PE-mouse IgG1 (all from Becton Dickinson); PE-CD83 (from Coulter/Immunotech, Miami, FL); and FITC-mouse IgG2 and PE-CD4 (both from Sigma, St Louis, MO). Primary antibodies were directed toward a panel of cell surface markers and compared with the appropriate isotype-matched controls.
RESULTS
TNFα expands the number of human DC and upregulates the expression of costimulatory molecules.
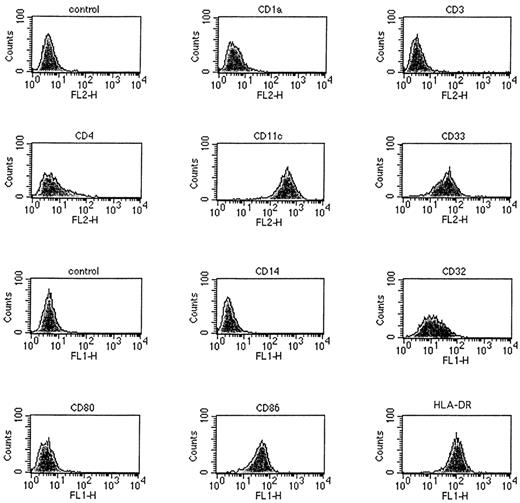
To avoid the disadvantages of serum (eg, potential exposure to pathogens and sensitization to irrelevant antigens), we have refined a method to generate DC from human peripheral blood leukocytes (PBL) that uses serum-free XVIVO-15 medium (SFM). Because DC have been shown to develop from both myeloid and lymphoid progenitors,17,27,28,30,38 39 we first attempted to increase the yield of DC from PBMC by deleting the commonly used E-rosetting step of DC purification to preserve precusors present in the CD2+ cell fraction. DC precursors were enriched by adherence to plastic and the adherent cells were then cultured for 14 days in SFM with added GM-CSF and IL-4. The DC generated in this culture displayed phenotypic and morphologic characteristics of mature DC. As shown in Fig 1, DC generated under these culture conditions were either partially or entirely positive for CD4, CD11c, CD32, CD33, CD86, and HLA-DR cell surface markers but were negative for CD1a, CD3, CD14, and CD80 expression.
Surface phenotype of metrizamide gradient-separated DC detected by a panel of antihuman FITC- or PE-labeled antibodies and FACS analysis. DC were generated from PBMC cultured for 14 days in XVIVO-15 serum-free medium containing GM-CSF and IL-4. The x-axis is a logarithmic scale of fluorescence intensity and the y-axis represents counts. DC showed high-level expression of MHC class II (HLA-DR), CD86, and CD11c molecules.
Surface phenotype of metrizamide gradient-separated DC detected by a panel of antihuman FITC- or PE-labeled antibodies and FACS analysis. DC were generated from PBMC cultured for 14 days in XVIVO-15 serum-free medium containing GM-CSF and IL-4. The x-axis is a logarithmic scale of fluorescence intensity and the y-axis represents counts. DC showed high-level expression of MHC class II (HLA-DR), CD86, and CD11c molecules.
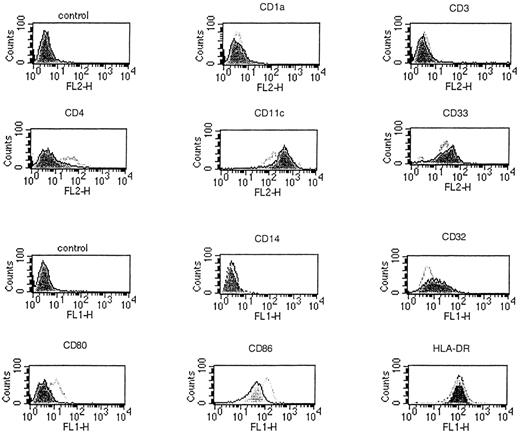
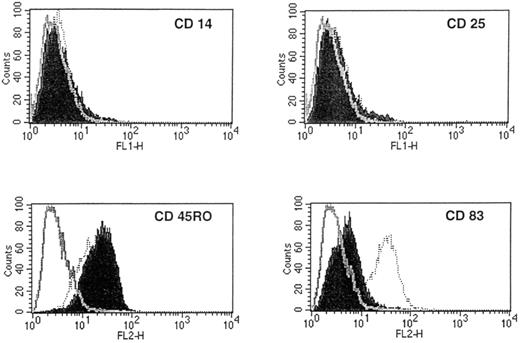
In a preliminary experiment, we found that 14-day DC treated with TNFα exhibited the characteristic cellular projections and veils, which showed continuous extension, retraction, and reorientation. As demonstrated by the seven representative experiments of Table1, the further addition of TNFα at day 7 resulted in a twofold higher yield of harvested DC compared with non–TNFα-containing DC cultures at day 14 (2.77 × 105 ± 0.35 v1.32 × 105 ± 0.3 per 5 × 106 PBL plated initially). When compared with non–TNFα-supplemented cultures, TNFα-treated DC demonstrated an increase in surface expression of CD4, CD80, and CD86, with a concomitant decrease in CD11c, CD32, and CD33 levels (Fig 2). As shown in Fig 3, TNFα-treated DC also exhibited high-level expression of CD83, a marker of mature DC, as reported previously.40 The addition of TNFα to GM-CSF/IL-4–driven DC did not markedly alter the high level of expression of CD45RO, and these DC remained CD14− and CD25− (Fig 3). Collectively, these data showed that the addition of recombinant TNFα could enhance the expression of costimulatory molecules on and increase the number of GM-CSF/IL-4–driven DC in vitro.
Yields of DC Cultured in the Presence or Absence of TNFα
| Experiment No. . | TNFα Treatment (×105)* . | |
|---|---|---|
| − . | +-151 . | |
| 1 | 1.0-152 | 2.6 |
| 2 | 1.9 | 2.9 |
| 3 | 1.2 | 2.5 |
| 4 | 1.3 | 3.5 |
| 5 | 1.5 | 2.8 |
| 6 | 1.1 | 2.5 |
| 7 | 1.3 | 2.7 |
| Mean ± SD | 1.3 ± 0.3 | 2.8 ± 0.4 |
| 8 | 1.2 | 3.0 |
| 2.3-153 | ||
| 0.9-155 | ||
| Experiment No. . | TNFα Treatment (×105)* . | |
|---|---|---|
| − . | +-151 . | |
| 1 | 1.0-152 | 2.6 |
| 2 | 1.9 | 2.9 |
| 3 | 1.2 | 2.5 |
| 4 | 1.3 | 3.5 |
| 5 | 1.5 | 2.8 |
| 6 | 1.1 | 2.5 |
| 7 | 1.3 | 2.7 |
| Mean ± SD | 1.3 ± 0.3 | 2.8 ± 0.4 |
| 8 | 1.2 | 3.0 |
| 2.3-153 | ||
| 0.9-155 | ||
*Fourteen-day cultures of DC generated in the presence of human recombinant GM-CSF and IL-4 as described in the Materials and Methods.
Recombinant human TNFα (10 ng/mL) was added to DC cultures at day 7.
Number of DC harvested at day 14 (per 5 × 106 PBMC initially plated).
TNFα was removed from DC cultures after 4 days and the cells were replated in fresh SFM with GM-CSF and IL-4 (without additional TNFα).
TNFα was removed from DC cultures after 2 days and the cells were replated in fresh SFM with GM-CSF and IL-4 (without added TNFα).
Phenotypic changes in cytokine-driven DC when cultured in the presence of TNFα detected by a panel of antihuman FITC- or PE-labeled antibodies and FACS analysis. PBMC were cultured in XVIVO-15 serum-free medium containing GM-CSF and IL-4 for 7 days. TNFα was then added at day 7 and the cultures were allowed to proceed for an additional 7-day period. When compared with non–TNFα-supplemented DC cultures (shaded histograms), the addition of TNFα resulted in positive cell surface expression of CD80 and an upregulated expression of CD86 costimulatory molecules (open histograms). The x-axis is a logarithmic scale of fluorescence intensity and the y-axis represents counts.
Phenotypic changes in cytokine-driven DC when cultured in the presence of TNFα detected by a panel of antihuman FITC- or PE-labeled antibodies and FACS analysis. PBMC were cultured in XVIVO-15 serum-free medium containing GM-CSF and IL-4 for 7 days. TNFα was then added at day 7 and the cultures were allowed to proceed for an additional 7-day period. When compared with non–TNFα-supplemented DC cultures (shaded histograms), the addition of TNFα resulted in positive cell surface expression of CD80 and an upregulated expression of CD86 costimulatory molecules (open histograms). The x-axis is a logarithmic scale of fluorescence intensity and the y-axis represents counts.
TNFα-treated DC express a high level of CD83. Compared with non–TNFα-supplemented 14-day DC cultures (shaded histograms), the addition of TNFα (at day 7) resulted in strong expression of the mature DC marker, CD83, by FACS analysis (dotted open histograms). Compared with isotype-matched control monoclonal antibody staining, both TNFα-treated and non–TNFα-treated DC did not show a notable change in high-level CD45RO expression and remained negative for CD14 and CD25 expression. The x-axis is a logarithmic scale of fluorescence intensity and the y-axis represents counts. Similar results were obtained in a second repeat experiment.
TNFα-treated DC express a high level of CD83. Compared with non–TNFα-supplemented 14-day DC cultures (shaded histograms), the addition of TNFα (at day 7) resulted in strong expression of the mature DC marker, CD83, by FACS analysis (dotted open histograms). Compared with isotype-matched control monoclonal antibody staining, both TNFα-treated and non–TNFα-treated DC did not show a notable change in high-level CD45RO expression and remained negative for CD14 and CD25 expression. The x-axis is a logarithmic scale of fluorescence intensity and the y-axis represents counts. Similar results were obtained in a second repeat experiment.
TNFα enhances the capacity of DC to mediate primary allo-stimulation of and soluble antigen presentation to T cells.
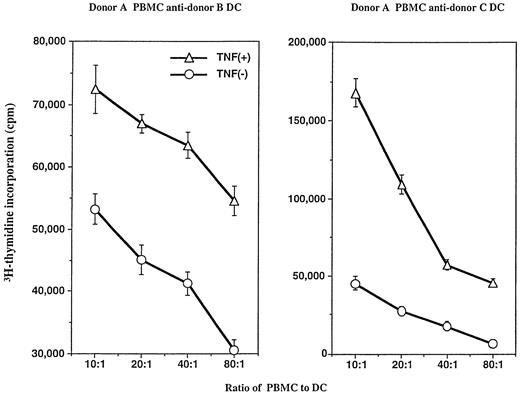
We next tested DC function in both allogeneic mixed leukocyte response (MLR) and soluble antigen presentation assays. DC generated in the presence of GM-CSF and IL-4 with or without TNFα were compared. The number of DC isolated by metrizamide gradient separation that were added from the two separate cultures (ie, with and without TNFα addition) in the functional assays were equal based on morphology (veiled appearance), size (large), and coexpression of high level CD86 and HLA-DR, but negative for CD14. Different numbers of DC from two separate donors were cultured with a fixed number of allogeneic PBMC. As shown in Fig 4, 14-day cultured DC generated in the presence of TNFα (when added at day 7) possessed enhanced capacity to stimulate a primary allogeneic MLR (ie, a further 8-fold increase in stimulation indices).
TNFα-treated DC are more stimulatory than non–TNFα-cultured DC in a primary, 6-day allogeneic mixed leukocyte reaction. PBMC from two separate donors (B and C) were used to generate DC; a single PBMC donor (A) served as the source of responder T cells in the assay. Various responder: stimulator (R:S) ratios were included, as described in the Materials and Methods. DC cultured in the presence of TNFα stimulated greater T-cell proliferative responses at all R:S ratios tested. The SEM of triplicate wells was always less than 15% of the mean. Experiments were repeated five times with similar results.
TNFα-treated DC are more stimulatory than non–TNFα-cultured DC in a primary, 6-day allogeneic mixed leukocyte reaction. PBMC from two separate donors (B and C) were used to generate DC; a single PBMC donor (A) served as the source of responder T cells in the assay. Various responder: stimulator (R:S) ratios were included, as described in the Materials and Methods. DC cultured in the presence of TNFα stimulated greater T-cell proliferative responses at all R:S ratios tested. The SEM of triplicate wells was always less than 15% of the mean. Experiments were repeated five times with similar results.
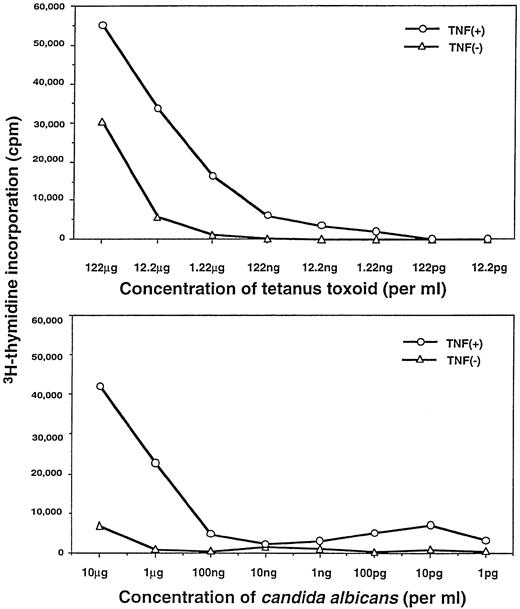
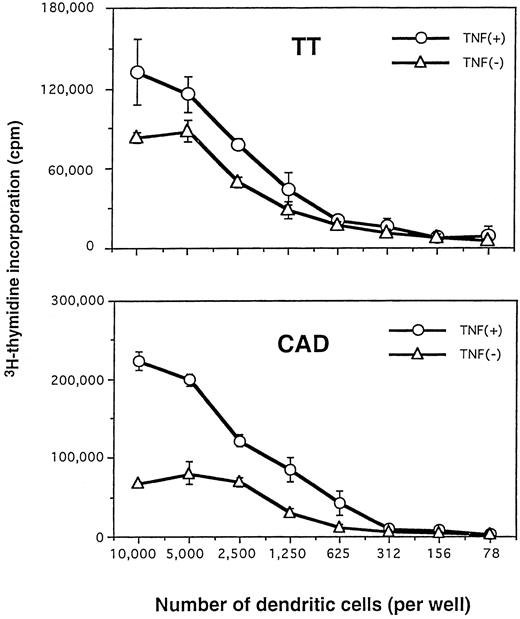
We then compared TNFα-treated versus non–TNFα-treated DC for their capacity to present TT and CAD antigens to autologous CD4+T cells. As shown in Fig 5, the TNFα-treated DC were considerably more efficient at presenting lower concentrations of soluble TT and CAD antigen(s) (eg, further 10- to 100-fold increases in stimulation indices) to purified autologous CD4+ T cells. In addition, at the same dose of antigen(s), markedly fewer TNFα-treated DC were required to trigger specific proliferative responses of autologous CD4+ T cells (Fig6). This finding was demonstrated most clearly at points at which the T-cell response had already plateaued with increasing numbers of non–TNFα-treated DC. By light microscopy, the culture plates of TNFα-treated DC displayed a stronger capacity to cluster T cells than those of non–TNFα-treated DC (data not shown).
TNFα-treated DC possess enhanced antigen-presenting function. Proliferative responses of purified autologous CD4+ T cells to different concentrations of soluble antigens presented by autologous DC were measured at day 6. Non–TNFα-treated or TNFα-treated DC were pulsed with either TT (upper) or CAD (lower), as described in the Materials and Methods. The background cpm of the proliferative response in the absence of antigens pulsed on DC (ie, unpulsed DC plus CD4+ T cells or the autologous MLR; upper, +TNFα = 5,236 cpm; −TNFα = 942 cpm; lower, +TNFα = 4,102 cpm, −TNFα = 929 cpm) were subtracted. Experiments were repeated five times with similar results.
TNFα-treated DC possess enhanced antigen-presenting function. Proliferative responses of purified autologous CD4+ T cells to different concentrations of soluble antigens presented by autologous DC were measured at day 6. Non–TNFα-treated or TNFα-treated DC were pulsed with either TT (upper) or CAD (lower), as described in the Materials and Methods. The background cpm of the proliferative response in the absence of antigens pulsed on DC (ie, unpulsed DC plus CD4+ T cells or the autologous MLR; upper, +TNFα = 5,236 cpm; −TNFα = 942 cpm; lower, +TNFα = 4,102 cpm, −TNFα = 929 cpm) were subtracted. Experiments were repeated five times with similar results.
Fewer numbers of TNFα-treated DC can efficiently present soluble antigens to autologous CD4+ T cells. Purified CD4+ T cells were cultured with different numbers of DC generated in the presence or absence of TNFα. DC were pulsed with 12.2 μg/mL TT (upper) or 10 μg/mL CAD (lower), as described in the Materials and Methods. Proliferative responses were measured on day 6. Experiments were repeated five times with similar results.
Fewer numbers of TNFα-treated DC can efficiently present soluble antigens to autologous CD4+ T cells. Purified CD4+ T cells were cultured with different numbers of DC generated in the presence or absence of TNFα. DC were pulsed with 12.2 μg/mL TT (upper) or 10 μg/mL CAD (lower), as described in the Materials and Methods. Proliferative responses were measured on day 6. Experiments were repeated five times with similar results.
TNFα treatment of DC reduces but does not eliminate phagocytic function.
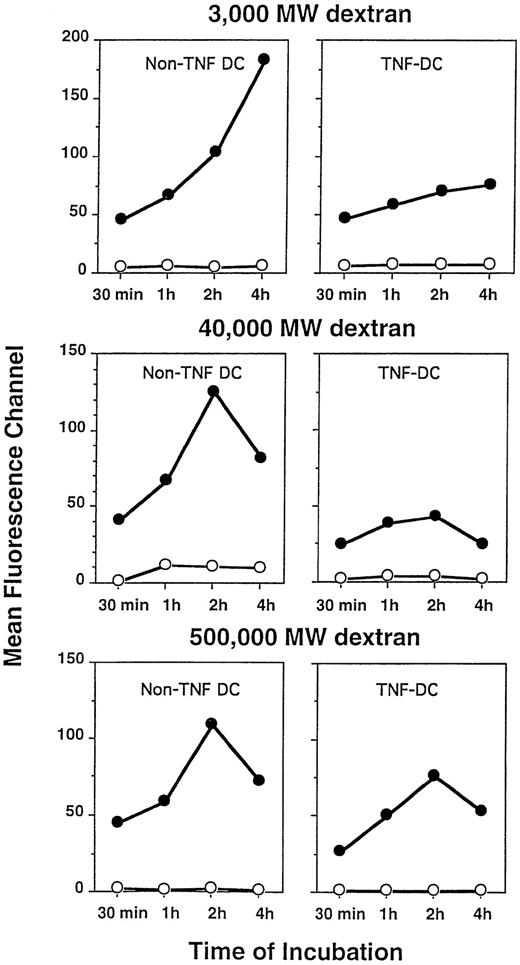
We next tested the effect of exogenous TNFα on the phagocytic function of DC cultured in GM-CSF and IL-4 by evaluating by FACS the uptake of FITC-labeled dextran particles of differing molecular weights. The amount of dextran particles incorporated by TNFα-treated DC on a per cell basis was less compared with non–TNFα-treated DC as measured by mean channel fluorescence (Fig7). However, the overall percentages of both TNFα-treated and non–TNFα-treated DC showing uptake of dextran particles of all three molecular weight sizes were comparable (∼80%) for at least up to the 4 hours of analysis (data not shown). Phagocytosis was measurable at 37°C but not at 4°C. Thus, the addition of TNFα resulted in DC that were still capable of phagocytic activity, albeit at a modestly reduced level, compared with DC cultured in the absence of this additional cytokine.
Phagocytosis of FITC-labeled dextran particles by DC treated with or without TNFα. Although TNFα-treated DC displayed a lower degree of phagocytosis on a per cell basis as measured by fluoresence intensity, the overall percentages of DC capable of dextran particle uptake were equivalent between DC cultured with or without TNFα. DC were incubated for the indicated periods at 4°C (○) or 37°C (•) with FITC-dextran of varying molecular weights. DC were washed free of unbound particles and were then analyzed by FACS, as described in the Materials and Methods. Data are expressed as mean fluorescence channel (MFC) calculated by FACS computer software.
Phagocytosis of FITC-labeled dextran particles by DC treated with or without TNFα. Although TNFα-treated DC displayed a lower degree of phagocytosis on a per cell basis as measured by fluoresence intensity, the overall percentages of DC capable of dextran particle uptake were equivalent between DC cultured with or without TNFα. DC were incubated for the indicated periods at 4°C (○) or 37°C (•) with FITC-dextran of varying molecular weights. DC were washed free of unbound particles and were then analyzed by FACS, as described in the Materials and Methods. Data are expressed as mean fluorescence channel (MFC) calculated by FACS computer software.
Removal of TNFα from DC cultures reduces its enhancing effects on the expression of costimulatory molecules and on primary allo-stimulation of and soluble antigen presentation to T cells.
The effect of removing TNFα at different time points during DC culture was next evaluated. TNFα was added to 7-day PBMC cultured in SFM with GM-CSF and IL-4. Two or 4 days later, cells in these DC cultures were then washed to remove cytokines and replated in fresh SFM with GM-CSF and IL-4 for an additional 5 or 3 days, respectively. The 14-day DC cultures were then analyzed for phenotype and T-cell stimulatory capacity in vitro. When compared with DC cultured in the presence of TNFα for 7 days, removal of TNFα resulted in reduced surface expression of the mature DC marker, CD83, as well as the costimulatory molecules, CD80 and CD86 (Fig8). As shown in Table 1, the yield of DC harvested at day 14 was also reduced after removal of TNFα compared with a DC culture containing TNFα for the entire 7-day period (see experiment no. 8). Figure 9 shows the effect of early removal of TNFα from the DC cultures on stimulation of antitetanus and primary allogeneic T-cell responses in vitro. When compared with DC cultured in the presence of TNFα for a 7-day period, removal of TNFα from the DC cultures after 2 or 4 days resulted in significant reductions in both T-cell responses.
Removal of TNFα from DC cultures results in reduced levels of CD80, CD86, and CD83 expression. TNFα added to 7-day DC cultures was subsequently removed by washing the cells 2 and 4 days later [denoted as TNF day 2(−) and TNF day 4(−), respectively]. The cultures were then replated in fresh SFM with added GM-CSF and IL-4 for an additional 5 or 3 days, respectively. Comparisons were made with DC cultured without TNFα (denoted Non-TNF DC) and those cultured in TNF for the complete 7-day period [denoted TNF day 7(+)]. FACS analysis of mean fluorescence channel is depicted. The results are representative of two experiments.
Removal of TNFα from DC cultures results in reduced levels of CD80, CD86, and CD83 expression. TNFα added to 7-day DC cultures was subsequently removed by washing the cells 2 and 4 days later [denoted as TNF day 2(−) and TNF day 4(−), respectively]. The cultures were then replated in fresh SFM with added GM-CSF and IL-4 for an additional 5 or 3 days, respectively. Comparisons were made with DC cultured without TNFα (denoted Non-TNF DC) and those cultured in TNF for the complete 7-day period [denoted TNF day 7(+)]. FACS analysis of mean fluorescence channel is depicted. The results are representative of two experiments.
Removal of TNFα from DC cultures results in reduced levels of antitetanus and primary allogeneic T-cell stimulation in vitro. Purified CD4+ T cells were cultured with DC generated in the presence of TNFα [denoted TNF DC day 7(+)], absence of TNFα (denoted Non-TNF DC), or after the removal of TNFα from the cultures at 2 days [denoted TNF DC day 2(−)] or 4 days [denoted TNF DC day 4(−)]. Proliferative responses were measured on day 6 at a DC to T-cell ratio of 1:20. The SEM of triplicate wells was always less than 15% of the mean. T cells alone = 1,534 ± 186 cpm; T cells + PHA = 30,081 ± 1,979 cpm. The results are representative of two experiments.
Removal of TNFα from DC cultures results in reduced levels of antitetanus and primary allogeneic T-cell stimulation in vitro. Purified CD4+ T cells were cultured with DC generated in the presence of TNFα [denoted TNF DC day 7(+)], absence of TNFα (denoted Non-TNF DC), or after the removal of TNFα from the cultures at 2 days [denoted TNF DC day 2(−)] or 4 days [denoted TNF DC day 4(−)]. Proliferative responses were measured on day 6 at a DC to T-cell ratio of 1:20. The SEM of triplicate wells was always less than 15% of the mean. T cells alone = 1,534 ± 186 cpm; T cells + PHA = 30,081 ± 1,979 cpm. The results are representative of two experiments.
DISCUSSION
Prior studies have identified proliferating progenitors within the CD34+ cell fraction of human peripheral blood that can be driven with cytokines, particularly GM-CSF and IL-4, to develop into potent dendritic cells over a 1- to 2-week period in culture.25,26,28 DC can also be generated from relatively small subpopulations of myeloid and lymphoid cells in the presence of cytokines.17,27,28,30,38,39 Early studies demonstrated that the combination of GM-CSF and IL-4 could facilitate the generation of relatively large numbers of dendritic cells from the adherent fraction of PBMC in media containing FCS.33 Bender et al41 and Romani et al32 modified the methods to generate mature DC that involved cytokines combined with special conditioned medium. In these studies, the DC progenitor population of cells was first depleted of CD2+ cells, which were later reported to contain DC precursors.38 39
In the current study, we focused on strategies to generate larger numbers of functional DC with more potent T-cell stimulatory activity. Adherent PBMC were first plated in SFM with GM-CSF and IL-4 for 7 days, followed by the addition of TNFα to these cultures for another 7 days. Neither culture was depleted of CD2+ or CD14+ DC precursors. Seven days of continuous TNFα exposure (added at day 7 of DC culture) was optimal to obtain increased yield and function of DC. Cells from DC cultures that contained TNFα were found to express increased surface expression of the costimulatory molecules CD80 and CD86, as well as the mature DC marker CD83. Earlier studies by Zhou and Tedder40 had demonstrated that the combination of GM-CSF, IL-4, and TNFα could drive the differentiation of CD14+ blood monocytes to mature CD83+ DC with potent T-cell allostimulatory capacity in vitro.40 We found that early removal of TNFα from DC cultures resulted in a lower cell yield, a reduced cell surface expression of CD80, CD83, and CD86 molecules, and an inferior capacity to trigger allogeneic and antitetanus CD4+ T-cell responses. Thus, the continued presence of TNFα (for at least 7 days) in combination with GM-CSF and IL-4 was necessary for achieving optimum effects on DC. In additional studies (not shown), removal of TNFα from the DC cultures at day 14 did not impact on (ie, reverse) the TNFα-induced changes for at least another 2 to 3 days (ie, day-16 or -17 cultures). Beyond this point, in serum-free medium, the viability of DC decreased in cultures with or without the addition of TNFα. We were able to generate nearly 3 × 105 DC per 5 × 106 PBMC, which was on average twofold greater than the cultures prepared in the absence of TNFα. It is conceivable that TNFα played a role in the maintenance of DC viability in culture, because it has been reported that this cytokine can inhibit spontaneous apoptosis of DC in vitro.31 Indeed, we have observed that DC will undergo apoptosis from day 11 of culture in SFM with GM-CSF and IL-4 in the absence of TNFα (not shown). In contrast, we have not observed such an apoptotic event in cultures containing added TNFα at least until day 16. Increased numbers of DC in cultures containing TNFα could also be the result of the activity of this cytokine on certain DC precursor subsets that are not dependent on GM-CSF but would differentiate into DC in the presence of TNFα.42 However, because differences in cell yields were not apparent until several days after TNFα addition (ie, after day 11 of culture), it is believed that lack of apoptosis was the predominant effect responsible for the increased DC yield in the TNFα-containing cultures.
We have compared DC cultured with and without TNFα for expression of certain cell surface molecules, phagocytic activity, and capacity to stimulate a primary MLR as well as to present defined antigens to CD4+ responder T cells. We confirmed that adherent PBMC cultured in SFM containing GM-CSF and IL-4 resulted in cells with typical DC morphology (veils) and phenotype. These DC also demonstrated efficient uptake of exogenous dextran particles via fluid-phase macropinocytosis and could trigger CD4+ T-cell responses to TT and CAD antigens in vitro. However, when TNFα was added at day 7, the DC produced by day 14 were more stimulatory to T cells and displayed increased expression of the costimulatory molecules, CD80 and CD86. These molecules have been shown to play differential roles in stimulating Th1 versus Th2 cell responses,43 including those leading to tumor rejection.44 Although we found that these DC were somewhat reduced in their capacity to engulf dextran particles on a per cell basis, overall percentages (∼80%) of DC that were positive for particle uptake over a 4-hour time course were similar between the two cultures. In some instances, TNFα had been shown to have no significant effect on the uptake levels of similar tracer molecules.36
One of the mechanisms that may contribute to the more efficient stimulatory capacity of DC generated in the presence of added TNFα is their enhanced capacity for clustering T cells in an antigen-independent fashion.45 Indeed, we observed in cultures that TNFα-treated DC could mediate a pronounced capacity for clustering T cells when compared with non–TNFα-treated DC (data not shown). Some investigators reported that TNFα decreased the capacity of antigen processing and presentation of DC.33 The enhancement of DC function with TNFα in our study may be due to the timing and duration of its addition to the cultures with GM-CSF and IL-4. It may be considered that TNFα has differing effects on certain stages of DC maturation, because TNFα has been shown to bidirectionally modulate the viability of hematopoietic progenitor cells in vitro37 and shorter exposure times to this cytokine were shown to be either inhibitory or have no effect24 33 (data not shown).
Koch et al46 reported that populations of cultured Langerhans cells (LC) were not completely inactive in processing and presenting native proteins and that populations of spleen DC obtained by a standard method involving overnight culture were able to process intact protein to some, albeit lesser, extent. These findings were attributed to the fact that populations of mature DC are heterogeneous. Moreover, small numbers of immature DC coexisting within populations of mature DC, such as cultured LC or spleen DC, could account for the residual antigen processing activity. The data reported by Sallusto and Lanzavecchia33 obtained with human DC derived from monocytes suggest that the interplay of various cytokines may be more complex. In these studies, TNFα induced the downregulation of processing activity and the upregulation of T-cell allostimulatory function in human blood-derived DC. This finding would imply that, rather than being inhibitory, TNFα was a crucial trigger for the process of DC maturation. In our study, 7-day incubation of DC with a relatively low concentration of TNFα improved DC presentation of soluble TET and CAD antigens at a time when their capacity to capture dextran particles was depressed on a per cell basis. It is conceivable that DC cultures remain heterogeneous and that subsets of residual (partly) immature DC within populations of mature DC were endowed with biologically significant ability to handle native protein antigen(s).
Several mechanisms have been reported to contribute to the efficient antigen presentation by DC, including a capacity for clustering T cells in an antigen-independent fashion45; the expression of high levels of MHC molecules, allowing presentation of more T-cell determinants1,2; the high expression of adhesion and costimulatory molecules and the low surface charge,1,2which may lower the number of determinants required for T-cell activation; the high level of fluid-phase pinocytosis47; and the expression of functional FcγR.48 In addition, DC have been shown to produce numerous cytokines with potent activity on a variety of immune cells.16,17 In this regard, we attempted to determine whether there existed differential expression of various cytokines between TNFα-treated and non–TNFα-treated DC. For this analysis, we elected to evaluate cytokines with known T-cell activities that included activation and proliferation (ie, IL-1α, IL-4, IL-6, IL-7, IL-12, IL-15, interferon-γ, and GM-CSF). Reverse transcription-polymerase chain reaction assays performed on FACS-sorted TNFα-cultured and non–TNFα-cultured DC (>95% purity) did not show differences in the levels of transcript expression between these two DC groups, although this methodology does not show actual levels of secreted cytokines (data not shown). On the basis of the findings from this current study, we have begun to compare in vitro the capacity of TNFα-treated and non–TNFα-treated DC to process and present known tumor peptides3,4,18-20 versus whole tumor cell lysates5-7 to autologous T cells from advanced cancer patients.
ACKNOWLEDGMENT
The authors thank Dr Satwant Narula, Dr Mary Ellen Rybak, and Chris DeLuca of Schering-Plough Research Institute for the generous supplies of recombinant human GM-CSF and IL-4 and thank Dr Douglas Fraker of the University of Pennsylvania Medical Center for the gift of recombinant human TNFα. We also thank Michelle Walsh of the U-M GCRC and Sandra Hoffmann of the U-M Blood Bank for leukapheresis samples. The critical review of the manuscript by Dr Laurence Boxer is greatly appreciated.
Supported by grants from the National Institutes of Health (Grants No. 1 P01 CA59327 and M01-RR00042), from the DOD/USAMRMC (DAMD17-96-1-6103), and from the US Army Research Office (DAAG55-97-1-0239).
Address reprint requests to James J. Mulé, PhD, Department of Surgery, University of Michigan Medical Center, 1520c MSRB-1, 1150 W Medical Center Dr, Ann Arbor, MI 48109-0666.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 8. Removal of TNFα from DC cultures results in reduced levels of CD80, CD86, and CD83 expression. TNFα added to 7-day DC cultures was subsequently removed by washing the cells 2 and 4 days later [denoted as TNF day 2(−) and TNF day 4(−), respectively]. The cultures were then replated in fresh SFM with added GM-CSF and IL-4 for an additional 5 or 3 days, respectively. Comparisons were made with DC cultured without TNFα (denoted Non-TNF DC) and those cultured in TNF for the complete 7-day period [denoted TNF day 7(+)]. FACS analysis of mean fluorescence channel is depicted. The results are representative of two experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4652/4/m_blod41203008x.jpeg?Expires=1769539242&Signature=AUjRc4C3JfUr9LFihbhMGQf21HJdM-WaiwWTzFpvhZmWDL6TLnyD7Dr9LolQHFLtHXnM1hDc4XK2oJORDHx8z3X8FpnUOwwisbkGGxs6LtANWd5mCe1fIf2-WVdskUeb9PtrxKPeSDwrSJq9LUbUfQyBDT7oYA1sCAhVGQ6~6b00zoUCzra3yJlc2ct33Hui8gi2RIfqW3vJRUcXQ0dsPPFAIaWrLVWAxnPASjqibrLIQ7fvJnKiM9Uq1W7paF1-OmTSeZb2drbWRO0VwckfebTCY7kNIXsIzTl-CEjZGp-Y85rca8AeRbzY7J9N7MuZEkyrHZm05x4~1P9~~-w9BA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Removal of TNFα from DC cultures results in reduced levels of antitetanus and primary allogeneic T-cell stimulation in vitro. Purified CD4+ T cells were cultured with DC generated in the presence of TNFα [denoted TNF DC day 7(+)], absence of TNFα (denoted Non-TNF DC), or after the removal of TNFα from the cultures at 2 days [denoted TNF DC day 2(−)] or 4 days [denoted TNF DC day 4(−)]. Proliferative responses were measured on day 6 at a DC to T-cell ratio of 1:20. The SEM of triplicate wells was always less than 15% of the mean. T cells alone = 1,534 ± 186 cpm; T cells + PHA = 30,081 ± 1,979 cpm. The results are representative of two experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4652/4/m_blod41203009x.jpeg?Expires=1769539242&Signature=0w4lVPCztDA7pnw~PPAQefGHd6awPa6U-9iXPDwJmZvYwZP5pmDAmi2lRYUG3oL-81Uu2tuYdUi8HxHTMJI~QyLPzboRXHzA9wyudPeDKWEvW3eAtXZhQtduy5y7gaUgl8s1b6aQhHBt5hXwTaMK0G0w8wUxEb199OXwNJ5bjK05HkXFTkX6Ha6NPK83PWq7Ig4AKF434gEUYEz1pvSpkR~Wine2ltJeqhRE16756mh~vxf3hJ12bOlKytUImEkgtBRXvJbWvPc4wBUDadDuII1RIEHlQccacNPWswIZM-sFLh82Y9howtMR2pC7uocLFBuy0AJOgEJSLXW6unJZpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal