Abstract
Activation of the mitogen-activated protein kinase (Erk) and c-Jun terminal kinase is a well-documented mechanism for the seven transmembrane spanning receptors. We have previously shown that thrombin stimulation of the T-leukemic cell line Jurkat induced a transient increase in [Ca2+]i and tyrosine phosphorylation of several cellular proteins. Here, we have analyzed p42-44 MAPK, JNK and p38 MAPK activation using Jurkat T-cell lines deficient in either the tyrosine kinase p56Lck (JCaM1) or the tyrosine phosphatase CD45 (J45.01). Our results demonstrate that p56Lck and CD45 exert a negative control on thrombin-induced p38 MAPK activation and [Ca2+]i release in Jurkat cells. Thrombin receptor expression was identical on the different cell lines as assessed by FACS analysis. Tyrosine phosphorylation of p38 MAPK was drastically increased after thrombin stimulation of JCaM1 or J45.01 cells, as compared with parental cells (JE6.1). P42-44 MAPK and JNK activity also enhanced after thrombin treatment of JE6.1 and JCaM1 cell lines, whereas basal kinase activity was higher in J45.01 cells and was not further stimulated by thrombin. Thrombin and thrombin receptor agonist peptide-induced [Ca2+]imobilization paralleled p38 MAPK activation in JCaM1 and J45.01 cells. Moreover, reconstitution of J45.01 and JCaM1 cell lines with either CD45 or Lck is accompanied by restoration of a normal thrombin-induced [Ca2+]i response and p38MAPK phosphorylation. These data show that a component of the T-cell receptor signaling pathway exerts a negative control on thrombin-induced responses in Jurkat T cells. Accordingly, we found that thrombin enhanced tyrosine phosphorylation of p56Lck and decreased p56Lck kinase activity in J45.01 cells. Our results are consistent with a negative role for p56Lck on thrombin-induced [Ca2+]i release and p38 MAPK activation in Jurkat T-cell lines.
THE THROMBIN RECEPTOR is a member of the seven transmembrane receptor family that can transduce mitogenic stimulation by coupling to heterotrimeric G proteins, Gi and/or Gq,1-4 leading to activation of adenylate cyclase and phospholipase C respectively. Receptor activation occurs through proteolysis by thrombin at a specific site in the N-terminal portion of the receptor unmasking a sequence that functions as a ligand for the receptor.5,6 Synthetic peptides corresponding to this sequence mimic the action of thrombin.6-9 T-leukemic cell lines stimulated with thrombin or the thrombin receptor agonist peptide show increased cytoplasmic free calcium, phospholipase C stimulation, protein kinase C activation and as a consequence NFκB activation.1,10 We have previously shown that thrombin and thrombin receptor agonist peptide-stimulated tyrosine phosphorylation of several cellular proteins and more particularly proteins with molecular mass of 38, 42, 56, and 70 kD in Jurkat T cells.1Owing to the well-established effect of thrombin on p42-44 MAPK in different cell lines,11-14 it is likely that the two former proteins corresponded to members of the MAP kinase family.
The MAP kinases refer to a family of apparented serine/threonine kinases, including p42-44 MAPK encoded by the Erk2 and Erk1 gene that are activated by tyrosine and threonine phosphorylation in response to mitogenic stimuli15-17 and the JNK and p38 MAPK, which are also activated by tyrosine and threonine phosphorylation after stress or triggering of heterotrimeric G-protein–coupled seven-transmembrane–spanning receptors.18,19 These latters included both receptors that couple to Gi or to Gq, or both. In this line, it has been proposed that Gqα-mediated MAPK activation is PKC dependent and p21ras independent,20 whereas the Gi-coupled pathway is Gβγ mediated, p21rasdependent, and PKC-independent.21,22 In addition, thrombin has been found to increase tyrosine kinase activity of src kinases in human platelets and hamster CCL39 fibroblasts.23,24 It is also well established that Src family members are involved in the activation of the Ras signaling pathway through adaptors molecules such as Shc24 or p36 in T cells.25 Furthermore, it was recently proposed that thrombin induced a transient rise in both p38 MAPK tyrosine phosphorylation and activity in platelets, suggesting a role for this MAPK in thrombin-mediated signaling events in platelets.26
This study took advantage of the availability of different Jurkat cell lines deficient in either the tyrosine kinase p56Lck or the tyrosine phosphatase CD45 to investigate the role of components of the T-cell receptor (TCR) signaling pathway on thrombin responses in Jurkat cell lines. We found that the tyrosine kinase p56Lck exerted a negative regulation on thrombin-induced [Ca2+]irelease and p38 MAPK activation in Jurkat T cells.
MATERIALS AND METHODS
Cells and reagents.
Jurkat leukemic cell lines JCaM1, J45.01, JE6.1, and J45/CD45 (clone LB3.3-3) were kindly provided by Arthur Weiss (University of California, San Francisco) and have been described elsewhere.27 JCaM1/Lck were obtained from Jean Philippe Breittmayer (INSERM U343, Nice, France). Cells were maintained in RPMI 1640 medium/5% fetal calf serum (FCS) at 37°C, as previously described.2 Biotin-conjugated (4G10) antiphosphotyrosine antibody was purchased from UBI (Upstate Biotechnology, Lake Placid, NY), phospho-p38 MAPK (Tyr 182) or (Thr 180, Tyr 182), and phospho-p44/42 MAPK (Tyr 204) antibodies from New England Biolabs (Beverly, MA), anti-p56Lck from Santa Cruz Biotechnology (Santa Cruz, CA). Peroxidase-conjugated secondary antibody was purchased from DAKO. P38 MAPK and JNK antibodies were kind gifts of Benoit Derijeard (Centre de Biochimie CNRS-INSERM, Nice, France). ATAP2 MoAb was a generous gift from Lawrence F. Brass (University of Pennsylvania, Philadelphia). Bovine thrombin was obtained from Sigma and the highly potent thrombin receptor agonist peptide Ala-pfluoro-Phe-Arg-Cha-homo-Arg-Tyr-NH228 was purchased from Neosystem (Strasbourg, France).
Flow cytometry.
Cells were first incubated with the antithrombin receptor MoAb ATAP2 (1/200, 30 minutes, 4°C) followed by a biotin-conjugated goat anti-mouse IgG secondary antibody (1/500, 30 minutes, 4°C) and by streptavidin-phycoerythrin (1/500, 30 minutes 4°C). Analyses were performed by flow cytometry on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Cytosolic-free Ca2+ measurements.
Cytoplasmic free calcium levels were determined using the fluorescent dye indo-1 and an ATC 3000 cytofluorograph, as previously described.29-31 Briefly, cells were incubated for 1 hour with 4 μmol/L indo-1 in a buffer containing 140 mmol/L NaCl, 5 mmol/L KCl, 0.7 mmol/L MgCl2, 0.7 mmol/L CaCl2, 20 mmol/L HEPES, 10 mmol/L glucose, and 0.1% bovine serum albumin (BSA) (pH 7.4) (calcium buffer) at a final concentration of 5 × 106 cells/mL. After a fivefold dilution in the same medium, the mean violet/blue ratio of 3,000 cells was determined every 15 seconds after the addition of effectors.
Tyrosine protein phosphorylation and immunoblotting analysis.
Jurkat cells (3 × 106 cells/condition) were stimulated without or with either 100 nmol/L thrombin or 2.5 μmol/L thrombin receptor agonist peptide for the indicated times at 37°C. Stimulation was terminated by chilling rapidly the cells in liquid nitrogen. Cells were solubilized in lysis buffer containing 50 mmol HEPES, pH 7.4, 150 mmol/L NaCl, 20 mmol/L EDTA, 10 mmol/L sodium orthovanadate, 100 mmol/L NaF, 1% Nonidet P-40 (NP-40), and a cocktail of protease inhibitors (5 μg/mL aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride, 1 μmol/L pepstatin) for 30 minutes on ice and then centrifuged at 4°C for 15 minutes at 13,000 rpm. Supernatants were analyzed on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to Immobilon membrane (Millipore, Bedford, MA). Membranes were then blocked for 2 hours at room temperature with 3% (wt/vol) BSA and probed overnight with biotin-conjugated (4G10) antiphosphotyrosine antibody or phospho-specific p38 MAPK antibody or phospho-specific p44/42 MAPK antibodies. Blots were further incubated with horseradish peroxidase (HRP)-conjugated secondary antibody and immunoreactivity was detected by enhanced chemiluminescence.
P38 MAPK, p42-44 MAPK, JNK, and p56Lck activity assays.
Cell lysates were prepared as described earlier. After centrifugation, supernatants were incubated at 4°C for 18 hours with anti-p38 MAPK, anti-JNK, anti-p42/44 MAPK, or anti-p56Lck antibodies preadsorbed to protein A-Sepharose. Immune complexes were washed four times with lysis buffer and once with kinase buffer A (20 mmol/L HEPES, pH 7.4, 10 mmol/L MgCl2, 1 mmol/L dithiothreitol, 10 mmol/L p-nitrophenyl phosphate). Beads were finally resuspended in 40 μL kinase buffer containing 5 μg of recombinant ATF2 (p38 MAPK and JNK) or 5 μg of MBP (p42-44 MAPK) and [γ-32P]ATP (50 μmol/L, 5 μCi). Reactions were initiated by addition of ATP. After incubation at 30°C for 30 minutes, assays were terminated by the addition of 4 μL of 10× Laemmli sample buffer. The samples were heated at 95°C for 5 minutes and analysed by SDS/12% polyacrylamide gel electropheresis (PAGE). The gels were dried and subjected to autoradiography. P56Lck immunoprecipitates were performed as described earlier, except that the incubation was performed in buffer B (50 mmol/L HEPES, pH 6.8, 5 mmol/L MnCl2, 5 mmol/L MgCl2) containing 10 μg of enolase.
RESULTS
Cell-surface thrombin receptor expression.
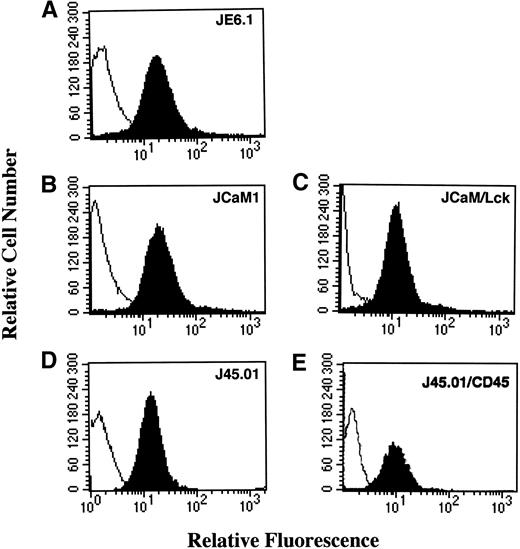
Expression of the thrombin receptor on different Jurkat cell lines was assessed by flow cytometry, using the monoclonal antibody (MoAb) ATAP2. Thrombin receptor was observed on the surface of the different Jurkat cell lines JE6.1 (parental) and variant clones JCaM1, JCaM1/Lck, J45.01, and J45.01/CD45 with a similar level of expression (Fig 1).
Thrombin receptor expression in different Jurkat cell clones. Cells were first stained with the antihuman thrombin receptor ATAP2 MoAb (black area) or an isotype matched control antibody (white area), followed by biotinylated goat antimouse antibody and phycoerythrin-streptavidin. Stained cells were analyzed with a flow cytometer FACScan (Becton Dickinson) gated to eliminate nonviable cells.
Thrombin receptor expression in different Jurkat cell clones. Cells were first stained with the antihuman thrombin receptor ATAP2 MoAb (black area) or an isotype matched control antibody (white area), followed by biotinylated goat antimouse antibody and phycoerythrin-streptavidin. Stained cells were analyzed with a flow cytometer FACScan (Becton Dickinson) gated to eliminate nonviable cells.
Thrombin and thrombin receptor agonist peptide induced a rapid increase in cellular protein tyrosine phosphorylation in different Jurkat cell lines.
Parental cell line JE6.1, p56Lck-deficient cell line JCaM1, and CD45-deficient cell line J45.01 were incubated for different times at 37°C with either 100 nmol/L thrombin or 2.5 μmol/L of the highly potent thrombin receptor agonist peptide Ala-pFluoro-Phe-Arg-Cha-homo-Arg-Tyr-NH2.28Cell lysates were immediately prepared and analysed by immunoblotting with the antiphosphotyrosine antibody 4G10. Figure 2A shows that thrombin induced a rapid and significant increase in the tyrosine phosphorylation of proteins with apparent molecular weights of 38 and 36 kD. Tyrosine phosphorylation of these proteins was observed within 15 seconds and was maintained for at least 1 minute. Significant differences in the level of tyrosine phosphorylation of these proteins as well as other (more particularly in the 60-kD region) were, however, observed in the three cell lines. Indeed, stimulation of p38 phosphorylation was only observed in JCaM1 and J45.01 cell lines, whereas phosphorylation of a p42/44-kD protein was observed to various extent in the three cell lines. Moreover, the phosphorylation of a 36-kD protein increased drastically in both deficient clones as compared with JE6.1. The same results were obtained when these different cell lines were stimulated by the thrombin receptor agonist peptide (Fig 2B).
Induction of tyrosine phosphorylation of cellular proteins by thrombin and thrombin receptor agonist peptide in different Jurkat cell clones. Cells were stimulated for the indicated times with 100 nmol/L thrombin (A) or 2.5 μmol/L thrombin receptor agonist peptide (B). Proteins from cell lysates were separated by SDS-PAGE and transferred to Immobilon membranes for Western blotting with biotin-conjugated 4G10 antibody. Blots were further incubated with horseradish peroxidase-conjugated secondary antibody and immunoreactivity was detected by enhanced chemiluminescence.
Induction of tyrosine phosphorylation of cellular proteins by thrombin and thrombin receptor agonist peptide in different Jurkat cell clones. Cells were stimulated for the indicated times with 100 nmol/L thrombin (A) or 2.5 μmol/L thrombin receptor agonist peptide (B). Proteins from cell lysates were separated by SDS-PAGE and transferred to Immobilon membranes for Western blotting with biotin-conjugated 4G10 antibody. Blots were further incubated with horseradish peroxidase-conjugated secondary antibody and immunoreactivity was detected by enhanced chemiluminescence.
Characterization of the low-molecular-weight thrombin-stimulated tyrosine-phosphorylated proteins.
Several thrombin-stimulated tyrosine-phosphorylated proteins have been identified in different studies, including p42-44 MAPK11and p38 MAPK in human platelets.32 To identify the proteins with apparent molecular weight of 38 and 42 to 44 kD, we used specific antiphosphotyrosine antibodies, that specifically recognized tyrosine phosphorylated p42-44 MAPK and p38 MAPK.
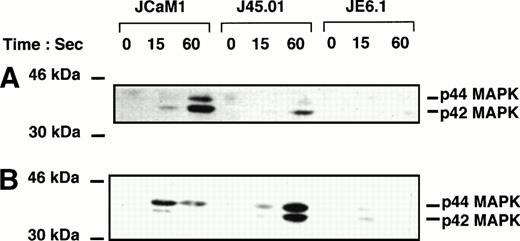
Figure 3 shows tyrosine phosphorylation of p42-44 MAPK after thrombin and thrombin receptor agonist peptide stimulation. Thrombin (100 nmol/L) and thrombin receptor agonist peptide (2.5 μmol/L) poorly stimulated tyrosine phosphorylation of p42–44 MAPK in JE6.1. By contrast, thrombin induced a significant increase in p42–44 MAPK tyrosine phosphorylation in J45.01 and in JCaM1 (Fig 3A). P42-44 MAPK was phosphorylated on tyrosine residues within 15 seconds. Identical results were obtained with thrombin receptor agonist peptide (Fig 3B), even though the ratio of p44 versus p42 phosphorylation was different in thrombin receptor agonist peptide-stimulated cells versus thrombin-treated cells.
Effect of thrombin and thrombin receptor agonist peptide on p42-44 MAPK tyrosine phosphorylation in different Jurkat clones. Cells were stimulated for the times indicated with 100 nmol/L thrombin (A) or 2.5 μmol/L thrombin receptor agonist peptide (B). Proteins from cell lysates were separated by SDS-PAGE and transferred to Immobilon membranes for Western blotting with phospho-specific p42-44 MAPK antibody. Development was performed as described in Fig 1.
Effect of thrombin and thrombin receptor agonist peptide on p42-44 MAPK tyrosine phosphorylation in different Jurkat clones. Cells were stimulated for the times indicated with 100 nmol/L thrombin (A) or 2.5 μmol/L thrombin receptor agonist peptide (B). Proteins from cell lysates were separated by SDS-PAGE and transferred to Immobilon membranes for Western blotting with phospho-specific p42-44 MAPK antibody. Development was performed as described in Fig 1.
Tyrosine phosphorylation of p38 MAPK after thrombin or thrombin receptor agonist peptide treatment was also assessed using highly specific antiphosphotyrosine p38 MAPK antibody. Thrombin (Fig 4A) and thrombin receptor agonist peptide (Fig 4B) induced a significant increase in p38 MAPK tyrosine phosphorylation in both JCaM1 and J45.01 cells after a 1-minute incubation. As expected from the results of Fig 2, p38 MAPK tyrosine phosphorylation was barely detectable in JE6.1 after thrombin or thrombin receptor agonist peptide stimulation. Increase in the phosphorylation of p38 MAPK cannot be accounted for by differences in the level of p38 MAPK, as approximatively the same amounts of p38 MAPK were detected by Western blot in each condition (Fig 4C). Densitometric scanning of the p38 phosphorylated band in the different Jurkat clones is shown in Fig 4D. There was a significant increase in p38MAPK phosphorylation in thrombin and agonist peptide induced JCaM1 and J45.01 clones, as compared with JE6.1 cells, even though basal p38MAPK phosphorylation was higher in agonist peptide-treated cells. Thrombin induced a rapid activation of p42-44 MAPK, P38 MAPK, and JNK in Jurkat cell lines.
Effect of thrombin and thrombin receptor agonist peptide on p38 MAPK tyrosine phosphorylation. Cells were stimulated for the times indicated with 100 nmol/L thrombin (A) or 2.5 μmol/L thrombin receptor agonist peptide (B). Proteins from cell lysates were subjected to SDS-PAGE as described above and transferred to Immobilon membranes for Western blotting using phospho-specific p38 MAPK antibody. Equal amounts of p38 MAPK were detected in each condition (C). Densitometric scanning of the p38 phosphorylated band present in (A) (thrombin-stimulated Jurkat clones) and (B) (thrombin receptor agonist peptide stimulated Jurkat clones) are shown. Results are representative of three experiments.
Effect of thrombin and thrombin receptor agonist peptide on p38 MAPK tyrosine phosphorylation. Cells were stimulated for the times indicated with 100 nmol/L thrombin (A) or 2.5 μmol/L thrombin receptor agonist peptide (B). Proteins from cell lysates were subjected to SDS-PAGE as described above and transferred to Immobilon membranes for Western blotting using phospho-specific p38 MAPK antibody. Equal amounts of p38 MAPK were detected in each condition (C). Densitometric scanning of the p38 phosphorylated band present in (A) (thrombin-stimulated Jurkat clones) and (B) (thrombin receptor agonist peptide stimulated Jurkat clones) are shown. Results are representative of three experiments.
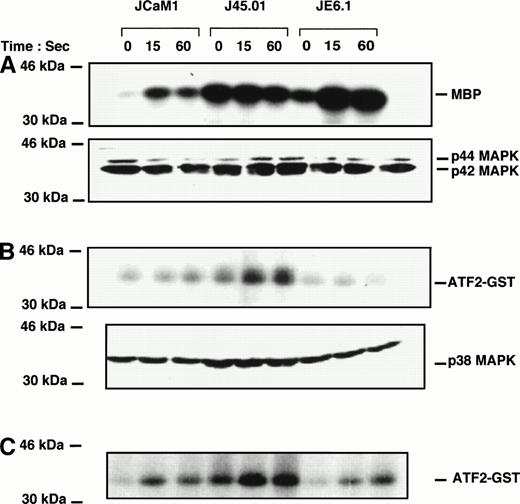
Thrombin induced a rapid increase in p42-44 MAPK activity in JCaM1 and JE6.1 within 15 seconds of stimulation. In J45.01, basal level of p42-44 MAPK activity was elevated, and thrombin failed to stimulate Erk1/Erk2 further (Fig 5A). Surprisingly, although p42-44 tyrosine phosphorylation was poorly detected upon thrombin treatment of JE6.1 cells (Fig 3), kinase activity was significantly increased (Fig 5A). However, this increase in MAP kinase activity could reflect phosphorylation on Thr of p42-44 MAPK, as the antibody used in the experiment presented in Fig 3 recognized specifically the tyrosine-phosphorylated form of p42-44 MAPK. In JCaM1, p42-44 MAPK tyrosine phosphorylation correlated with the kinase activity. However, we did not find a strict correlation between p42-44 MAPK phosphorylation and activity in both J45.01 and JE6.1 clones. Here again, phosphorylation on threonine residues might explain some discrepancies observed between tyrosine phosphorylation of p42-44 MAPK and kinase activity, but the simplest explanation for these differences is likely to come from the different features of the antibodies used in each type of experiment. Nevertheless, variations in kinase activity were not caused by differences in the amount of proteins loaded on the gels (Fig 5A, bottom).
Effect of thrombin on p42/44, p38 MAPK, and Jun kinase activities in different Jurkat clones. Cells were stimulated with 100 nmol/L thrombin for the times indicated. Cell lysates were prepared as described in Materials and Methods and incubated overnight with anti-p42/44 MAPK (A) , anti-p38 MAPK (B), or anti-JNK antibodies (C) preadsorbed to protein A-Sepharose. Immune complexes were washed and kinase activities were determined using myelin basic protein for p42-44 MAPK and ATF2-GST for p38 MAPK and JNK. Equal amounts of p42/44 MAPK and p38 MAPK were immunoprecipitated in each condition (A and B, bottom).
Effect of thrombin on p42/44, p38 MAPK, and Jun kinase activities in different Jurkat clones. Cells were stimulated with 100 nmol/L thrombin for the times indicated. Cell lysates were prepared as described in Materials and Methods and incubated overnight with anti-p42/44 MAPK (A) , anti-p38 MAPK (B), or anti-JNK antibodies (C) preadsorbed to protein A-Sepharose. Immune complexes were washed and kinase activities were determined using myelin basic protein for p42-44 MAPK and ATF2-GST for p38 MAPK and JNK. Equal amounts of p42/44 MAPK and p38 MAPK were immunoprecipitated in each condition (A and B, bottom).
We then looked for p38 MAPK activity in thrombin-stimulated Jurkat cells. Basal p38 MAPK activity was higher in J45.01 cells than in JE6.1 or JCaM1 cells (Fig 5B). This higher basal activity could perfectly be explained by the higher level of phosphorylated p38 MAPK in J45. 01 cells (Fig 4A). Thrombin failed to induce P38 MAPK activity in JE6.1 according to the fact that p38 MAPK was not phosphorylated in these cells (Figs 2 and 4). Conversely, thrombin induced a significant increase in p38 MAPK activity in both deficient cell lines.
P56Lck exerts a negative control on thrombin-induced p38 MAPK activation in Jurkat cells.
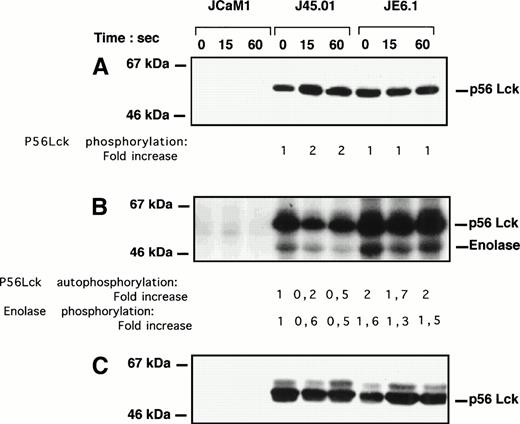
As p56Lck is critical for signaling via the TcR34 and TcR triggering induced a total loss of thrombin response,1 we sought to analyze the effect of thrombin on p56Lck phosphorylation and activity. The level of p56Lck phosphorylation was visualized after a 1-minute thrombin stimulation after immunoprecipitation, followed by Western blotting with a rabbit polyclonal anti-p56Lck antibody. Thrombin stimulated p56Lck tyrosine phosphorylation in J45.01 cells within 15 seconds, whereas level of p56Lck phosphorylation remained unchanged in JE6.1 (Fig 6A). As expected, p56Lck phosphorylation was undectectable in JCaM1 cells (Fig 6A). Basal p56Lck phosphorylation was approximatively twofold lesser in J45.01 cells as compared with JE6.1 cells. Furthermore, thrombin-induced phosphorylation of p56Lck was accompanied by a significant decrease in p56Lck autophosphorylation (50% to 80%) and kinase activity (40% to 50%) in J45.01 cells, as judged by the phosphorylation of enolase (Fig6B). Again inhibition of p56Lck kinase activity in J45.01 cells did not reflect differences in the amount of immunoprecipitated p56Lck (Fig6C).
p56Lck tyrosine phosphorylation and activity in different Jurkat cell clones. Cells were stimulated with 100 nmol/L thrombin for the times indicated. (A) Cell lysates were immunoprecipitated with 4G10 antibody preadsorbed on protein A-Sepharose. Immunoprecipitated proteins were subjected to SDS-PAGE and transferred to Immobilon membranes for Western blotting with anti-p56Lck antibody. Development was performed as described above. (B) Cell lysates were immunoprecipitated with anti-p56Lck antibody preadsorbed to protein A-Sepharose. After extensive washing, p56Lck activity was determined with enolase as exogenous substrate. (C) Equal amounts of p56Lck were immunoprecipitated in JE6.1 and J45.01 cells. Note the lack of p56Lck and p56Lck activity in JCaM1.
p56Lck tyrosine phosphorylation and activity in different Jurkat cell clones. Cells were stimulated with 100 nmol/L thrombin for the times indicated. (A) Cell lysates were immunoprecipitated with 4G10 antibody preadsorbed on protein A-Sepharose. Immunoprecipitated proteins were subjected to SDS-PAGE and transferred to Immobilon membranes for Western blotting with anti-p56Lck antibody. Development was performed as described above. (B) Cell lysates were immunoprecipitated with anti-p56Lck antibody preadsorbed to protein A-Sepharose. After extensive washing, p56Lck activity was determined with enolase as exogenous substrate. (C) Equal amounts of p56Lck were immunoprecipitated in JE6.1 and J45.01 cells. Note the lack of p56Lck and p56Lck activity in JCaM1.
Thrombin-induced [Ca2+]i release is increased in J45.01 and JCaM1 cells.
Figure 7 depicts a time course of [Ca2+]i release after thrombin and thrombin receptor agonist peptide stimulation. Thrombin induced a transient increase in [Ca2+]i, with a peak at 45 seconds, in the three cell lines. This rapid increase in [Ca2+]i after thrombin stimulation has been ascribed solely to the release from internal stores1,2 and is mediated by the heterotrimeric pertussis toxin insensitive Gq protein.1 However, the peak and amount of [Ca2+]i released were drastically increased (twofold to threefold) in J45.01 and JCaM1 cells as compared with parental cell lines JE6.1 (Fig 7A). Maximal stimulation was observed for 20 nmol/L thrombin, whatever the cell lines (Fig 7B). The kinetics and dose-response curves for thrombin receptor agonist peptide-induced [Ca2+ ]i release in the three cell types were identical to those observed in the presence of thrombin (Fig 7C and D). However, [Ca2+]i increase was always significantly higher in J45.01 cells, as compared with JCaM1 cells (Fig7A through D).
Effect of thrombin and thrombin receptor agonist peptide on [Ca2+]i in Jurkat clones deficient in either the tyrosine kinase p56Lck or tyrosine phosphatase CD45. The cell lines used were the leukemic cell line Jurkat (clone JE6.1, ⊡) and two variants lacking p56Lck (JCaM1, ▴) or the CD45 molecule (J45.01, ⊠). Cells were loaded with indo-1 as described in Materials and Methods. Thrombin 100 nmol/L or thrombin receptor agonist peptide (2.5 μmol/L) was added at 0 time and fluorescence monitored as a function of time. (A and C) Time course of thrombin and thrombin receptor agonist peptide effects. (B and D) Dose-response curves for thrombin (2 to 100 nmol/L) and thrombin receptor agonist peptide (0.1 to 2 μmol/L) effects. Results are representative of three different experiments.
Effect of thrombin and thrombin receptor agonist peptide on [Ca2+]i in Jurkat clones deficient in either the tyrosine kinase p56Lck or tyrosine phosphatase CD45. The cell lines used were the leukemic cell line Jurkat (clone JE6.1, ⊡) and two variants lacking p56Lck (JCaM1, ▴) or the CD45 molecule (J45.01, ⊠). Cells were loaded with indo-1 as described in Materials and Methods. Thrombin 100 nmol/L or thrombin receptor agonist peptide (2.5 μmol/L) was added at 0 time and fluorescence monitored as a function of time. (A and C) Time course of thrombin and thrombin receptor agonist peptide effects. (B and D) Dose-response curves for thrombin (2 to 100 nmol/L) and thrombin receptor agonist peptide (0.1 to 2 μmol/L) effects. Results are representative of three different experiments.
Reconstitution of JCaM1 and J45.01 cells.
We then looked for thrombin-induced [Ca2+]iresponse and p38 MAPK phosphorylation after retransfection of CD45 and Lck-deficient Jurkat cell lines. Consistent with the results depicted in Fig 7, J45.01 and JCaM1 cell lines exhibited a huge increase in thrombin-induced [Ca2+]i response, as compared with JE6.1 cells (Fig 8A). This response was lost in cells stably retransfected with CD45 and Lck.
Effect of thrombin on CD45 and Lck reconstituted deficient clones. (A) Thrombin-induced [Ca2+]i responses. JE6.1 (□), J45.01 (⊞), J45.01/CD45 (⧫), JCaM1 (▴), or JCaM1/Lck (◊) were stimulated with 100 nmol/L thrombin and fluorescence analyzed as a function of time. (B and C) Thrombin-mediated phosphorylation of p38MAPK. Cells were stimulated with 100 nmol/L thrombin for the times indicated. Proteins from cell lysates were subjected to SDS-PAGE as described in the legend to Fig 4 and were transferred to Immobilon membranes for Western blotting using phospho-specific p38 MAPK antibody. Equal amounts of p38 MAPK were detected in each condition (not shown).
Effect of thrombin on CD45 and Lck reconstituted deficient clones. (A) Thrombin-induced [Ca2+]i responses. JE6.1 (□), J45.01 (⊞), J45.01/CD45 (⧫), JCaM1 (▴), or JCaM1/Lck (◊) were stimulated with 100 nmol/L thrombin and fluorescence analyzed as a function of time. (B and C) Thrombin-mediated phosphorylation of p38MAPK. Cells were stimulated with 100 nmol/L thrombin for the times indicated. Proteins from cell lysates were subjected to SDS-PAGE as described in the legend to Fig 4 and were transferred to Immobilon membranes for Western blotting using phospho-specific p38 MAPK antibody. Equal amounts of p38 MAPK were detected in each condition (not shown).
Moreover, there was a good correlation between thrombin-induced [Ca2+]i response and p38 MAPK phosphorylation. Indeed, reconstitution of J45.01 and JCaM1-deficient cell lines with either CD45 or p56Lck was accompanied by loss of thrombin-mediated p38MAPK phosphorylation (Fig 8B and C).
DISCUSSION
Thrombin effects on platelets, fibroblasts, and vascular smooth muscle cells have been well documented.35 Thrombin plays a central role on platelets, causing aggregation and secretion of granules and on fibroblasts, eliciting mitogenic responses. On vascular smooth muscle cells, thrombin enhances endothelial permeability and induces the production of both inflammatory factors36 and growth factors.37 Although thrombin also causes chemotaxis and adhesion of monocytes and neutrophiles,38 its action on components of the immune system has remained poorly documented. The recent characterization of the thrombin receptor on T lymphocytes or T-leukemic cell lines underscores the potential role of thrombin at sites of hemostatic stress and inflammation.1,2,10 39
We have examined signaling events after thrombin stimulation of Jurkat cell lines either deficient in the p56Lck tyrosine kinase or the CD45 tyrosine phosphatase. We report here that thrombin activated the three MAPK pathways in Jurkat cells. P42-44 MAPK activation is a well-characterized mechanism for G-protein–coupled receptor.20,40,41 Accordingly, we show that thrombin induced tyrosine phosphorylation and activation of p42-44 MAPK in Jurkat T-cell lines. As previously reported in platelets,11thrombin responses are rapid and transient, maximal within 15 seconds after stimulation with the protease or the thrombin receptor agonist peptide. P42-44 MAPK and JNK activation were observed to various extent both in p56Lck and CD45-deficient cell lines and in parental cell lines, suggesting that thrombin-induced p42-44 MAPK and JNK activity might be largely independent on p56Lck or CD45 expression.
Conversely, phosphorylation and activation of p38 MAPK was drastically higher in p56Lck and CD45-deficient cell lines, suggesting that one or more components of the TcR signaling pathway exert a negative control on thrombin-induced p38 MAPK activation in Jurkat cells. Furthemore, thrombin stimulation of Jurkat cells resulted in an increase in [Ca2+]i release, which was drastically enhanced in cell lines deficient in either the p56Lck or the CD45 molecule. In addition, thrombin and thrombin receptor agonist peptide-induced [Ca2+]i release was significantly higher in J45.01 cells than in JCaM1 cells. The reasons for which J45.01 cells exibited higher levels of [Ca2+]i than JCaM1 cells in response to thrombin or thrombin receptor agonist peptide is unknown. This effect cannot be accounted for by differences in thrombin receptor expression in JE6.1, JCaM1, JCaM1/Lck, J45.01, or J45.01/CD45 clones. However, one may supposed that besides p56Lck other signaling molecules regulated by the CD45 tyrosine phosphatase may exert a negative control on thrombin responses in Jurkat cells, a good candidate for this type of effect being p59Fyn. Finally, we also found that a first treatment of JE6.1 cells with an anti-CD3 MoAb (OKT3) totally impaired further stimulation by thrombin or the thrombin receptor agonist peptide2 (not shown), whereas after a prior stimulation with OKT3, JCaM1, and J45.01 cells were still able to respond fully to thrombin and thrombin receptor agonist peptide (not shown). Taken together, these findings suggest a cross-talk between signaling components of the TcR and those of the thrombin receptor, controling at least [Ca2+]i release and p38 MAPK activation. Interestingly, reconstitution of JCaM1 and J45.01 cells with either p56Lck or CD45 was sufficient to restore normal thrombin responses (ie, identical to those observed in JE6.1 cells).
Activation of the p38 MAP kinase cascade by seven-transmembrane receptors is a recently described mechanism of intracellular signaling by this family of receptors.32,42 On the basis of our findings on an enhanced response to thrombin in deficient cell lines, we hypothesized that the increase in thrombin-induced p38 MAPK activation and [Ca2+]i release could be due to the lack of p56Lck expression and to a decrease of p56Lck activity in JCaM1 and J45.01, respectively. Accordingly, we found that thrombin stimulated tyrosine phosphorylation of p56Lck in J45.01, but not in JE6.1 cells. This increase in p56Lck tyrosine phosphorylation was accompanied by a significant decrease in kinase activity. Weiss and Littman34 recently proposed that the basal state of activation of the TcR signaling pathway is an equilibrium between positive and negative regulatory signals. Thus it appears that inhibition of p56Lck kinase activity could be the mechanism by which thrombin induced [Ca2+]i release and p38 MAPK activation in Jurkat cells. Consistently, lack of p56Lck or decreased p56kinase activity upon thrombin stimulation of J45.01 cells was accompanied by an increase in [Ca2+]i release and p38 MAPK activation. However, other components of the TcR signaling pathway such as p59Fyn may also be important in thrombin signaling, especially in view of the higher thrombin response in J45.01 cells.
In this study we also observed tyrosine phosphorylation of a 36-kD protein after thrombin and thrombin receptor agonist peptide stimulation. Phosphorylation of p36 paralleled that of p38 MAPK and was also strongly increased in JCaM1 and J45.01 cell lines. Several observations support the notion that p36/Lnk mediates interaction between Grb2 and PLCγ1 and links the TcR to the ras pathway.43 Moreover, in HEL cells and platelets, a 36-kD molecule associates with and is phosphorylated by Csk.44This association is thought to relocate the cytosolic kinase to the particulate fraction in contact with members of the src family of tyrosine kinases. If the 36-kD protein phosphorylated on tyrosine upon thrombin and thrombin receptor agonist peptide stimulation corresponds to p36/Lnk, an attractive hypothesis would be that phosphorylated p36 can bind and relocate Csk to the plasma membrane where it could in turn inactivate p56Lck by phosphorylation. This would bring a good explanation for the decrease in p56Lck activity observed in J45.01 cells treated with thrombin. Accordingly, preliminary results indicate that thrombin induced relocation of Csk in Jurkat T cells (not shown).
In summary, our data demonstrate a cross-talk between components of the TcR and the thrombin receptor signaling pathways in Jurkat T cells. We also show that the tyrosine kinase p56Lck is likely to exert a negative regulation on thrombin-induced [Ca2+]irelease and p38 MAPK activation in T lymphocytes. This model may have important physiological implications more particularly concerning thrombin responses in preactivated or anergized T cells on sites of hemostatic stress and inflammation. Thus thrombin receptor expression in T cells may be particularly relevant to a role in preactivated or anergized T lymphocytes, where TcR signaling is impaired. This hypothesis is in line with the apparent activation-induced regulation of thrombin receptor mRNA expression in peripheral blood lymphocytes.1
NOTE ADDED IN PROOF
While this manuscript was in revision, Joyce et al reported an enhanced thrombin receptor signaling in a TCR-negative T-cell line and T-cell lines deficient in either p56Lck or CD45.45
ACKNOWLEDGMENT
We thank Jean-François Peyron for helpful discussion and Aurore Grima for illustration work. JE6.1, JCaM1, J45.01, and J45.01 reconstituted cells (LB3.3-3) were kindly provided by Arthur Weiss (University of California, San Francisco). We are undebted to Lawrence F. Brass for the kind gift of human thrombin receptor antibodies.
Supported by the Ligue Nationale contre le Cancer, by the Féderation Nationale des Entreprises Françaises dans la Lutte contre le Cancer, and by Grant No. 6684 from the Association pour la Recherche Contre le Cancer.
Address reprint requests to Dr Patrick Auberger, CJF INSERM 96.05, Faculté de Médecine, Av de Valombrose, 06107 Nice Cédex 02, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



![Fig. 7. Effect of thrombin and thrombin receptor agonist peptide on [Ca2+]i in Jurkat clones deficient in either the tyrosine kinase p56Lck or tyrosine phosphatase CD45. The cell lines used were the leukemic cell line Jurkat (clone JE6.1, ⊡) and two variants lacking p56Lck (JCaM1, ▴) or the CD45 molecule (J45.01, ⊠). Cells were loaded with indo-1 as described in Materials and Methods. Thrombin 100 nmol/L or thrombin receptor agonist peptide (2.5 μmol/L) was added at 0 time and fluorescence monitored as a function of time. (A and C) Time course of thrombin and thrombin receptor agonist peptide effects. (B and D) Dose-response curves for thrombin (2 to 100 nmol/L) and thrombin receptor agonist peptide (0.1 to 2 μmol/L) effects. Results are representative of three different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4232/4/m_blod41132007x.jpeg?Expires=1763569677&Signature=IJpmQ4MHPvkagj7VTD8dUdCLdRGzVB34ClDXZ6HKQOjacQwwIn0jqd7V8vm3GSj28nE7KFJAnsaIdL861qDUzliwNnrrDtj8xlJ5EkC-fVhIiYi~Rh1trCZeCvnlCYYaaeidPmp0huN6p4HTMPLHyC-Dsxbq8KWc5OT65H6E6DYHJ~A6ikcd2jrfX7YfxMAUw9Ct2wINLoBeUrkFznXkf3JT2ITvnvuvelBcefua3tD59AqYlBqLtkzwpgePEwJ1c4z-6zhQm5ehtWu60Y4LJx0DwxTU78QKfuEf7nSO70e-RjiZzFp3kIcil0IWLJ6iL6raAmIGzT7fDqiMdBDJ9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effect of thrombin on CD45 and Lck reconstituted deficient clones. (A) Thrombin-induced [Ca2+]i responses. JE6.1 (□), J45.01 (⊞), J45.01/CD45 (⧫), JCaM1 (▴), or JCaM1/Lck (◊) were stimulated with 100 nmol/L thrombin and fluorescence analyzed as a function of time. (B and C) Thrombin-mediated phosphorylation of p38MAPK. Cells were stimulated with 100 nmol/L thrombin for the times indicated. Proteins from cell lysates were subjected to SDS-PAGE as described in the legend to Fig 4 and were transferred to Immobilon membranes for Western blotting using phospho-specific p38 MAPK antibody. Equal amounts of p38 MAPK were detected in each condition (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4232/4/m_blod41132008w.jpeg?Expires=1763569677&Signature=Eg5utTGrybq98J8VKLhfETzS-0U9PTeDWBGLS~NoCPuUcI3GkgRWp-ji~clOwjw3aKcYMOPxD9DCuF7cD6R-8iS0TUn0rUXz5j8r5aCFzgkUIg-jD--vhRP9g~HSovdKnLc43suN4KjFzsVir1FznRY~odD4nI7Zcs74meHQBG4ObY~wo6-B7Mc9Y28miX80UVvuC3DOhEApI8I~sPdTZdxP5RKtgAY2~ljeSsFkn5lFPYRO4FreyIz8eF8m-HzmWYUhcBWBD8FNnbQ9sbVtU6VOMivSS~n9fhkCInAriQmNs4kFCqQQyoY4Ug3vd6rYgjBKiAHNRI2texO7AqfJ9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal