Abstract
We have identified and characterized a human β (CC) chemokine, designated HCC-4, that is most closely related to HCC-1 and which demonstrates chemotactic activity for monocytes. Northern analysis of multiple tissue blots and of activated monocytes mRNA shows expression of a 500-bp mRNA. A 1,500-bp mRNA was highly expressed in monocytes activated 12 hours in the presence of interleukin-10 (IL-10) but was absent in monocytes activated for only 1 hour regardless of the presence or absence of IL-10. The upregulation of expression in the presence of IL-10 is in contrast to the downregulatory effects of IL-10 on expression of most other chemokines. Recombinant HCC-4 demonstrated chemotactic activity for human monocytes and THP-1 monocyte cells but not for resting lymphocytes or neutrophils. HCC-4 also induced a Ca2+ flux in THP-1 cells that was desensitized by prior exposure to RANTES. Taken together, these data indicate that HCC-4 is a novel chemokine whose expression is uniquely upregulated by IL-10.
ALTHOUGH THE BIOLOGY of interleukin-10 (IL-10) is very complex1 it has been associated with a number of anti-inflammatory effects. The anti-inflammatory properties of IL-10 include downregulation of adhesion and costimulatory molecules, inhibition of NO synthesis, and the downregulation of IL-1, IL-6, and tumor necrosis factor-α (TNF-α) production by monocytes.2 IL-10 has also been shown to upregulate the expression IL-1 receptor antagonist (IL-1ra), apparently through stabilization of its mRNA.3
The family of small peptide cytokines known as chemokines, in contrast, is generally associated with proinflammatory processes.4Indeed, a characteristic biological function of the chemokines is their ability to induce the trafficking of leukocytes into the site of an inflammatory response.4,5 In addition to these activities, various chemokines have been found to stimulate granule release by basophils and eosinophils6-8 and to upregulate the expression of adhesion markers. In keeping with this concept, expression of many of the various chemokines has been found to be upregulated by inflammatory cytokines such as IL-1, TNF-α, and interferon-γ (IFN-γ)9-12 and downregulated by the anti-inflammatory cytokine IL-10.9,10 12-15
In contrast to the large body of work supporting the role of chemokines in the initiation and effector phases of inflammation, there has been very little evidence suggesting an anti-inflammatory role for chemokines. We report here a previously uncharacterized human β (CC) chemokine that is upregulated in the presence IL-10 and that is active on monocytes. Because this chemokine is most closely related to HCC-116 and its uncharacterized splice variant HCC-3 (GenBank accession no. Z70293), we have designated it HCC-4.
MATERIALS AND METHODS
Bioinformatics.
A human expressed sequence tag (EST) with a high degree of homology to the β chemokines was identified using a chemokine consensus sequence as the basis for a TBLASTN search of the public database of ESTs (dbEST). Nucleic acid sequence analysis and editing was performed using the Sequencher (Genecodes Corp, Ann Arbor, MI). Prediction of the signal peptide cleavage site was made through the SignalP server (http://www.cbs.dtu.dk/services/SignalP).17 The nucleotide and protein sequences of human HCC-4 have been deposited in GenBank under the accession no. U91746. Phylogenetic analysis was performed using the Clustal W program.18
Nucleotide sequencing and sequence analysis.
The cDNA clone 77539 (corresponding to GenBank accession nos. T58847and T58775, encoding HCC-4) was purchased from Research Genetics Inc (Birmingham, AL). The nucleotide sequence of HCC-4 was then confirmed by automated sequencing using an Applied Biosystems 373 sequencer (Foster City, CA). The individual sequences obtained were then assembled into a contiguous sequence (contig) using Sequencher.
Tissue distribution and cellular expression of HCC-4 mRNA.
The tissue distribution of HCC-4 mRNA was assessed by Northern blot analysis of multiple tissue blots (Clonetech, Palo Alto, CA) and multiple tissue dot-blots (BioChain Institute, San Leandro, CA). Blots were hybridized with the full-length (1.5 kb) HCC-4 probe labeled with the DIG-High Prime kit (Boehringer Mannheim, Indianapolis, IN) according to the manufacturer's instructions and washed under high stringency conditions (65°C, 0.2× SSC). Expression was confirmed using a smaller probe corresponding to the coding region of HCC-4. The expression of HCC-4 was also analyzed by Southern blot of cDNA libraries using a 32P-labeled, full-length probe as previously described.19 Human monocyte mRNA was obtained from elutriated human monocytes activated with LPS (5 μg/mL) for either 1 hour or 12 hours in the presence of recombinant human IL-10 (200 U/mL) or the neutralizing anti–IL-10 monoclonal antibody (MoAb) 19F1 (10 μg/mL). The Northern analysis of the human monocytes was performed with the same probe used in the library blots.
Expression of recombinant HCC-4.
A DNA sequence encoding amino acids 24-120 of HCC-4 (corresponding to the predicted mature protein) was expressed in Escherichia colias a fusion protein with a 30-kD leader protein. After solubilization and refolding of the inclusion bodies, the fusion protein was cleaved with factor Xa and purified using Fast Flow S cation exchange column chromatography followed by C4 reverse-phase high-performance liquid chromatography (HPLC) column chromatography. The amino-terminal sequence was confirmed by sequencing and preparations obtained were greater than 97% pure as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Endotoxin levels were less than 0.1 ng/mg of protein.
Biological activity of recombinant HCC-4.
The biological activity of the recombinant protein was analyzed by microchemotaxis using a 48-well Boyden chamber (Neuroprobe, Cabin John, MD) as previously described.20 Ca2+ flux upon chemokine stimulation was measured using indo-1-AM (Calbiochem, La Jolla, CA) loaded THP-1 cells and a Photon Technologies spectrofluorometer (South Brunswick, NJ) with an excitation wavelength of 350 nm. Recombinant human RANTES was obtained from R&D Systems (Minneapolis, MN).
RESULTS
Identification of the human CC chemokine HCC-4 and localization to chromosome 17.
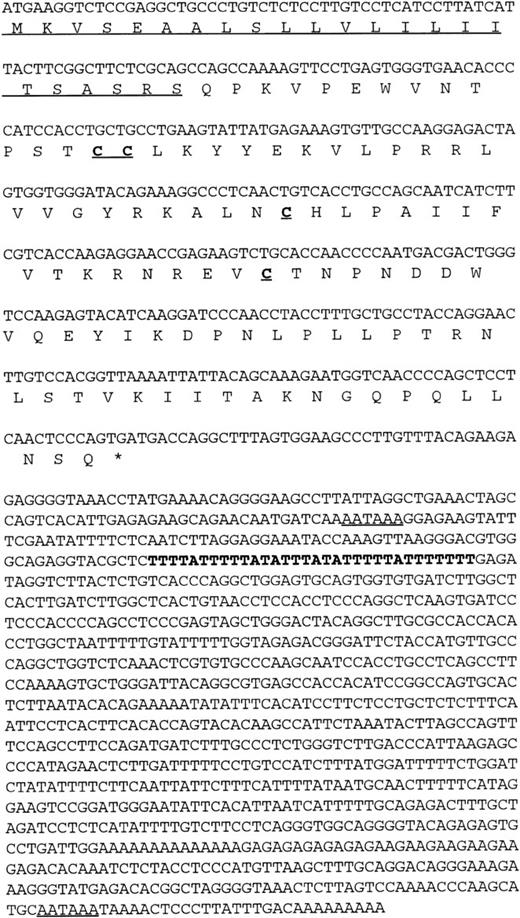
Using a chemokine consensus sequence as the basis for a TBLASTN search of the public EST database (dbEST) a single EST (GenBank accession no.T58847) was identified that encoded a novel human CC chemokine. TheT58847 sequence represents the 5′ end of IMAGE clone 77539, which originated from a cDNA library of adult liver. Using this sequence as the basis for a subsequent BLASTN search of dbEST showed no additional overlapping sequences (ie, no additional, contiguous sequences could be found); however, a 3′ sequence (GenBank accession no.T58775) was found by querying GenBank with the IMAGE clone number. This 3′ sequence contained an AATAAA polyadenylation signal near its end, indicating that the clone was likely to be full-length. To confirm this, clone 77539 was obtained and sequenced in its entirety. The results confirmed that the clone was indeed fulllength and that it encoded a novel β (CC) chemokine (Fig 1). Interestingly, the full-length sequence was found to contain two polyadenylation (polyA) signals as well as a potential mRNA instability sequence (Fig 1). The first polyA signal precedes the instability sequence, raising the possibility of two differentially regulated mRNAs depending on which polyA site is used. Indeed, we have observed shorter cDNA clones that are identical to clone 77539, except that it terminates in a polyA tail just downstream from the first polyA signal and lacks the putative instability sequence. The predicted amino acid sequence obtained from the ORF of clone 77539 shows a typical β chemokine sequence containing four conserved cysteines in the expected positions (Fig 1).
Nucleic and amino acid sequence of human HCC-4. The putative signal peptide is underlined as are the two potential polyA signal sequences present in the 3′ UTR. An AU-rich element in the 3′ UTR is indicated in bold. These sequence data have been submitted to GenBank under the accession no. U91746.
Nucleic and amino acid sequence of human HCC-4. The putative signal peptide is underlined as are the two potential polyA signal sequences present in the 3′ UTR. An AU-rich element in the 3′ UTR is indicated in bold. These sequence data have been submitted to GenBank under the accession no. U91746.
To determine the chromosomal localization of HCC-4, we searched the human gene map consortium data at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/SCIENCE96/ResTools.html) using the full-length HCC-4 cDNA sequence. This search found a single sequence tagged site (STS; GenBank accession no. G07028) that is identical to the last 300 bases of the 1.5-kb HCC-4 cDNA (data not shown), including a short repeat sequence of less than 40 bases. This STS has been mapped to a region between 58 and 65 centimorgans on chromosome 17 by the Whitehead Institute Center for Genome Research using a yeast artificial chromosome panel. Chromosome 17 is also known to contain a cluster of other CC chemokines.5,21 Recently, Naruse et al22 have reported characterizing a yeast artificial chromosome contig of human chromosome 17q11.2. Contained within this contig was a gene identical to HCC-4 that they designated NCC-4 and described as CC chemokine-like; however, the open reading frame of this molecule was not reported and there no further characterization of the putative chemokine was provided.22
Expression of HCC-4 mRNA and its regulation by IL-10.
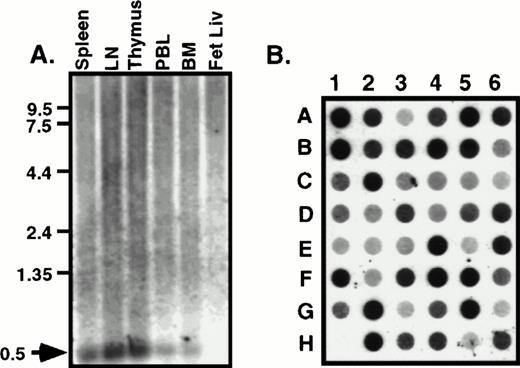
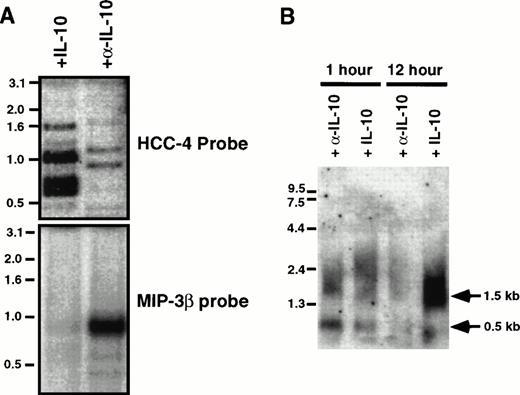
The tissue distribution of HCC-4 was determined using commercially available multiple tissue Northern blots. Surprisingly, we failed to detect the 1,500-bp mRNA predicted from the size of the IMAGE clone we had previously obtained. Instead, a 500-bp mRNA was detected in several human immune tissues (Fig 2A). This smaller message most likely results from use of the first polyA signal sequence. Indeed, as discussed above, we have isolated cDNA clones from several cDNA libraries that do appear to use this first polyA+ signal. The results of the Northern blot analysis were extended and confirmed by dot-blot analysis of a number of human tissues (Fig 2B). Identical results were obtained using a smaller probe consisting of the coding region alone (data not shown). To determine what cell types might express HCC-4, we performed Southern blotting of a number of human cDNA libraries constructed at DNAX. The results of these experiments suggest weak expression by some lymphocytes, including natural killer (NK) cells, γδ T cells, and some T-cell clones (data not shown). However, the highest level of expression detected was in lipopolysaccharide (LPS) + IFN-γ–activated human monocytes (Fig 3A). Interestingly, the amount of HCC-4 cDNA detected in a similar library of monocytes activated with IFN-γ and LPS in the presence of IL-10 was found to be much higher than in a library of the same cells activated as before, but without IL-10 and in the presence of a neutralizing anti–IL-10 MoAb (Fig 3A). In contrast, probing this same library with a cDNA encoding the recently reported IL-10–inhibitable chemokine macrophage inflammatory protein (MIP)-3β19 showed this chemokine to be virtually absent from monocytes activated in the presence of IL-10 (Fig 3A). The laddering effect in the HCC-4 cDNA blot (and weakly in the MIP-3β blot; Fig 3A and B) results from the variously sized inserts present in each library and has been observed for other molecules, particularly when their mRNA exceeds 1.0 kb in length23 (our unpublished observations).
HCC-4 is expressed in multiple tissue types. RNA from human immune tissues (A) or a multiple tissue dot-blot (B) were probed with a full-length HCC-4 probe. The 500-bp HCC-4 mRNA detected is indicated with an arrow and appropriate size markers are shown. Dot blot legend: A1, left atrium; B1, right atrium; C1, left ventricle; D1, right ventricle; E1, interventricle septum; F1, pericardium; G1, human DNA; H1, plasmid DNA; A2, frontal lobe; B2, temporal lobe; C2, occipital lobe; D2, parietal lobe; E2, thalamus; F2, pons; G2, cerebellum; H2, spinal cord; A3, parotid; B3, esophagus; C3, stomach; D3, small intestine; E3, colon; F3, rectum; G3, liver; H3, gallbladder; A4, throat; B4, bronchial trachea; C4, left lung; D4, right lung; E4, diaphragm; F4, skeletal muscle; G4, tongue; H4, adipose tissue; A5, kidney; B5, bladder; C5, prostate; D5, testis; E5, uterus; F5, breast; G5, ovary; H5, placenta; A6, thyroid; B6, pancreas; C6, adrenal; D6, tonsil; E6, thymus; F6, spleen; G6, lymph node; H6, appendix.
HCC-4 is expressed in multiple tissue types. RNA from human immune tissues (A) or a multiple tissue dot-blot (B) were probed with a full-length HCC-4 probe. The 500-bp HCC-4 mRNA detected is indicated with an arrow and appropriate size markers are shown. Dot blot legend: A1, left atrium; B1, right atrium; C1, left ventricle; D1, right ventricle; E1, interventricle septum; F1, pericardium; G1, human DNA; H1, plasmid DNA; A2, frontal lobe; B2, temporal lobe; C2, occipital lobe; D2, parietal lobe; E2, thalamus; F2, pons; G2, cerebellum; H2, spinal cord; A3, parotid; B3, esophagus; C3, stomach; D3, small intestine; E3, colon; F3, rectum; G3, liver; H3, gallbladder; A4, throat; B4, bronchial trachea; C4, left lung; D4, right lung; E4, diaphragm; F4, skeletal muscle; G4, tongue; H4, adipose tissue; A5, kidney; B5, bladder; C5, prostate; D5, testis; E5, uterus; F5, breast; G5, ovary; H5, placenta; A6, thyroid; B6, pancreas; C6, adrenal; D6, tonsil; E6, thymus; F6, spleen; G6, lymph node; H6, appendix.
Expression of HCC-4 mRNA is increased in the presence of IL-10. (A) Two cDNA libraries were made from pools of elutriated human monocytes stimulated for 1, 2, 6, 12, and 24 hours with LPS (5 μg/mL) and IFN-γ (200 U/mL) in the presence of IL-10 (200 U/mL) or the neutralizing anti–IL-10 MoAb 19F1 (α-IL-10; 10 μg/mL). The cDNAs were treated with restriction enzymes to release their inserts and analyzed by Southern blotting with either a 32P-labeled cDNAs for HCC-4 or MIP-3β, as indicated. For reference, the prominent MIP-3β band is approximately 800 bp in length. (B) Total RNA was obtained from elutriated human monocytes activated as described above for 1 or 12 hours. Ten micrograms of total RNA (per lane) was analyzed by Northern blotting with a 32P-labeled HCC-4 probe. The sizes of the two HCC-4 messages found are indicated with arrows. The equivalence of loading was assessed by ethidium bromide staining of the RNA.
Expression of HCC-4 mRNA is increased in the presence of IL-10. (A) Two cDNA libraries were made from pools of elutriated human monocytes stimulated for 1, 2, 6, 12, and 24 hours with LPS (5 μg/mL) and IFN-γ (200 U/mL) in the presence of IL-10 (200 U/mL) or the neutralizing anti–IL-10 MoAb 19F1 (α-IL-10; 10 μg/mL). The cDNAs were treated with restriction enzymes to release their inserts and analyzed by Southern blotting with either a 32P-labeled cDNAs for HCC-4 or MIP-3β, as indicated. For reference, the prominent MIP-3β band is approximately 800 bp in length. (B) Total RNA was obtained from elutriated human monocytes activated as described above for 1 or 12 hours. Ten micrograms of total RNA (per lane) was analyzed by Northern blotting with a 32P-labeled HCC-4 probe. The sizes of the two HCC-4 messages found are indicated with arrows. The equivalence of loading was assessed by ethidium bromide staining of the RNA.
To confirm the apparent upregulation of HCC-4 by IL-10, a Northern blot of elutriated human monocytes activated with LPS for either 1 or 12 hours in the presence of either IL-10 or anti–IL-10 MoAb was performed. This Northern blot analysis also showed two differently sized HCC-4 mRNA transcripts. A smaller transcript of approximately 500 bp, consistent with the band observed in tissue Northern blots (Fig2A), was detected in monocytes activated for 1 hour, but not in monocytes activated for 12 hours, whereas a larger message of approximately 1.5 kb, corresponding to the size of the original IMAGE clone, was dramatically increased after 12 hours of activation, but only in the presence of IL-10 (Fig 3B). The amount of 0.5-kb mRNA expressed after 1 hour of activation was relatively unaffected or slightly decreased in the presence of IL-10. The results of this Northern blot confirm the results of the library Southern blot, because HCC-4 mRNA was found to be more abundant in activated monocytes cultured in the presence of IL-10. Furthermore, they suggest that the increase seen in the cDNA libraries is largely attributable to an increase in HCC-4 mRNA levels later in moncyte activation.
As previously mentioned, a potential mRNA instability sequence is present between the first and second polyA+ signal sequences of the 1.5-kb clone (Fig 1). This suggests the possibility that expression of the 1.5-kb mRNA is regulated by this sequence and that IL-10 provides a signal necessary for its stability. This would also explain why the 0.5-kb message is relatively unaffected by IL-10, because it would lack the putative destabilization sequence. This finding is also consistent with the lack of a 1.5-kb message in the Northern blot of human immune tissues (Fig 2A), which presumably express negligible levels of IL-10. Future experiments will address the regulation of HCC-4 in greater detail.
Biological activity of HCC-4.
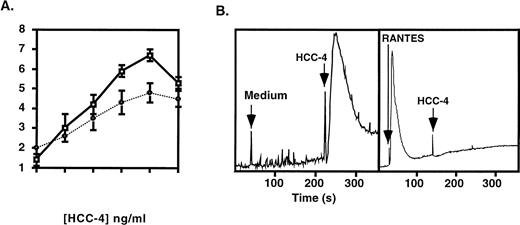
To determine if HCC-4 possessed chemotactic activity we produced a recombinant protein containing amino acids 24-120 of the full-length molecule. Recombinant protein was purified by HPLC and the amino-terminus was confirmed by sequencing. The recombinant HCC-4 demonstrated a dose-dependent ability to attract resting human monocytes (Fig 4A) and THP-1 human monocyte cells (Fig 4A, dashed line), with a peak response occurring at 1 μg/mL. Purified resting human T cells, B cells, and neutrophils were also tested but demonstrated no apparent response (data not shown). Controls for chemokinesis showed that the activity observed was indeed chemotaxis and not chemokinesis (data not shown).
Monocytes respond to recombinant HCC-4. Recombinant HCC-4 was used at the indicated concentrations in (A) microchemotaxis assays with human monocytes (□) and THP-1 cells (•) or (B) Ca2+ flux assay with THP-1 monocyte cells. The results shown are representative of three independent experiments. The chemotatic index (number of cells migrated/background) was calculated from the total cell count of five high power fields (1,000× at eyepiece) from duplicate wells. For calcium flux, the reponse of THP-1 cells to a HCC-4 concentration of 10−7 mol/L is shown. Desensitization by RANTES was at the same concentration. Flux buffer alone was used as a negative control. The results are displayed as the ratio of emmission 400:490 nm versus time.
Monocytes respond to recombinant HCC-4. Recombinant HCC-4 was used at the indicated concentrations in (A) microchemotaxis assays with human monocytes (□) and THP-1 cells (•) or (B) Ca2+ flux assay with THP-1 monocyte cells. The results shown are representative of three independent experiments. The chemotatic index (number of cells migrated/background) was calculated from the total cell count of five high power fields (1,000× at eyepiece) from duplicate wells. For calcium flux, the reponse of THP-1 cells to a HCC-4 concentration of 10−7 mol/L is shown. Desensitization by RANTES was at the same concentration. Flux buffer alone was used as a negative control. The results are displayed as the ratio of emmission 400:490 nm versus time.
In addition to chemotaxis, recombinant HCC-4 induced a Ca2+flux in THP-1 monocytes that was desensitized by prior incubation with RANTES (Fig 4B). The Ca2+ flux in response to HCC-4 could be observed at concentrations as low as 10−9 mol/L (data not shown) and was dose-dependent with a maximal response observed at 10−6 mol/L (Fig 4B). Experiments are presently underway to more precisely identify the specific receptor(s) used by HCC-4.
DISCUSSION
We have presented here the first characterization of this novel CC chemokine. We have shown that is expressed in a number of human tissues under normal circumstances, but whose expression by activated monocytes is dramatically increased in the presence of IL-10. We have designated this chemokine HCC-4 to denote is relationship to HCC-1. A previously uncharacterized chemokine was already designated HCC-2 (now Leukotactin-1),24 and the designation HCC-3 has also been used for an apparent splice variant (uncharacterized) of HCC-1 (Gen Bank accession no. Z70293).
HCC-4 is the first chemokine identified whose message is strongly increased in the presence of IL-10. This suggests that expression of HCC-4 mRNA would increase in the presence of IL-10. In contrast, other chemokines whose regulation by IL-10 has been examined are either downregulated by IL-10 or unaffected by its presence.9,10,12-15 Interestingly, IL-10 seems to accomplish the downregulation of chemokines through destabilization of their mRNAs.10 However, IL-10 can also act to stabilize mRNAs, as in the case of IL-1ra,3 and this may be the way in which IL-10 regulates the expression of the 1.5-kb HCC-4 mRNA. Future experiments will address the precise mechanism by which HCC-4 is regulated.
An exception to the general IL-10 inhibition of chemokine expression is monocyte chemotactic protein-1 (MCP-1), whose message in unstimulated monocytes was found to be slightly increased in the presence of IL-10.22 However, once the monocytes were activated by LPS, MCP-1 mRNA was found to be downregulated in the presence of IL-10.25 Thus, HCC-4 is uniquely upregulated by IL-10 and would most likely be present in a microenvironment that would be inhibitory for other known chemokines. This, in turn, suggests a unique biological role for HCC-4.
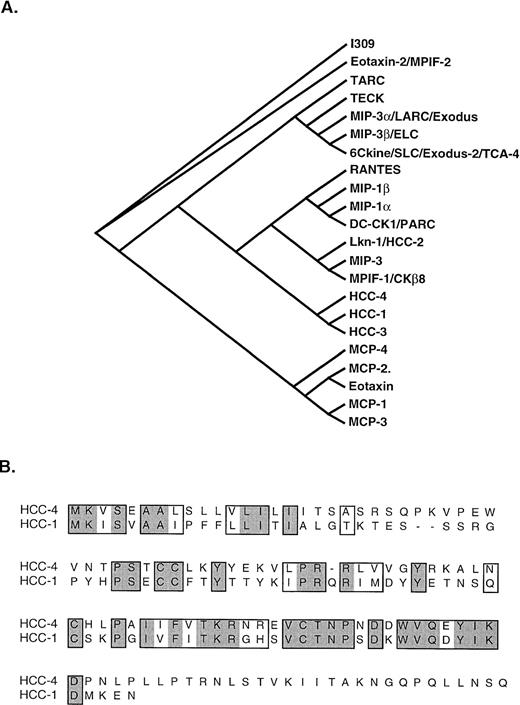
Phylogenetic analysis of HCC-4 shows it to be most closely related to the chemokine HCC-1 (Fig 5). This is most intriguing, because, like HCC-4, HCC-1 mRNA is expressed in a wide range of tissues and the protein shows activity on monocytes.16 Unlike most chemokines, HCC-1 was shown to be expressed at high levels in normal serum (1 to 10 nmol/L); however, little is known about the regulation of this gene.16
Phylogenic analysis of HCC-4 and alignment with HCC-1. (A) Phylogenic analysis of the relationship of HCC-4 to other human CC chemokines is shown. HCC-3 (GenBank accession no. Z70293) is a potential splice variant of HCC-1. MIP-3 (GenBank accession no. P55773) is a potential splice variant of MPIF-1. (B) CLUSTAL alignment of HCC-4 and HCC-1. Similar amino acid residues are boxed, whereas identities are boxed and shaded.
Phylogenic analysis of HCC-4 and alignment with HCC-1. (A) Phylogenic analysis of the relationship of HCC-4 to other human CC chemokines is shown. HCC-3 (GenBank accession no. Z70293) is a potential splice variant of HCC-1. MIP-3 (GenBank accession no. P55773) is a potential splice variant of MPIF-1. (B) CLUSTAL alignment of HCC-4 and HCC-1. Similar amino acid residues are boxed, whereas identities are boxed and shaded.
The region of highest homology between HCC-1 and HCC-4 extends from Cys-3 beyond Cys-4 and terminates just before a series of three prolines separated by two intervening amino acid residues. Just beyond these prolines a potential N-linked glycosylation site (NLS) is found in the HCC-4 sequence. It is not known whether this site is used in vivo, but antibodies are being raised to HCC-4 to facilitate such an analysis as well as to isolate the native form of the protein. It is possible that C-terminal modifications might modulate the biological activity of HCC-4, although we have observed that supernatant from HCC-4–transfected COS cells also exhibits chemotactic activity for monocytes, but not for lymphocytes.
In addition to its chemotatic effects on human monocytes and THP-1 monocyte cells, we also demonstrated that HCC-4 can cause a Ca2+ flux in THP-1 cells. This flux was maximal at 1 μmol/L and was completely inhibited by prior exposure of THP-1 to RANTES, suggesting that HCC-4 shares a receptor with RANTES. However, HCC-4 was unable to desensitize the RANTES response (data not shown); thus, HCC-4 may bind only a subset of RANTES receptors present on the surface of THP-1.
Historically, chemokines have been associated with the initiation and augmentation of inflammatory responses. They have been shown to attract inflammatory leukocytes, to activate neutrophils and granulocytes, and to be upregulated by cytokines of an inflammatory nature while being downregulated by anti-inflammatory cytokines. Other molecules whose expression is regulated by IL-10 in this fashion are associated with the resolution of an inflammatory response (reviewed in de Vries and de Waal Malefyt2). We suggest, by analogy, that HCC-4 may not be involved in the initiation of an inflammatory response, but rather in its resolution. Although this idea would at first appear to be in conflict with the ability of HCC-4 to attract monocytes, this is not necessarily the case.
Several observations suggest that the primary biological activity of HCC-4 and related chemokines may not be chemotaxis, but might instead be some other function. First, the in vitro chemotactic effects of HCC-4 on monocytes require relatively high concentrations when compared with other chemokines. Similarly, HCC-1, the chemokine most closely related to HCC-4, has no demonstrated chemotactic effects on monocytes,16 although, like HCC-4, it can generate a Ca2+ flux in these cells. Second, the mRNA for both HCC-1 and HCC-4 is expressed constitutively in a wide range of tissues16 (Fig 2B), and the HCC-1 protein is present at high levels in normal serum.16 Finally, as already discussed, increased expression of HCC-4 in the presence of IL-10 is not consistent with continued infiltration of inflammatory cells.
One possible alternative is that HCC-4 competes for binding to chemokine receptors used by inflammatory chemokines, thus regulating their activity. Their presence in the circulation (or in tissue) at high levels would then inhibit the directed migration of cells in response to chemokines such as MIP-1α and RANTES, thus slowing or blocking continued infiltration of inflammatory leukocytes. In any case, HCC-4 and related chemokines have a number of unusual characteristics that suggest that they do not function like typical chemokines (if such a thing exists) and that may lead us to discover novel functions for these intriguing molecules.
NOTE ADDED IN PROOF
While this manuscript was in press an article appeared describing HCC-4 as liver-expressed chemokine (LEC). The authors report two LEC mRNAs which are identical to the HCC-4 mRNAs.26
ACKNOWLEDGMENT
The authors acknowledge Anne-Marie O'Farrell and René de Waal Malefyt for providing mRNA blots for Northern analysis and Junichi Naganuma for assistance with expression analysis. We also acknowledge the contribution of R&D Systems in the production of recombinant HCC-4.
DNAX Research Institute is supported by Schering-Plough Corp.
Address reprint requests to Albert Zlotnik, PhD, DNAX Research Institute, 901 California St, Palo Alto, CA 94304; e-mail:zlotnik@dnax.org.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal