Abstract
The Caco-2 cell line grown in bicameral chambers was used to study the effect of transferrin in the basal chamber on the transepithelial transport of iron. We have shown that when iron was offered as59Fe on the apical surface of the Caco-2 cells, transport of 59Fe into the basal chamber was stimulated by 50 μmol/L apotransferrin. Here, we examined the effect on59Fe transport of lower concentrations of apotransferrin, as well as the effects on transport of ovo-, cobalt-, and ferri-transferrin and of iron chelators with an affinity for iron greater than that of transferrin. The stimulation of 59Fe transport was more sensitive to the presence of apotransferrin with a Km of 0.078 ± 0.008 μmol/L compared with ferri-transferrin with a Km of 1.24 ± 0.39 μmol/L (P < .006). 59Fe transport was less sensitive to diethylenetriaminopenta-acetic acid (DTPA) than apotransferrin with Kms of 1.52 ± 0.70. The chelator nitrilotriacetic acid (NTA) exhibited no stimulation of59Fe transport. Analysis of laser scanning confocal micrographs showed that apotransferrin labeled with Texas Red is internalized by Caco-2 cells from the basal side and localizes in distinct vesicles above the nucleus. The sensitivity of apotransferrin in stimulating Fe transport suggests a unique interaction of apotransferrin with the basal surface of the intestinal epithelium.
THE TRANSPORT OF IRON across the intestinal epithelium occurs in three phases: an uptake phase during which iron is transported from the lumen of the intestine into an apical compartment of the epithelial cells; a transport phase during which iron is transported across the cell to the serosal or basolateral surface of the cell; and a transfer phase during which the iron is transported across the basolateral membrane to the plasma. This report advances our understanding of the mechanisms operating in the transfer phase.
The mechanism(s) controlling the transfer phase are enigmatic. The role of transferrin (Tf), the plasma iron transport protein, in the transfer phase has been evaluated in a variety of studies.1-3 Transferrin receptors are present on the basolateral surface of the intestinal epithelium and undergo recycling as in other cells.4,5 These receptors may be involved in iron uptake into intestinal epithelium for normal cellular metabolism. The question addressed by a variety of studies is whether transferrin, presumably interacting through these receptors, is directly involved in iron release. There is evidence that iron transported across the intestinal mucosa is released into the portal circulation in a low molecular weight form to be taken up by the liver and then released from the liver onto transferrin.6 Recent evidence from studies with the hypotransferrinemic mouse also suggests that transferrin is not involved in release and that three distinct, although as yet unidentified, species of nontransferrin iron can be detected.2,3 Other studies suggest a constitutive release of iron from the epithelial cells with the released iron rapidly binding to transferrin in a process dependent on a dialyzable moiety, presumably bicarbonate.7 The Caco-2 cell line, when grown as a polarized cell layer in bicameral chambers, has been used to delineate various aspects of the uptake and transfer phases of intestinal iron transport by other groups8-14 and by us.15-18 We have previously shown that the transfer phase is facilitated by the presence of apotransferrin (apo-Tf) in the basal chamber16 and recently we have reported that apo-Tf undergoes a different endocytic cycle than ferri-transferrin (FeTf).18
The present studies were initiated to determine the specificity and sensitivity of the effect of apotransferrin and if apotransferrin could be visualized by laser scanning confocal microscopy within the Caco-2 cells. As part of these studies, we determined if the stimulation of Fe transport by apo-Tf merely reflected exchange of iron from the small portion of ferri-transferrin in the apo-Tf preparations with iron within the cells. In addition, we determined the effect of ovo-transferrin (ovo-Tf), which is purported not to interact with the transferrin receptor, and cobalt-Tf, as Co bound to Tf, is kinetically inert.19 Finally, to determine if apo-Tf were serving merely as a chelator of iron, we examined the effect on the transfer phase of chelators with a greater affinity for iron than transferrin. We found that apo-Tf has unique characteristics that allow apo-Tf to enhance the transfer phase of the process of iron absorption.
MATERIALS AND METHODS
Cell culture.
Caco-2 cells, from American Type Culture Collection #HTB37 (Rockville, MD), were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS; GIBCO, Gaithersburg, MD), 1% nonessential amino acids, and antibiotics/antimycotic (100 U/mL penicillin-G, 100 U/mL streptomycin, and 250 ng/mL fungizone; GIBCO). Cells were grown in Transwell bicameral chambers with 3 μmol/L pore size membrane (Costar, Cambridge, MA) coated with collagen. The collagen film was applied to the filter as 50 μL of collagen solution (0.1 mg/mL, 60% ethanol; rat tail, type I; Boehringer, Mannheim, Germany) and then the Transwells were inverted and dried under a sterile laminar air flow. Formation of a monolayer was monitored by measuring the transepithelial electrical resistance (TEER) with a Millicell electrical resistance system (Millipore, Bedford, MA). Cell layers were used only after TEER had increased to greater than 250 Ohms.cm2, a level indicating the formation of an intact monolayer.15-18,20 21
Transport of 59Fe into the basal chamber.
The transport of 59Fe from the apical chamber across the Caco-2 cells into the basal chamber was studied as previously described.15,16 First, the cell monolayers were depleted of serum proteins by washing the monolayers twice with Dulbecco's modified Eagle medium and then incubating the monolayers overnight with Dulbecco's modified Eagle medium without FBS. Next, 1 μmol/L59Fe (59FeCl3, 110-740 Bq/mg; NEN-Dupont, Boston, MA) was offered to the apical surface in 1 mmol/L ascorbate in 50 mmol/L HEPES buffered saline solution (HBS), pH 7.4, prepared from 193 mmol/L HEPES diluted with an isotonic salt solution consisting of 130 mmol/L NaCl, 10 mmol/L KCl, 1 mmol/L CaCl2, and 1 mmol/L MgSO4. The basal chamber buffer was HBS to which were added apotransferrin (apo-Tf), ovo-transferrin (ovo-Tf), cobalt-transferrin (Co-Tf), bovine serum albumin (BSA), diethylenetriaminopentacetate (DTPA), DTPA-dextran, or nitrilotriacetate (NTA) at the indicated concentrations. The TEER was monitored during the course of the incubations and was constant in the presence of these buffers for at least 2 hours. Apo-Tf was prepared from human Tf (Boehringer, Mannheim, Germany), which was brought to pH 5.0 with HCl, dialyzed extensively against Chelex beads (Bio-Rad, Hercules, CA) and then titrated to pH 7.0 with NaOH. The Fe saturation, measured by monitoring the absorption at 280 nm and 470 nm, was always less than 2%. Ovo-Tf was obtained from Canadian Lysozyme (Abbottford, British Columbia, Canada) and apo-ovo-Tf prepared as for apo-Tf. Co2-Tf was prepared by the addition of CoCl2 to apo-Tf in the presence of 0.04 mol/L NaHCO3 and the preparation allowed to stand at 4°C for 7 days.22 The saturation of Tf with Co, determined by measuring the absorption at 405 nm and using an extinction coefficient of 8,900 mol/L-1cm-1, was always 94% to 96%. BSA, DTPA, and NTA were obtained from Sigma Chemicals (St Louis, MO) and DTPA-dextran from Molecular Probes (Eugene, OR). The moles of DTPA coupled to dextran were determined by addition of 59Fe citrate at different concentrations to a solution of dextran-DTPA. After incubation at 25°C for 60 minutes, the dextran-DTPA–59Fe was separated from unbound 59Fe citrate by ultracentrifugation through a Amicon UM-10 filter (Amicon, Beverly, MA), the radioactivity of the bound 59Fe determined, and the moles of DTPA per mole of dextran calculated assuming one 59Fe bound per DTPA. The lot of dextran-DTPA used was determined to have 17.1 ± 0.12 mol of DTPA per mole of dextran.
Uptake of 59Fe from the basal chamber.
In some experiments, 59Fe uptake into the cells was measured from 59Fe,125I-transferrin in the basal chamber. In these experiments59Fe,125I-transferrin was prepared as previously described,23 added to the basal chamber at various concentrations up to 10 μmol/L for 60 minutes at 37°C, the cells harvested as previously described,15 and the59Fe radioactivity in the cells determined. In preliminary experiments uptake into the cells was linear for at least 2 hours and was not affected by the presence of Fe(II) ascorbate in the apical chamber. Nonspecific binding of 59Fe and125I-transferrin was determined from the linear portion of the binding curves.24
Laser scanning confocal microscopy.
Texas Red-labeled Tf (Tf-TxR) was obtained from Molecular Probes. The Tf-TxR was rendered Fe-free as previously described for fetal calf serum (FCS).15 Briefly, the pH of 2 mL of a 2-mmol/L solution of Tf-TxR was lowered to 4.5 with HCl in the presence of 0.5 g of Chelex resin (Bio-Rad), the pH of the solution was slowly increased to 7.4 by the addition of small aliquots of NaOH with constant stirring. The resin was removed by sedimentation, the apotransferrin filter sterilized, and kept at −20°C until used.
Apo-Tf-TxR was offered on the basolateral surface of Caco-2 cells for 20 minutes. Transferrin was maintained in its apo form by the addition of 1 mmol/L desferroxiamine (DFO; Ciba-Geigy, Summit, NJ) an iron chelator. After this incubation period, the cell layer was washed with HBS and fixed with paraformaldehyde following Bacallao's protocol for confocal microscopy.25 The cell monolayer was also stained with ToPro-1 (Molecular Probes) to show the nucleus. Caco-2 cell monolayers were observed under a Laser Scanning Confocal Microscope (LSCM). The LSCM used consists of a Bio-Rad MRC 1024 scan head connected to a Nikon Diaphot microscope (Hercules, CA). Illumination of specimens is by Ar/Kr and Argon lasers. A 60×, 1.4 NA Nikon objective was used to observe the Caco-2 cell monolayer. The images at different wavelengths were recorded in sequential mode. Image analysis was accomplished with NIH-Image v1.60 software (NIH Image is a public domain program, developed at the US National Institutes of Health, available by anonymous FTP from zippy.nimh. nih.gov).
Statistical analysis and curve fitting.
Statistical analyses were performed using Instat v2.04 (Graph Pad Software, San Diego, CA) using an unpaired t-test. Curve fitting was performed with GraFit Software (Erithacus Software, Ltd) using nonlinear least-squares regression analysis.
RESULTS
The sensitivity of the transport of 59Fe from Caco-2 cells to apotransferrin.
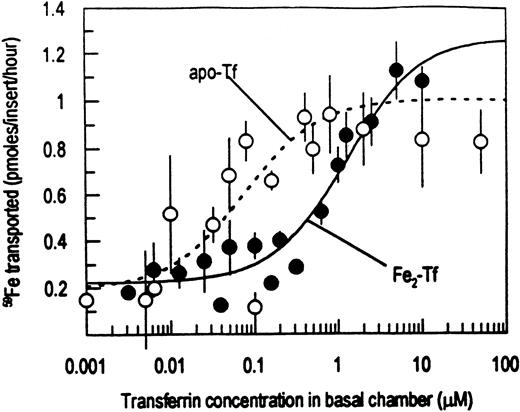
Caco-2 cells, grown to confluency as determined by measurement of TEER >250 ohms · cm2, were offered 59Fe ascorbate on the apical surface with different concentrations of apo-Tf in the basal chamber. The transport of 59Fe into the basal chamber was measured after 1 hour. Preliminary experiments for these studies, as also noted previously,16 showed linear59Fe transport into the basal chamber for at least 2 hours after an initial lag period of about 15 minutes. As shown in Fig 1, the transport was sensitive to the concentration of apo-Tf. A constitutive release of about 0.211 ± 0.049 pmoles of 59Fe/insert/hour was noted in the absence of apo-Tf (mean ± standard deviation [SD] of 12 experiments). The transport of 59Fe did not significantly increase until apo-Tf concentrations of greater than 0.01 μmol/L apo-Tf were added to the basal chamber. Maximal stimulation occurred by about 0.4 μmol/L apo-Tf. As noted in Table 1, the data for apo-Tf showed a Km of about 0.08 μmol/L and a Vmax of about 0.8 pmoles of 59Fe/insert/hour. Addition of NaHC03 to the basal chamber did not affect the transport of Fe at any concentration of apo-Tf (data not shown). The basal chamber contained only apo-Tf, serum proteins were removed before the 59Fe transport experiment (see Materials and Methods). At all concentrations of apo-Tf added, the 59Fe released into the basal chamber was precipitated with 60% ammonium sulfate. Hence, the released 59Fe was all bound to Tf. Also, we have shown using high-performance liquid chromatography (HPLC) that when apo-Tf was present in the basal chamber, only a59Fe-Tf peak is observed in the chromatogram.16When apo-Tf was presented covalently linked to Sepharose beads, no stimulation was noted even at apo-Tf concentrations of 10 μmol/L (data not shown).
Caco-2 cells grown in bicameral chambers and depleted of serum proteins were offered 1 μmol/L 59Fe ascorbate in the apical chamber with the basal chamber containing either 0 to 50 μmol/L apotransferrin (○) or 0 to 10 μmol/L ferri-transferrin (•). The cells were incubated at 37°C for 1 hour and the moles of59Fe transported into the basal chamber were determined. Presented are the mean ± SD of triplicates for three experiments. When not apparent, the error bars are smaller than the symbols. The curves were fit to the data with Michaelis-Menton kinetics using nonlinear regression analysis.
Caco-2 cells grown in bicameral chambers and depleted of serum proteins were offered 1 μmol/L 59Fe ascorbate in the apical chamber with the basal chamber containing either 0 to 50 μmol/L apotransferrin (○) or 0 to 10 μmol/L ferri-transferrin (•). The cells were incubated at 37°C for 1 hour and the moles of59Fe transported into the basal chamber were determined. Presented are the mean ± SD of triplicates for three experiments. When not apparent, the error bars are smaller than the symbols. The curves were fit to the data with Michaelis-Menton kinetics using nonlinear regression analysis.
The Km and Vmax for59Fe Transport Into the Basal Chamber by Caco-2 Cells
| Basal Chamber Addition . | Km (μmol/L) . | P . | Vmax (pmoles 59Fe/ insert/hour) . | P . |
|---|---|---|---|---|
| None | not applicable | 0.21 ± 0.05 | ||
| Apotransferrin | 0.078 ± 0.0078 | 0.81 ± 0.19 | <.0001 | |
| Ovotransferrin | 0.076 ± 0.0072 | .85 | 0.24 ± 0.05 | .38 |
| Ferri-transferrin | 1.25 ± 0.39 | .006 | 1.05 ± 0.10 | <.001 |
| Cobalt-transferrin | 0.14 ± 0.042 | .07 | 1.08 ± 0.07 | <.0001 |
| DTPA | 1.52 ± 0.70 | .02 | 2.94 ± 0.34 | <.0001 |
| Dextran-DTPA | 1.71 ± 0.13 | .02 | 1.40 ± 0.25 | <.0001 |
| Basal Chamber Addition . | Km (μmol/L) . | P . | Vmax (pmoles 59Fe/ insert/hour) . | P . |
|---|---|---|---|---|
| None | not applicable | 0.21 ± 0.05 | ||
| Apotransferrin | 0.078 ± 0.0078 | 0.81 ± 0.19 | <.0001 | |
| Ovotransferrin | 0.076 ± 0.0072 | .85 | 0.24 ± 0.05 | .38 |
| Ferri-transferrin | 1.25 ± 0.39 | .006 | 1.05 ± 0.10 | <.001 |
| Cobalt-transferrin | 0.14 ± 0.042 | .07 | 1.08 ± 0.07 | <.0001 |
| DTPA | 1.52 ± 0.70 | .02 | 2.94 ± 0.34 | <.0001 |
| Dextran-DTPA | 1.71 ± 0.13 | .02 | 1.40 ± 0.25 | <.0001 |
Caco-2 cells were grown in bicameral chambers and 59Fe transport into the basal chamber measured as described in Materials and Methods. The Fe transport determined for conditions in which the basal chamber contained varying concentrations either of apotransferrin, ovotransferrin, ferri-transferrin, cobalt-transferrin, DTPA, or dextran-DTPA. The rates were then fit with Michaelis-Menton kinetics and the Km and Vmax determined. Km= sensitivity of effect. Vmax = magnitude of effect at satutating concentrations. Shown are the mean ± SD for three experiments (with 6 to 8 points in triplicate for each experiment) except for the no addition (constitutive) rate of release, which is the mean of 12 experiments (n = 36). The values for Km for apotransferrin were compared with those for the other basal chamber additions by a one-sided student t test. The Vmaxs for all additions were compared by a one-sidedt test with the no addition rate of release. In addition the Vmax for both DTPA and dextran-DTPA were significantly different from apo-Tf (P = .005 and P = .03, respectively).
Fe2-Tf also stimulated release of 59Fe as shown in Fig 1. The Vmax was not significantly greater than that for apo-Tf. However, stimulation of 59Fe transport required about a 16-fold higher concentration of Fe2-Tf than with apo-Tf. With Fe2-Tf in the basal chamber, 59Fe transport continued at about the constitutive level until about 0.6 μmol/L Fe2Tf with a Km of 1.2 μmol/L and a Vmax of 1.1 pmoles of 59Fe/insert/hour. In contrast to apo-Tf stimulated 59Fe transport, only about 50% of the released 59Fe could be precipitated by 60% ammonium sulfate at the Vmax for Fe2-Tf.
Does stimulation of 59Fe transport reflect exchange of Fe by the Caco-2 cells with Fe on Fe-Tf in the basal chamber?
Despite the effort to maintain apo-Tf as the Fe depleted moiety, it is difficult to maintain apo-Tf fully depleted of bound Fe, as the affinity of Tf for Fe is high and Fe is ubiquitous in reagents. Hence, it is possible that Fe release from the Caco-2 cells represented merely exchange of Fe from the cell for Fe being delivered by Tf in the basal chamber. Spectrophotometric determination of Fe on apo-Tf just before the addition of apo-Tf to the basal chamber suggested no more than 1% to 2% Fe-Tf in the apo-Tf preparation. Nonetheless, the following experiments were undertaken to exclude the possibility that the stimulation of Fe release from the cells merely represented exchange of intracellular Fe with Fe carried on Tf added to the basal chamber. In these studies, the rate of 59Fe uptake into cells was measured from 59Fe-Tf in the basal chamber. If transport merely represented exchange of Fe from Fe being taken up from Fe-Tf in the basal chamber, then the rate of transport out of the cell should be similar to the rate of 59Fe transport into the cell. The uptake of 59Fe from 59Fe2-Tf in the basal chamber showed saturation after 1 μmol/L59Fe2-Tf. The data were also fitted with Michaelis-Menton kinetics and exhibited a Km = 0.733 ± 0.197 μmol/L and a Vmax = 19.8 ± 1.6 pmoles of59Fe/insert/hour (mean ± SD of three experiments). At the Km for apo-Tf and assuming that the apo-Tf contained 1% to 2% Fe-Tf based on spectrophotometric determination, the concentration of Fe-Tf would be about 0.0008 to 0.0016 μmol/L and the predicted Fe uptake of about 0.02 to 0.04 pmoles of Fe/insert/hour would be far less than the observed constitutive release. Likewise, at the concentration of apo-Tf required to reach the Vmax for apo-Tf, the predicted uptake of Fe from the 1% to 2% Fe-Tf present would be approximately 0.1 to 0.2 pmoles of Fe/insert/hour; this uptake rate is about eightfold to 16-fold less than the observed transport rate into the basal chamber. Hence, it appears unlikely that exchange of Fe between the small fraction of Fe in the apo-Tf preparation is accounting for the transport into the basal chamber.
The effect of Co-Tf, ovo-Tf, and BSA on Fe transport.
Transferrin carrying other metals, homologues of transferrin, and other metal binding proteins can participate in Fe uptake and release from various cells and do so with characteristics different from Tf. It was of interest then to see if any of these other proteins added to the basal chamber could effect Fe transport. For example, Co bound to Tf is kinetically inert.19 Hence, if the Fe binding sites were occupied with Co and if these sites on Tf were important in the stimulation of Fe transport, then 59Fe transport into the basal chamber should be inhibited by Co-Tf. As seen in Table 1, Co-Tf did not inhibit, but stimulated the rate of transport. Unfortunately, the preparations of Co-Tf always contained 4% to 6% unoccupied metal binding sites and this amount of apo-Tf could account for the observed increase in Vmax. This conclusion is supported by the finding that all of the 59Fe released in the presence of Co-Tf was precipitated by 60% ammonium sulfate.
The effect of ovo-Tf in the basal chamber was examined because ovo-Tf has an affinity for Fe similar to that of Tf, but is reported to interact poorly with the transferrin receptor.26 27 Hence, the addition of ovo-Tf would supply a protein, which binds Fe analogously to Tf but which should not interact with the transferrin receptor. To our surprise, when we investigated 59Fe uptake from 1 μmol/L 59Fe-ovo-Tf in the basal chamber, the Caco-2 cells exhibited an uptake of about 10 pmoles59Fe/insert/hour. This uptake was similar to that observed from 59Fe2-Tf in the basal chamber. Despite the Caco-2 cells being able to acquire 59Fe from ovo-Tf, apo-ovo-Tf had a slight, nonsignificant effect of 59Fe transport into the basal chamber. The slight effect had a sensitivity similar to that of apo-Tf and was not inhibited by the presence of antibodies, which block the binding of ovo-Tf to the ovo-Tf receptor (kindly provided by Dr Anne B. Mason, Department of Biochemistry, University of Vermont College of Medicine, Burlington, VT). Again, all of the 59Fe released from the cells in the presence of ovo-Tf was precipitated with 60% ammonium sulfate, suggesting that the released 59Fe was bound to the protein.
Finally, the effect of BSA in the basal chamber was examined because of the known metal binding sites on BSA and the inability of BSA to interact with the transferrin receptor. No increase in constitutive release was observed until high concentrations of BSA were added and no dose response curve was observed. At 2 and 10 μmol/L BSA, the59Fe transport rate was 0.360 ± 0.059 and 0.31 ± 0.021 pmoles/insert/hour, respectively (mean ± SD of three experiments with triplicates for each concentration).
The effect of metal chelators on 59Fe transport.
The effect of two chelators, NTA and DTPA, on Fe transport was examined. The Ka for Fe(III) for these chelators is 1024 for NTA and 1028 for DTPA.28These values compare with the Ka of 1021 of Tf for Fe. NTA had no effect on transport until 40 μmol/L at which concentration the observed transport of 59Fe was 0.393 ± 0.049 pmoles/insert/hour (mean ± SD of three experiments with triplicates for each concentration). As noted in Table 1, DTPA did stimulate transport with a Km of about 1.5 μmol/L and a Vmax of nearly 3 pmoles/insert/hour, the highest observed rate of transport. To be certain that DTPA was not merely shifting equilibrium by acting as a sink for intracellular 59Fe, rates of transport were measured in the presence of 10 μmol/L DTPA and were found to be linear for at least 2 hours. This observation supports DTPA as having an effect on the rate of release of iron from the cells. To determine if the parameters measured for DTPA reflected merely the relatively low molecular weight of DTPA and hence its ability to find access to 59Fe, DTPA was also presented in the basal chamber bound to dextran of molecular weight 70,000 daltons. For the experiments using DTPA-dextran, the amount of DTPA per molecule of dextran was determined by titrating the DTPA-dextran with increasing amounts of 59Fe-citrate, separating the unbound59Fe from the DTPA-dextran bound 59Fe by ultrafiltration and then expressing the concentration of DTPA-dextran as molarity of DTPA. The Km for DTPA-dextran was not significantly different from DTPA alone and although the Vmax was less than for DTPA, the Vmax was significantly more than that observed for the various transferrins (Table 1).
To determine if apo-Tf and DTPA were stimulating transport from the same or different compartments, 59Fe transport was measured with both apo-Tf and DTPA in the basal chamber and compared with the transport with both entities alone. We found no additive effects of apo-Tf and DTPA, suggesting that apo-Tf and DTPA were stimulating59Fe transport from the same compartment.
The localization of apotransferrin by confocal microscopy.
Apotransferrin binds to the transferrin receptor with an affinity far less that Fe-transferrin.29-32 As a consequence, in many cell types, it is not possible to show an interaction with apo-Tf. Hence, given the exquisite sensitivity of apo-Tf in stimulating Fe transport from Caco-2 cells, it was important to show that apo-Tf could gain entry into the Caco-2 cells. The internalization from basolateral surface of apotransferrin into vesicles within the Caco-2 cells was assessed by laser scanning confocal microscopy using apo-Tf labeled with Texas Red.
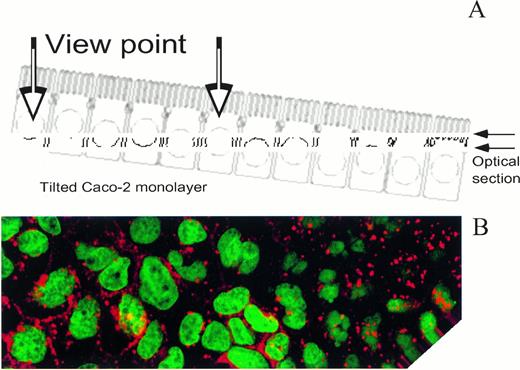
In these studies, as shown in Fig 2A, the Caco-2 cell monolayer was tilted in such a way that one could observe the apical or supranuclear side, at the right, and the basal or infranuclear side, at the left, of the cells in the same confocal micrograph. The scheme in Fig 2A depicts the viewpoint for observation and the angle at which the monolayer was tilted. Figure 2B is a typical fluorescent micrograph showing the Caco-2 cell nuclei, stained with ToPro-1, in green, and vesicles, containing apo-Tf–TxRed, in red. Because of the optical slicing power of laser confocal microscopy, it is possible to determine clearly if vesicles are above or below the nucleus. In similar studies (data not shown), Fe2-Tf–TxRed could be located in vesicles mainly beneath the nucleus.
Confocal microscopy of apotransferrin in a Caco-2 cell monolayer. Caco-2 cell monolayers were offered apo-Tf-TxRed from the basal side for 20 minutes. The cell layers were counterstained with ToPro-1 to show the nucleus in green and the monolayers observed by laser scanning confocal microscopy as described in Materials and Methods. Shown is a photomicroghraph of a section through a typical monolayer. As shown in the schematic (A), the cell layer was tilted so that the optical section (B) passed through the basal and apical portions of the cells constituting the monolayer. Note the vesicles above the nucleus at the right side of the figure. Because of the optical slicing power of laser confocal microscopy, it is possible to determine clearly in this figure if a vesicle is above or below the nucleus.
Confocal microscopy of apotransferrin in a Caco-2 cell monolayer. Caco-2 cell monolayers were offered apo-Tf-TxRed from the basal side for 20 minutes. The cell layers were counterstained with ToPro-1 to show the nucleus in green and the monolayers observed by laser scanning confocal microscopy as described in Materials and Methods. Shown is a photomicroghraph of a section through a typical monolayer. As shown in the schematic (A), the cell layer was tilted so that the optical section (B) passed through the basal and apical portions of the cells constituting the monolayer. Note the vesicles above the nucleus at the right side of the figure. Because of the optical slicing power of laser confocal microscopy, it is possible to determine clearly in this figure if a vesicle is above or below the nucleus.
DISCUSSION
During intestinal Fe absorption, the mechanism of the transfer phase, that is, how Fe is transported out of the basal surface of the intestinal epithelium, remains elusive. The solubility and reactivity of Fe dictate that Fe be bound to a cellular constituent during transport through cellular compartments.33 Recently, a divalent cation transporter (DCT1) has been implicated as a metal transporter involved in the uptake step.34 Other studies have suggested that a newly described protein, Mobilferrin, and a β3-integrin are also involved in the uptake process.35,36 Studies from our laboratory suggest that a moiety with a basic Iso-electric point (pI) and transferrin, but not ferritin, are involved in intracellular transport.16 Recently, we have reported that apo-Tf and FeTf undergo a different endocytic cycle.18 How then is Fe in various intracellular compartments transported across the basal surface and how do the observations presented here, as well as others studies, showing greater or lesser involvement of transferrin in iron transport comport with the intracellular handling of Fe?
The Caco-2 cells exhibit a constitutive rate of transport of Fe that was stimulated about fourfold by the presence of apo-Tf. While iron depletion of the cells did not significantly alter the constitutive rate, the stimulation of transport by apo-Tf was markedly enhanced by iron depletion.16 The effect of the apotransferrin added to the basal chamber was to stimulate the rate of release of Fe and not merely to shift the equilibrium. This observation was supported by the lack of stimulatory effect of (1) apo-Tf bound to Sepharose beads; (2) ovo-Tf, which has a Ka for Fe similar to apo-Tf; and (3) NTA, a chelator with a Ka for Fe greater than that of apo-Tf. The stimulation of Fe transport observed with Fe-Tf and Co-Tf represented the unsaturated metal binding sites present in the Fe-Tf and Co-Tf preparations.
Our studies are also compatible with the Fe transport, which occurs in the hypotransferrinemic mouse.2,3 As with the mice, the Caco-2 cells have a constitutive rate of iron transport in the absence of added apo-Tf. In the hypotransferrinemic mice, the intestinal mucosa senses the anemia and increases intestinal Fe absorption. In the model system of Caco-2 cells, the cells recognize the iron deficient state and increase transport.15 As noted previously,16 the Tf concentration in the hypotransferinemic mice is about 0.1 μmol/L, a level that will stimulate Fe transport from Caco-2 cells. In human plasma, Tf concentration is about 50 μmol/L. As only about one third of the iron binding sites on plasma Tf are occupied, there will be ample apo-Tf to stimulate Fe transfer. These observations suggest a model in which apo-Tf, or unoccupied Fe sites on Tf, reaches a compartment of newly absorbed iron within the epithelial cell and accelerates transfer of that iron out of the cell.
The sensitivity of transport to the presence of apo-Tf suggests that apo-Tf has a unique ability to interact with the basal surface of the Caco-2 cells. This hypothesis is supported in several ways. First, as detailed in the Results, the release of 59Fe from the cells could not be merely exchange of 59Fe in the cells for Fe on Tf in the basal chamber. The interaction of apo-Tf with the cells to stimulate transfer of 59Fe out of the cells need not be through the transferrin receptor. Recently transferrin has been noted to interact with heparin sulfate proteoglycan and this interaction can affect the rate of release of Fe from transferrin.37Second, the stimulation of 59Fe transfer is not an effect of the affinity of a chelator for Fe. DTPA with an affinity for Fe greater than that of Tf, had a Km for stimulating transport of 59Fe that was more than 15-fold greater than for apo-Tf. Neither ovo-Tf, with a Ka for Fe similar to apo-Tf, nor NTA, with a Ka greater than apo-Tf, had an effect on Fe release. These results suggest that apo-Tf has a greater ability to interact with the Fe release site than does a homologue of Tf or the chelators DTPA or NTA, which have a greater affinity for Fe. Third, using confocal microscopy, it was possible to show that apo-Tf proceeded into vesicular compartments within the Caco-2 cells and that these apo-Tf vesicles reached the apical portions above the nucleus, while in contrast, Fe2Tf was located primarily beneath the nuclei. As newly absorbed iron is found in intestinal epithelium at the terminal web, the ability of the cells to sort apo-Tf to this region may explain why apo-Tf stimulates iron transport. In toto these experiments suggest that apo-Tf has a unique mechanism independent of the traditional Tf receptor for interacting with the basal surface of Caco-2 cells.
Supported in part by Grants No. DK-41279 and DK-37866 from the National Institutes of Health, Bethesda, MD, and grants from the Center for Excellence in Cancer Research, Treatment, and Education, Louisiana State University Medical Center, Shreveport, LA.
Address reprint requests to Xavier Alvarez-Hernandez, PhD, 1501 Kings Highway, Shreveport, LA 71130.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal