Abstract
RAFTK, a novel nonreceptor protein kinase, has been shown to be involved in focal adhesion signal transduction pathways in neuronal PC12 cells, megakaryocytes, platelets, and T cells. Because focal adhesions may modulate cytoskeletal functions and thereby alter phagocytosis, cell migration, and adhesion in monocyte-macrophages, we investigated the role of RAFTK signaling in these cells. RAFTK was abundantly expressed in THP1 monocytic cells as well as in primary alveolar and peripheral blood-derived macrophages. Colony-stimulating factor-1 (CSF-1)/macrophage colony-stimulating factor (M-CSF) stimulation of THP1 cells increased the tyrosine phosphorylation of RAFTK; similar increases in phosphorylation were also detected after lipopolysaccharide stimulation. RAFTK was phosphorylated with similar kinetics in THP1 cells and peripheral blood-derived macrophages. Immunoprecipitation analysis showed associations between RAFTK and the signaling molecule phosphatidylinositol-3 (PI-3) kinase. PI-3 kinase enzyme activity also coprecipitated with the RAFTK antibody, further confirming this association. The CSF-1/M-CSF receptor c-fms and RAFTK appeared to associate in response to CSF-1/M-CSF treatment of THP1 cells. Inhibition of RAFTK by a dominant-negative kinase mutant reduced CSF-1/M-CSF–induced MAPK activity. These data indicate that RAFTK participates in signal transduction pathways mediated by CSF-1/M-CSF, a cytokine that regulates monocyte-macrophage growth and function.
THE MACROPHAGE IS a cell that serves critical functions in immune system defense, including the phagocytosis of microbial pathogens, the proteolytic processing and presentation of foreign antigens, and the elaboration of a repertoire of cytokines.1,2 The regulation of monocyte-macrophage (MM) production, maturation, and survival is subserved primarily through the growth factor colony-stimulating factor-1 (CSF-1)/macrophage colony-stimulating factor (M-CSF).3 CSF-1/M-CSF interacts with its cognate receptor, c-fms, a member of the protein tyrosine kinase family, and its activation leads to its rapid autophosphorylation and dimerization.4,5 Other downstream molecules that appear to participate in c-fms signal transduction and are phosphorylated after CSF-1/M-CSF treatment of MMs include Shc, Raf-1, c-cbl, phosphatidylinositol-3 (PI-3) kinase, and the protein tyrosine phosphatase 1C.5-11
Functional changes induced in MMs that may be important in host defense include alterations in the expression of surface molecules that mediate adhesion.12-14 Particular attention has been focused on the integrin family of surface receptors that facilitates the formation of focal adhesion contacts upon binding to certain extracellular ligands. Such focal adhesion contacts represent the interaction sites of intracellular signaling molecules and cytoskeletal proteins.15 CSF-1/M-CSF, which modulates αVβ5 integrin expression, enhanced the formation of focal contacts involving the cytoskeletal protein paxillin in human macrophages bound to vitronectin.16,17 The mechanism of this phenomenon is still obscure, because the focal adhesion kinase (FAK), which has been reported to serve as a critical molecule in forming such focal contacts, was not detected in human macrophages.16 18
We have recently identified and characterized a novel signaling molecule, the related adhesion focal tyrosine kinase (RAFTK). RAFTK, also termed Pyk2, CAK-β, and CADTK, appears to be a member of the FAK family based on its deduced amino acid sequence.19-22 RAFTK resembles FAK in that it has similar consensus motifs in the central kinase catalytic domain and also lacks a transmembrane region, myristylation sites, and SH2 and SH3 domains. Both of these signaling molecules have a proline-rich region in the carboxy-terminal domain that may function as binding domains for SH3-containing signaling proteins.23 Studies to date indicate that RAFTK may participate in several signaling pathways, including those involving calcium ion channels in neuronal cells, integrin activation in megakaryocytes, the Ras/MAPK and JNK pathways in response to UV irradiation, T-cell receptor (TCR) cross-linking, G protein stimulation, and via the cytokine tumor necrosis factor-α (TNF-α).20 24-27
Based on this background, we investigated whether RAFTK was expressed in the cells of MM lineage and whether it participated in CSF-1/M-CSF–induced signaling. In parallel, we studied the effects of treatment of MMs with the potent physiological activator of macrophages, bacterial lipopolysaccharide (LPS). We observed that RAFTK was expressed in the THP1 monocytic cell line and in peripheral blood-derived MMs as well as tissue-derived alveolar macrophages. Moreover, RAFTK was phosphorylated upon the treatment of mononuclear phagocytes with CSF-1/M-CSF or LPS and associated with PI-3 kinase and the CSF-1/M-CSF receptor, c-fms. These observations provide new data on CSF-1/M-CSF signaling.
MATERIALS AND METHODS
Cells and cell culture.
The permanent human monocytic cell line THP1 was obtained from the American Type Culture Collection (ATCC; Rockville, MD) and shown to be mycoplasma-free before expansion in culture. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 2 mmol/L glutamine, 100 μmol/mL sodium pyruvate, 1 mmol/mL nonessential amino acids, 50 μg/mL penicillin, and 50 μg/mL streptomycin. Primary human peripheral blood MMs were obtained by the phlebotomy of normal volunteers, after obtaining their informed consent, and isolated by Ficoll Hypaque density centrifugation, as previously described.28 MMs were plated on 24-well tissue culture plates (Costar, Cambridge, MA) for 24 hours, shaken at 150 RPM for 15 minutes and washed three times with Hank's balanced salt solution (HBSS) to remove the nonadherent cells. The adherent cells were cultured for an additional 7 days before their use. MM cultures were determined to contain greater than 95% macrophages by nonspecific acetate esterase staining (Sigma, St Louis, MO). All cells used in these studies were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 100 μmol/mL sodium pyruvate, 1 mmol/mL nonessential amino acids, 50 μg/mL penicillin, and 50 μg/mL streptomycin at 37°C, 5% CO2 under humidified atmosphere.
RAFTK transfectants.
Dominant-negative RAFTK kinase mutants were produced by electroporation of THP1 cells with 10 μg purified plasmids, and cells were maintained in G418 selection medium (DMEM, 10% FBS, 0.5 mg/mL G418). Controls consisted of a pcDNA vector without the RAFTK construct. The dominant-negative kinase mutant RAFTKm457 was generated by replacing Lys-(457) with Ala by site-directed mutagenesis.
Reagents and materials.
LPS from Escherichia coli was obtained from Sigma Chemical Co and recombinant human CSF-1/M-CSF was kindly provided by Genetics Institute (Cambridge, MA). The monoclonal antibodies against phosphotyrosine (4G10), the PI-3 kinase p85 regulatory subunit, and the polyclonal rabbit antisera to the human c-fms receptor were obtained from Upstate Biotechnology, Inc (Lake Placid, NY) and Santa Cruz Biotechnology (Santa Cruz, CA). Specific polyclonal antibodies to RAFTK were generated by immunizing New Zealand White rabbits with a bacterially expressed fusion protein consisting of GST and the carboxy terminus (amino acids 681-1,009) of human RAFTK cDNA subcloned into the pGEX-2T expression vector as described.19 High-titer RAFTK antiserum (R-4250) was used in the subsequent experiments, because it was shown to be specific and not cross-reactive with FAK in prior experiments.19 24
Electrophoresis reagents and nitrocellulose membranes were obtained from Bio-Rad Laboratories (Hercules, CA). All other chemicals, including the protease inhibitors pepstatin, antipain, chymostatin, leupeptin, aprotinin, sodium vanadate, and sodium fluoride, were obtained from Sigma. Because bacterial endotoxin is a potent regulator of MM function,29 all media and reagents were shown to be free of endotoxin contamination by the Limulus endotoxin assay (Sigma) before their use in cell cultures (<1 ng/mL).
Cell treatment and processing.
Cells were initially starved in serum-free DMEM for 16 hours and stimulated in HBSS at a density of 5 × 106/mL for the indicated time periods at 37°C with either LPS (2 μg/mL) or CSF-1/M-CSF (1,000 U/mL). For each timepoint, 20 × 106 cells were lysed in 1 mL of ice-cold modified RIPA buffer (50 mmol/L Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL of pepstatin, antipain, chymostatin, leupeptin, aprotinin, 10 mmol/L sodium vanadate, 10 mmol/L sodium fluoride, and 10 mmol/L sodium pyrophosphate) for 30 minutes at 4°C. Detergent-insoluble material was removed by centrifugation at 18,000g for 10 minutes at 4°C. Protein concentrations were determined by BioRad DC protein assay (Bio-Rad Laboratories). Cell lysates for the PI-3 kinase assays were performed using RIPA lysis buffer as previously described, without sodium deoxycholate.
Immunoprecipitation and Western blot analysis.
For the immunoprecipitation studies, identical amounts of protein from each sample were clarified by incubation with protein sepharose-A CL-4B (Pharmacia Biotech, Piscataway, NJ) for 1 hour at 4°C. After the removal of protein sepharose-A by brief centrifugation, the solution was incubated with different primary antibodies as detailed below for each experiment for 4 hours or overnight at 4°C. Immunoprecipitations of the antibody-antigen complexes were performed by incubation for 3 hours at 4°C with 75 μL of protein sepharose-A (10% suspension). Nonspecific bound proteins were removed by washing the sepharose beads three times with the modified RIPA buffer and three times with phosphate-buffered saline (PBS). The bound proteins were solubilized in 30 μL of 2× Laemmli buffer and boiled for 5 minutes. Samples were then run on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk protein and probed with primary antibody for 3 hours at room temperature or overnight at 4°C. Immunoreactive bands were visualized using horseradish peroxidase-conjugated secondary antibody and the enhanced chemiluminescent system (Amersham Corp, Arlington Heights, IL). Blots were stripped (2% SDS, 62.5 mmol/L Tris, and 100 mmol/L β-mercaptoethanol) for 30 minutes at 50°C and washed in Tris-buffered saline/0.5% Tween 20 (TBS-T) for 60 minutes before blocking and reprobing with primary antibodies.
In vitro PI-3 kinase assay.
Aliquots of cell lysates were normalized for protein concentration and then incubated overnight at 4°C with antibodies against RAFTK, PI-3 kinase, or control normal rabbit serum. Immune complexes were absorbed to sepharose-A beads for 3 hours at 4°C. Nonspecific binding was removed by washing three times with PBS 1% NP-40 and three times with 0.5 mol/L LiCl/0.5 mol/L Tris, followed by washing three times with TE buffer. Samples were resuspended in 20 μL TE buffer, 20 μL phosphoinositol (10 μg; Avanti Polar Lipids, Alabaster, AL), and 10 μL ATP mix (1 mmol/L HEPES, 10 μmol/L ATP, 1 μmol/L MgCl2, 5 μCi γ32 P-ATP) and incubated at room temperature for 10 minutes. The reaction was stopped by adding 60 μL of 2 mol/L HCL and 160 μL chloroform:methanol (1:1 vol/vol). Lipids were separated on oxalate impregnated silica TLC plates using a solvent system of chloroform:methanol:water:ammonium hydroxide (28%) (35:35:3.5:7). TLC plates were dried and subjected to autoradiography at −80°C.
Immune complex kinase assay for MAPK activity.
Aliquots of cell lysates normalized for protein concentration were incubated overnight at 4°C with antibodies against ERK1 and ERK2 (Santa Cruz Biotechnology). Immune complexes were then absorbed to sepharose-A beads for 3 hours at 4°C. Nonspecific binding was removed by washing three times with RIPA buffer followed by washing three times with kinase buffer (50 mmol/L HEPES, pH 7.4, 5 mmol/L MgCl2, and 20 mmol/L ATP). The complex was incubated in 30 μL kinase buffer containing 7 μg myelin basic protein (MBP; Upstate Biotechnology) and 5 μCi γ32P-ATP for 20 minutes at 30°C. The reaction was terminated by adding 4× Laemmli sample buffer and boiling samples for 5 minutes. Proteins were separated on 15% SDS-PAGE and detected by autoradiography.
RESULTS
RAFTK is expressed and phosphorylated in human MMs.
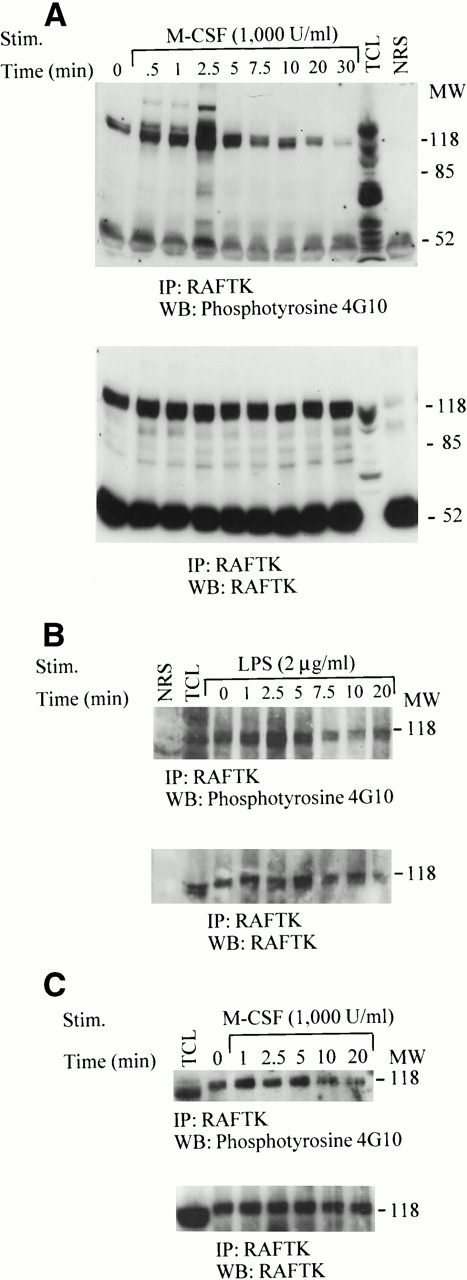
To further characterize the signaling pathways in human MMs that are involved in their growth, differentiation, and function, we used as a model the permanent monocytic cell line THP1 as well as primary peripheral blood-derived MMs. Analysis by immunoprecipitation showed an abundance of RAFTK protein in these cells (Fig 1A through C). There appeared to be low levels of constitutive phosphorylation of RAFTK under unstimulated culture conditions. Depending on the resolution of the gels, RAFTK was seen to migrate either as a single band or as a doublet (Fig 1A through C).
Tyrosine phosphorylation of RAFTK in THP1 monocytic cells and primary MMs. THP1 cells (20 × 106) were stimulated with either (A) 1,000 U/mL CSF-1/M-CSF or (B) 2 μg/mL LPS for the indicated time periods. (C) MMs (20 × 106) were allowed to adhere and mature in culture for 7 to 14 days before their stimulation with 1,000 U/mL CSF-1/M-CSF. Cell lysates prepared in RIPA buffer were subjected to immunoprecipitation with anti-RAFTK antibody or normal rabbit serum as a control. Anti-RAFTK immunoprecipitates were resolved by 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antiphosphotyrosine antibody (4G10) (top panel). The same blot was subjected to serial immunoblotting with anti-RAFTK antibody (bottom panel). TCL, total cell lysates; NRS, normal rabbit serum control.
Tyrosine phosphorylation of RAFTK in THP1 monocytic cells and primary MMs. THP1 cells (20 × 106) were stimulated with either (A) 1,000 U/mL CSF-1/M-CSF or (B) 2 μg/mL LPS for the indicated time periods. (C) MMs (20 × 106) were allowed to adhere and mature in culture for 7 to 14 days before their stimulation with 1,000 U/mL CSF-1/M-CSF. Cell lysates prepared in RIPA buffer were subjected to immunoprecipitation with anti-RAFTK antibody or normal rabbit serum as a control. Anti-RAFTK immunoprecipitates were resolved by 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antiphosphotyrosine antibody (4G10) (top panel). The same blot was subjected to serial immunoblotting with anti-RAFTK antibody (bottom panel). TCL, total cell lysates; NRS, normal rabbit serum control.
We then addressed whether certain stimuli associated with mononuclear phagocyte activation modulated RAFTK phosphorylation. Our preliminary experiments determined that 1,000 U/mL CSF-1/M-CSF and 2 μg/mL LPS were optimal concentrations for the stimulation of RAFTK in THP1 cells and primary macrophage cultures (data not shown). As seen in Fig 1A and B (top panels), an increase in the tyrosine phosphorylation of RAFTK was specifically observed in THP1 cells after their treatment with either CSF-1/M-CSF or LPS. The membrane was then stripped and reprobed with anti-RAFTK antibody to confirm that equivalent amounts of RAFTK were loaded in each lane (Fig 1A and B, bottom panels).
To determine the time course of the tyrosine phosphorylation of RAFTK, THP1 cells or MMs were stimulated with CSF-1/M-CSF or LPS and harvested at the times indicated in Fig 1. The phosphotyrosine levels in the RAFTK immunoprecipitates of the CSF-1/M-CSF–treated THP1 cells showed a strong peak at 2.5 minutes that appeared to decrease in intensity at later time points (Fig 1A, top panel). The membrane was then stripped and reprobed with anti-RAFTK antibody to confirm that equivalent amounts of RAFTK were loaded in each lane (Fig 1A, bottom panel). There did not appear to be any changes in the levels of RAFTK protein to explain these fluctuations in the degree of tyrosine phosphorylation. However, we routinely see a slight difference in mobility between RAFTK detected in immunoprecipitates and that in total cell lysates that appears to migrate at a slightly faster rate.
LPS treatment of THP1 cells resulted in a maximum tyrosine phosphorylation of RAFTK within 2.5 minutes that then decreased with increasing time of stimulation (Fig 1B, top panel). The phosphotyrosine levels of RAFTK after LPS stimulation appear to have similar kinetics to those we observed after CSF-1/M-CSF stimulation.
CSF-1/M-CSF stimulation of primary MMs resulted in a peak tyrosine phosphorylation of RAFTK by 1 minute, which gradually decreased over time (Fig 1C, top panel). Anti-RAFTK immunoblotting of RAFTK immunoprecipitates showed that the approximately 120-kD phosphoprotein corresponded to the RAFTK protein and remained constant between samples (Fig 1A through C, bottom panels).
RAFTK associates with the PI-3 kinase.
Because RAFTK, like FAK, is believed to act as a platform kinase site for the coalescence of signaling and adaptor molecules at sites of focal adhesions, we examined RAFTK immunoblots for associating coprecipitating proteins. Using immunoprecipitation analysis, we observed a specific association of RAFTK with PI-3 kinase, an important enzyme in the modulation of phosphoinositol signaling30 31(Fig 2A and B). Time course studies after either CSF-1/M-CSF or LPS treatment of THP1 cells demonstrated that the PI-3 kinase-RAFTK association increased over time of stimulation and gradually decreased to background levels at longer stimulation times (data not shown). Similar findings were observed after phorbol 12-myristate 13-acetate (PMA) treatment (not shown). This association between RAFTK and PI-3 kinase was confirmed by an in vitro kinase assay (Fig 3). These studies demonstrated that PI-3 kinase activity increased and migrated with RAFTK immunoprecipitates after CSF-1/M-CSF or LPS stimulation of THP1 cells (Fig 3). We did not detect PI-3 kinase activity in the normal rabbit serum control immunoprecipitates.
Association of RAFTK with PI-3 kinase in THP1 cells. THP1 cells (20 × 106) were stimulated with either (A) CSF-1/M-CSF (1,000 U/mL) or (B) LPS (2 μg/mL) for the indicated time periods. Cell lysates prepared in RIPA buffer were subjected to immunoprecipitation with anti-RAFTK antibody. Anti-RAFTK immunoprecipitates were resolved by 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti–PI-3 kinase antibody. TCL, total cell lysates.
Association of RAFTK with PI-3 kinase in THP1 cells. THP1 cells (20 × 106) were stimulated with either (A) CSF-1/M-CSF (1,000 U/mL) or (B) LPS (2 μg/mL) for the indicated time periods. Cell lysates prepared in RIPA buffer were subjected to immunoprecipitation with anti-RAFTK antibody. Anti-RAFTK immunoprecipitates were resolved by 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti–PI-3 kinase antibody. TCL, total cell lysates.
PI-3 kinase activity associates with RAFTK in THP1 cells. THP1 cells were stimulated with either 1,000 U/mL or 2 μg/mL LPS for 2 minutes and lysed in RIPA buffer without sodium deoxycholate. Lysates were immunoprecipitated with either anti-RAFTK, normal rabbit serum control, or anti–PI-3 kinase p85 antibody. Immune complexes were absorbed to sepharose-A beads for 3 hours, washed, and subjected to PI-3 kinase assay. Lipids were extracted using methanol:chloroform (1:1) and spotted on oxalate-impregnated silica gel TLC plates. Samples were subjected to ascending chromatography using a methanol:chloroform:water:ammonium hydroxide solvent system. TLC plates were dried and samples were visualized by autoradiography.
PI-3 kinase activity associates with RAFTK in THP1 cells. THP1 cells were stimulated with either 1,000 U/mL or 2 μg/mL LPS for 2 minutes and lysed in RIPA buffer without sodium deoxycholate. Lysates were immunoprecipitated with either anti-RAFTK, normal rabbit serum control, or anti–PI-3 kinase p85 antibody. Immune complexes were absorbed to sepharose-A beads for 3 hours, washed, and subjected to PI-3 kinase assay. Lipids were extracted using methanol:chloroform (1:1) and spotted on oxalate-impregnated silica gel TLC plates. Samples were subjected to ascending chromatography using a methanol:chloroform:water:ammonium hydroxide solvent system. TLC plates were dried and samples were visualized by autoradiography.
RAFTK associates with the c-fms receptor upon mononuclear phagocyte cell activation with CSF-1/M-CSF.
Because CSF-1/M-CSF stimulation of THP1 cells and primary macrophages appeared to have rapid effects on RAFTK phosphorylation, we examined whether RAFTK may directly associate with the c-fms receptor. We observed a specific association of RAFTK with the c-fmsreceptor upon CSF-1/M-CSF treatment of the cells (Fig 4A and B). Associations were detected in blotting experiments of the THP1 cell lysates that were immunoprecipitated with RAFTK antisera followed by c-fmsimmunoblotting. We identified immunoreactive bands at both 135 and 150 kD that correspond to the mobility of the c-fms receptor (Fig4B). The reciprocal experiment of c-fms immunoprecipitation followed by RAFTK immunoblotting identified a prominent 120-kD molecule (Fig 4A). Interestingly, the RAFTK and c-fms association appeared to increase with longer times of CSF-1/M-CSF stimulation, but did not respond to PMA stimulation (data not shown).
Association of RAFTK with the c-fms receptor in THP1 cells. THP1 cells (20 × 106) were stimulated with 1,000 U/mL CSF-1/M-CSF for the indicated time periods. Cell lysates prepared in RIPA buffer were subjected to immunoprecipitation with either the anti-c-fms antibody (A) or anti-RAFTK antibody (B). Immune complexes were absorbed onto sepharose-A beads, washed and resolved by 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with either anti-RAFTK (A) or anti–c-fmsantibody (B). NRS, normal rabbit serum control.
Association of RAFTK with the c-fms receptor in THP1 cells. THP1 cells (20 × 106) were stimulated with 1,000 U/mL CSF-1/M-CSF for the indicated time periods. Cell lysates prepared in RIPA buffer were subjected to immunoprecipitation with either the anti-c-fms antibody (A) or anti-RAFTK antibody (B). Immune complexes were absorbed onto sepharose-A beads, washed and resolved by 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with either anti-RAFTK (A) or anti–c-fmsantibody (B). NRS, normal rabbit serum control.
Dominant-negative RAFTK kinase mutant reduces MAPK activity.
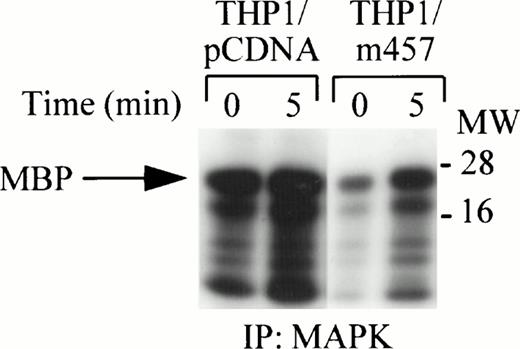
The RAFTK protein has been identified as an upstream mediator of the Ras pathway via Grb2-SOS interactions.20 THP1 cells expressing a dominant-negative kinase mutant, RAFTKm457, were used to determine if RAFTK participates in c-fms signaling through the ERK1/ERK2 pathway.5 MAP kinase activity was strongly activated after CSF-1/M-CSF treatment of THP1 cells expressing the RAFTKpcDNA control vector alone. However, MAP kinase activity in THP1 cells expressing the dominant-negative kinase mutant RAFTKm457 was decreased when compared with the control THP1 cells expressing the RAFTKpcDNA vector (Fig 5). Although MAPK activity was routinely reduced in RAFTKm457-expressing THP1 cells, there were no detectable differences in cell viability between the RAFTKm457- or RAFTKpcDNA-expressing THP1 cells to account for this finding.
Reduction of MAP Kinase activity by overexpression of a RAFTK dominant-negative kinase mutant. THP1 cells were stably transfected with the RAFTKpcDNA vector control or with RAFTKm457 dominant-negative kinase mutant. THP1 transfectants (20 × 106) were stimulated with CSF-1/M-CSF (1,000 U/mL), and then cell lysates were prepared in RIPA buffer. Lysates were subjected to immunoprecipitation with anti-ERK1 and ERK2 antibodies. Immune complexes were absorbed with sepharose-A beads and then washed and subjected to in vitro kinase assay for 30 minutes. Samples were subjected to 15% SDS-PAGE and to autoradiography at −80°C.
Reduction of MAP Kinase activity by overexpression of a RAFTK dominant-negative kinase mutant. THP1 cells were stably transfected with the RAFTKpcDNA vector control or with RAFTKm457 dominant-negative kinase mutant. THP1 transfectants (20 × 106) were stimulated with CSF-1/M-CSF (1,000 U/mL), and then cell lysates were prepared in RIPA buffer. Lysates were subjected to immunoprecipitation with anti-ERK1 and ERK2 antibodies. Immune complexes were absorbed with sepharose-A beads and then washed and subjected to in vitro kinase assay for 30 minutes. Samples were subjected to 15% SDS-PAGE and to autoradiography at −80°C.
DISCUSSION
Our studies indicate that human mononuclear phagocytes, including peripheral blood-derived MMs, express RAFTK, a recently identified signaling molecule that is a member of the FAK family. RAFTK appeared to participate in certain previously described signaling pathways after the activation of these cells. Treatment with CSF-1/M-CSF showed an increased phosphorylation of RAFTK in both the model THP1 monocytic cell line as well as in primary blood-derived MMs. Parallel studies using the potent macrophage stimulators (LPS) and the chemical activator PMA, an activator of protein kinase C (PKC), also showed RAFTK phosphorylation in macrophages in a time and concentration dependent manner (data not shown). In these studies, phosphorylated RAFTK appears to migrate either as a single band or as a doublet. It is likely that one of the doublet bands represents either a phosphorylated form or a degradation product of RAFTK produced by endogenous proteases found in abundance in cells of the macrophage/monocyte lineage. The degradation of the RAFTK protein by endogenous proteases has been previously described in platelets in an integrin-independent mechanism.32
The phosphorylation of RAFTK has been reported to result in its association with several well-characterized components of cellular signaling pathways, including src kinases, paxillin, and the adaptor molecule Grb2. In this study, we observed that RAFTK can associate with the enzyme PI-3 kinase and the CSF-1/M-CSF receptorc-fms.20,24,26,27,31 Grb2 is an adaptor protein that has the capacity to link with a number of kinases and substrates and functions by facilitating signaling through the creation of physical associations of such partners in enzymatic reactions.23 PI-3 kinase appears to modulate phosphoinositol metabolism in a variety of cell types, including mononuclear phagocytes, and is an important component of the tyrosine kinase-regulated signaling pathways that lead to cell proliferation.33,34 CSF-1/M-CSF has been reported to induce the direct association of the p85-α subunit of PI-3 kinase with the SH2 domain of Grb2 and Grb2-SOS complexes, thus supporting its role upstream of the Ras signaling pathway in monocytes.5,11 In addition, PI-3 kinase activation and the production of its metabolites have been suggested to be an upstream activator of the calcium-independent form of PKC.35 Our data demonstrate that PI-3 kinase associates with RAFTK and that this association appears to increase after stimulation with either LPS or CSF-1/M-CSF. We also found that PI-3 kinase enzymatic activity associates with RAFTK immunoprecipitated from CSF-1/M-CSF or LPS stimulated THP1 cell lysates. Previous reports have demonstrated that PDGF and cell adhesion stimulate the association of PI-3 kinase, via its SH2 domain, with FAK at Tyrosine 397.36 Future studies will focus on determining which of the PI-3 kinase p85 SH2 or SH3 domains mediates this observed association with RAFTK in MMs.
Our observations regarding RAFTK suggest that this recently identified signaling molecule could play a variety of roles in the transduction of MM signaling, particularly in light of prior studies of CSF-1/M-CSF–induced integrin expression and the subsequent formation of focal adhesion contacts.16 Recent studies of CSF-1/M-CSF–induced upregulation of αVβ5 integrin-dependent phosphorylation of paxillin in human macrophages showed a PKC-dependent mechanism.16 Our results complement those studies, in that we observed that LPS, PMA, or CSF-1/M-CSF induced the phosphorylation of RAFTK in THP1 cells and its subsequent association with paxillin.27
Our observation that RAFTK associated with the c-fms receptor in CSF-1/M-CSF stimulated THP1 cells contributes new information on signaling mediated by this growth factor in mononuclear phagocytes. Our results are of particular interest in light of the report of Kharbanda et al,37 who did not find a direct association betweenc-fms and FAK in CSF-1/M-CSF–stimulated primary macrophages. Together with these other data, our observations suggest that RAFTK and FAK may have different associations and roles in signaling in MMs, as they do in other cell types.20 24-27
Because RAFTK does not contain either the SH2 or SH3 binding domains commonly found in signaling molecules,19 it is likely that the c-fms-RAFTK association we observed may be mediated via an adaptor molecule such as Grb2. Grb2 has been previously reported to bind to both the c-fms receptor5 and to RAFTK.20 26 Future studies will aim to identify the nature of these interactions and whether Grb2 plays such a role in MMs.
Previously, c-fms has been reported to form associations with Grb1, Shc, and SOS1 in myeloid cells, suggesting that it signals through the Ras pathway.38 Our data showing RAFTK association with Grb2 (not shown) support the data of Lev et al,20 who found that Pyk2 associated with the adaptor proteins Grb2 and Shc upstream of the Ras/MAPK signaling pathway in PC12 neuronal cells.27 Pyk2 has recently been reported to activate the c-Jun N-terminal kinase signaling pathway after stress signals and the MAPK pathway after G protein stimulation.25,27 Because both c-fms and RAFTK are upstream activators of the Ras/MAPK pathway, it is possible that RAFTK acts as an intracellular link between divergent extracellular signals. For example, these signals may originate from extracellular matrix proteins that signal through integrin binding and from cytokines such as CSF-1/M-CSF that signal through c-fms, both resulting in an enhanced stimulation of this Ras/MAPK pathway. Alternatively, because RAFTK function has been linked to the cytoskeleton through its association with paxillin and focal adhesions,19,24 its association with c-fms may be due, in part, to c-fmsinternalization after CSF-1/M-CSF binding, which may also involve cytoskeletal interactions.39 Whereas our MAPK data (Fig 5) suggest that RAFTK may play an intermediary role linking c-fmsto the MAPK pathway and that MAPK activity was only partially reduced by the RAFTKm457 dominant-negative mutant, these data suggest that RAFTK is only one of several signaling molecules that act upstream to activate MAPK and contribute to the complex cascade leading to MAPK activation.
In summary, RAFTK appears to function in LPS and CSF-1/M-CSF signaling pathways in MMs through multiple downstream pathways, including PI-3 kinase, Ras/MAPK, and JNK.20,24,25 36 Although further studies are needed to characterize the sites and mechanisms of interaction among the currently identified molecules that associate with RAFTK, it seems clear that macrophages, like megakaryocytes and T cells in our prior work, prominently use RAFTK in cytokine-mediated pathways of activation that are linked to focal contact formation. The confluence of RAFTK, other kinases, and cytoskeletal molecules may provide a platform for the interactions of signaling molecules and adaptor proteins that regulate downstream signaling pathways and affect cell morphology by finely controlling certain components of the immune response, like adhesion or migration. It will be of interest to characterize how such cellular responses are triggered in the macrophage host defense against extracellular pathogens that are often coated with extracellular matrix proteins that bind to integrins, such as Pneumocystis carinii.
ACKNOWLEDGMENT
The authors are grateful to Janet Delahanty for her editing and the preparation of the figures as well as Jennifer McGrath and Nancy DesRosiers for their assistance with the figures. We thank our colleagues Zhong-Ying Liu and Jian-Feng Wang for their technical assistance. Finally, we appreciate Tee Trac and Youngsun Jung for their typing assistance and Delroy Heath for facilitating our receipt of the needed reagents for the experiments. We also thank the Cantley Lab for their help with the PI-3 kinase assays and the Genetics Institute for supplying CSF-1/M-CSF.
This manuscript is dedicated to the memory of Dananagoud Hiregowdara.
Supported in part by National Institutes of Health Grants No. HL 43510-07, HL 53745-02, HL 55187-01, and HL 51456-02. W.C.H. is supported by a David Geffen Foundation fellowship.
Address reprint requests to Jerome E. Groopman, MD, Chief, Division of Experimental Medicine, Harvard Institutes of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, 4 Blackfan Circle, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal