Abstract
We have performed a retrospective analysis of the development of T- and B-cell functions after HLA-nonidentical T-cell–depleted bone marrow transplantation (BMT) performed in 193 patients with severe combined immunodeficiency (SCID) at 18 European centers between December 1982 and December 31, 1993. One hundred sixteen of 193 patients were alive with evidence of engraftment 6 months after BMT. Development of T-cell function occurred earlier than B-cell function and was achieved more frequently up to the time of last follow-up. The median time to achieve normal T-cell function was 8.7 months, whereas the median time to achieve normal B-cell function was 14.9 months. Twenty-four patients died later than 6 months post-BMT, mainly due to chronic graft-versus-host disease (cGVHD) and/or viral infection. Absence of T-cell reconstitution 6 months after BMT, unlike absence of B-cell reconstitution, was associated with a poor outcome. Two additional factors were associated with a poor outcome: presence of cGVHD 6 months after BMT and B− SCID versus B+ SCID. However, two of these three factors remained as significant prognostic factors in a multivariate analysis: the absence of T-cell function and the presence of cGVHD 6 months after BMT. Analysis of the factors influencing the development of immune reconstitution showed that T- and B-cell functions occurred earlier and more frequently in B+ SCID versus B− SCID patients. Acute GVHD was associated with a slower development of T-cell function at 6 months, and cGVHD had a negative influence on the development of T-cell function afterwards, but neither acute nor chronic GVHD was found to influence the development of B-cell function. Once engraftment occurred, whether patients had or had not received Busulfan in the conditioning regimen did not influence the kinetics and quality of T-cell function development. In a multivariate study, two factors were found to influence the T-cell function 6 months after BMT: type of SCID and acute GVHD. The results of this retrospective analysis should lead to new protocols adapted to SCID disease, considering that disease-related as well as BMT-related parameters influence the development of immune function and thereby long-term outcome after HLA-nonidentical T-cell–depleted BMT.
BONE MARROW transplantation (BMT) is the treatment of choice for patients with severe combined immunodeficiency (SCID). It has been shown since 1968 that the results of HLA-identical BMT are excellent, characterized in most patients by full restoration of T- and B-cell function.1-4 Because most patients lack an HLA-identical related donor, HLA-nonidentical BMTs have been performed since 1981, when it became feasible to deplete marrow of T cells.5-11 In the last European survey, published in 1990,10 T-cell–depleted (TCD) HLA-nonidentical BMT from a related donor was associated with a 52% long-term survival. The latter study focused on the factors influencing engraftment and survival. Two factors had a significant effect on outcome of HLA-nonidentical BMT: the presence of a lung infection before BMT and the absence of a protective environment. The use of a conditioning regimen (CR) including Busulfan led to a higher engraftment rate but was not associated with any significant improvement in survival. However, the kinetics of the immune reconstitution was not studied.
As previously described, T-cell functions are not always optimally achieved and full B-cell function is lacking in almost 40% of cases.7 12-14 To assess prognostic factors that influence the development of T- and B-cell function after HLA-nonidentical TCD BMT in SCID patients, we retrospectively analyzed these parameters in a cohort of European patients transplanted before December, 31, 1993. The kinetics of the development of immune function and outcome as a function of immune status at 6 months post-BMT were assessed.
PATIENTS AND METHODS
Between January 1, 1983 and December 31, 1993, 193 SCID patients received a TCD BMT from an HLA-nonidentical related donor in 18 European centers. Data were retrieved from the European Bone Marrow Transplantation/European Society for Immuno-Deficiency registry. Long-term immune reconstitution was studied in 116 of these patients who were alive with engraftment (as defined by the presence of T lymphocytes of donor origin) at 6 months after BMT. Patients' type of SCID at diagnosis is given in Table 1, according to the classification of the World Health Organization (WHO) committee for immunodeficiencies.15
Distribution of the Patients Among the Different SCID Subgroups Before and After Haploidentical BMT
| . | No. of Patients . | Alive With Donor T-Cell Engraftment at 6 Mo Post-BMT . | Alive at Last Follow-Up (median, 6 yr) . |
|---|---|---|---|
| Absence of T lymphocytes B+ SCID | 107 | 71 | 62 |
| Absence of T and B lymphocytes B− SCID | 50 | 24 | 15 |
| Adenosine deaminase deficiency | 20 | 12 | 8 |
| Other types of SCID | 10 | 5 | 5 |
| Reticular dysgenesis | 6 | 4 | 2 |
| Total | 193 | 116 | 92 |
| . | No. of Patients . | Alive With Donor T-Cell Engraftment at 6 Mo Post-BMT . | Alive at Last Follow-Up (median, 6 yr) . |
|---|---|---|---|
| Absence of T lymphocytes B+ SCID | 107 | 71 | 62 |
| Absence of T and B lymphocytes B− SCID | 50 | 24 | 15 |
| Adenosine deaminase deficiency | 20 | 12 | 8 |
| Other types of SCID | 10 | 5 | 5 |
| Reticular dysgenesis | 6 | 4 | 2 |
| Total | 193 | 116 | 92 |
BMT characteristics.
One hundred one patients had received one BMT, 11 patients had received 2 BMT, and 4 patients had received 3 BMT because of previous lack of engraftment. Data were analyzed from the last BMT in recipients of 2 or 3 BMT. All donors were parents and were HLA 1 antigen to full haplotype mismatched.
Twenty-seven patients received no CR. Twelve patients received immunosuppressive agents alone (Cyclophosphamide at 200 mg/kg total dose [Cy] alone in 8 cases, Cy and antithymocyte globulin [ATG] in 2, and ATG alone in 2). Seventy-three patients received Busulfan (Bu; Bu at 8 mg/kg total dose alone in 4, Bu at 8 mg/kg total dose and Cy in 44, and Bu at 16 mg/kg total dose and Cy in 25). CR was not known in 4 occasions. In addition, 27 patients received monoclonal antibodies (MoAbs) to prevent graft rejection (anti-LFA1 antibody alone in 21 and anti-LFA1 and anti-CD2 antibodies in 6).16 17
As prevention against graft-versus-host disease (GVHD), all transplants were TCD. The method of depletion was E-rosetting with or without albumin gradient separation in 38 patients, soybean agglutination and E-rosetting or MoAbs with complement lysis in 71, and unrecorded in 7. Post-BMT GVHD prophylaxis was administered in patients who received E-rosetting–depleted bone marrow (60- to 180-day course of Cyclosporin A).8
Most patients were nursed within a protective environment (sterile isolator or laminar air flow). They all received prophylactic antimicrobial medication to suppress their intestinal microflora and intravenous immune globulin (IVIg) therapy weekly.
Analysis.
To assess the kinetics and quality of the development of T- and B-cell immune functions after BMT, T- and B-cell function were assayed separately and their degree of recovery arbitrarily classified as T+, T low, or T− and B+, B low, or B− (Table 2). This classification is based on the data obtained by simple and reproducible tests (see below) that were performed for all transplanted patients enrolled in this study in different laboratories. A specific questionnaire was sent to all participating centers to collect the relevant information. Normal and abnormal values were set according to individual laboratory values. Peripheral blood T-cell counts were determined by indirect immunofluorescence with specific MoAbs (CD3, CD2 CD4, and CD8).8 In vitro lymphocyte proliferation induced by mitogens and antigens were performed as described previously and evaluated by 3H-thymidine uptake.8 Serum Ig levels were measured by nephelometry. Serum antibodies to polio viruses, tetanus, and diphteria toxoids were detected with enzyme-linked immunosorbent assay tests.
Definition of T- and B-Cell Immune Functions Assessment Criteria
| T-cell function |
| T+: T cell counts >1,000/μL |
| and Responses to mitogens (PHA): positive (≥50,000 cpm) |
| and Responses to antigen (tetanus toxoid): positive (>15,000 cpm) |
| T−: Responses to mitogens: negative or low (<50,000 cpm) |
| and Responses to antigens: negative (<5,000 cpm) |
| T low: Any other combination |
| B-cell function |
| B+: Positive antibody responses at least to two immunization antigens* in the absence of Ig substitution |
| B−: Absence of antibody responses after immunization |
| and IgA: ≤0.1 g/L |
| B low: Any other combination |
| T-cell function |
| T+: T cell counts >1,000/μL |
| and Responses to mitogens (PHA): positive (≥50,000 cpm) |
| and Responses to antigen (tetanus toxoid): positive (>15,000 cpm) |
| T−: Responses to mitogens: negative or low (<50,000 cpm) |
| and Responses to antigens: negative (<5,000 cpm) |
| T low: Any other combination |
| B-cell function |
| B+: Positive antibody responses at least to two immunization antigens* in the absence of Ig substitution |
| B−: Absence of antibody responses after immunization |
| and IgA: ≤0.1 g/L |
| B low: Any other combination |
*Two antigens among polio viruses, tetanus, and diphtheria toxoids.
Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were assessed in all patients according to standard criteria.18
Chimerism analysis data of T- and B-cell populations were available only in a limited number of patients and therefore were not analyzed in this study. However, in all patients (n = 116), T cells of donor origin were detected 6 months after BMT by HLA typing, by fluorescence in situ hybridization for the Y chromosome and/or karyotyping in sex-mismatched transplants, and/or by using DNA analysis with microsatellite probes.19
The analysis was made on data available up until December 31, 1996. Data regarding immune reconstitution were collected for all the patients at 6, 12, and 24 months after BMT and at last follow-up.
Statistical analysis.
Differences between distribution of qualitative variables were analyzed using the χ2 test. Distribution of survival with engraftment was studied using the product-limit method. Comparisons of survival distribution were performed using the log rank test. Analysis of the variables influencing survival with engraftment was performed by means of a Cox proportional hazards model.20 Variables affecting the development of T- or B-cell function at 6 months after BMT were sought using a logistic regression model. Odd ratios (OR) were expressed with their 95% confidence intervals.
RESULTS
Kinetics and quality of T- and B-cell function development.
Among the 116 patients alive with evidence of engraftment 6 months after BMT, the proportion of patients with normal T-cell function, defined as T+, was significantly higher than the proportion of patients with normal B-cell function, defined as B+ (Fig 1).
Evolution of T-cell (A) and B-cell (B) functions after BMT. Last follow-up, 3 to 13 years. The number of patients with normal T-cell function was higher than the number of patients with normal B-cell function (41% v 26% at 6 months [P < .0001] and 94% v 69% at last follow-up [P < .001]). See criteria of definition of T- and B-cell functions in Table2.
Evolution of T-cell (A) and B-cell (B) functions after BMT. Last follow-up, 3 to 13 years. The number of patients with normal T-cell function was higher than the number of patients with normal B-cell function (41% v 26% at 6 months [P < .0001] and 94% v 69% at last follow-up [P < .001]). See criteria of definition of T- and B-cell functions in Table2.
From 6 months to 2 years post-BMT, an increasing number of patients achieved normal T-cell function (Fig 1). Although several patients also developed normal B-cell function during that period, the number of patients with normal B-cell function remained lower than the number of patients with normal T-cell function (Fig 1). The median time to achieve normal T-cell function was 8.7 months, whereas the median time to achieve normal B-cell function was 14.9 months. The T-cell function status at last follow-up was strongly influenced by the T-cell function at 6 months after BMT, because, among the 21 patients with absent T-cell function at 6 months, only 9 patients (43%) had developed normal T-cell function at last follow-up. In contrast, among the 93 patients with low or normal T-cell function at 6 months, 66 patients (71%) had developed persistent full T-cell function (P < .05). Among the 86 evaluable patients alive 2 years after BMT, only 12 patients had an incomplete development of T-cell function, of which only 2 had absent T-cell function. Five of 10 with low T-cell function at 2 years developed normal T-cell function afterwards. Persisting failure to develop adequate T-cell immunity led to the decision to perform a second BMT in 4 patients (3 developed normal T-cell function and 1 developed incomplete T-cell function associated with improved clinical condition).
As shown in Fig 2, the development of B-cell function was associated with T-cell function, because most of the patients with normal T-cell function 6 months after BMT also had normal B-cell function and most of those with incomplete T-cell reconstitution also had abnormal B-cell function. Moreover, the development of normal B-cell function was more frequent among patients with complete or incomplete T-cell function than among patients with absent T-cell function at 6 months after BMT (Fig 2).
Influence of T-cell function status 6 months after BMT on B-cell function development. At 6 months post-BMT, 22 of 47 patients with normal T-cell function (ie, T+) had a normal B-cell function (ie, B+), whereas 7 of 67 patients with incomplete or absent T-cell reconstitution (ie, T low or T−) had a normal B-cell function (P = .01). y axis, number of patients; x axis, time lapsed post-BMT. See criteria of definition of T- and B-cell functions in Table 2.
Influence of T-cell function status 6 months after BMT on B-cell function development. At 6 months post-BMT, 22 of 47 patients with normal T-cell function (ie, T+) had a normal B-cell function (ie, B+), whereas 7 of 67 patients with incomplete or absent T-cell reconstitution (ie, T low or T−) had a normal B-cell function (P = .01). y axis, number of patients; x axis, time lapsed post-BMT. See criteria of definition of T- and B-cell functions in Table 2.
Influence of immune function development on long-term survival.
Among the 116 patients alive 6 months after BMT, 24 patients later died (20%), 19 of which occurred during the 2 years after BMT. Death was due to GVHD and/or viral infection in 18 of these 19 patients and was unrecorded in 1. Five patients died more than 2 years post-BMT, 2 dying from immune dysfunction with severe auto-immunity involving the liver, 1 from GVHD, 1 after cerebral hemorrhage, and 1 from an unrecorded cause.
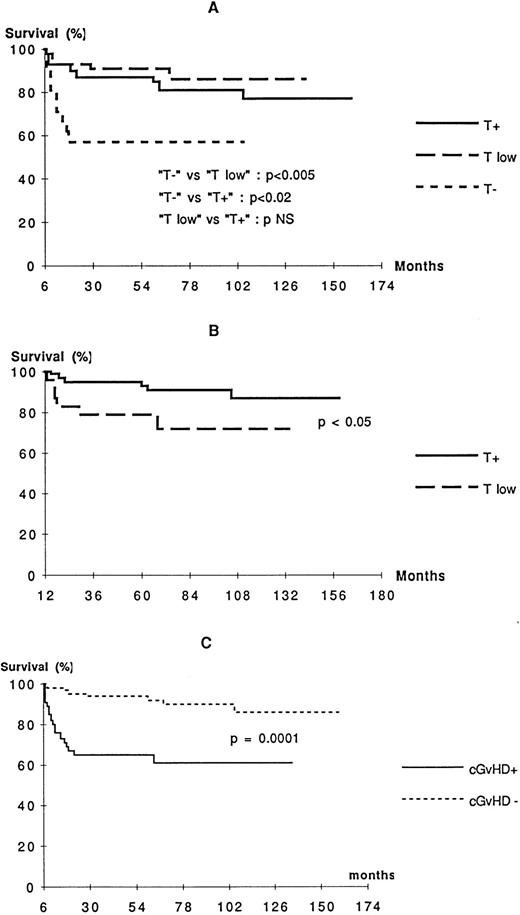
T-cell function status at 6 months after BMT influenced strongly the outcome. Patients from T− group did poorly, whereas a greater proportion of patients from the T low and T+ groups survived (Fig 3A). Although the prognosis for patients with incomplete T-cell function (T low) at 6 months was not different from those with complete reconstitution, those who were still T low at 12 months had a poorer prognosis (Fig 3B). In contrast, B-cell function status 6 months after BMT was found not to be associated with outcome (data not shown). The presence of cGVHD 6 months after BMT had the most significant negative influence on outcome (Fig 3C).
Probability of survival according to T-cell function status 6 months after BMT (A), T-cell function status 1 year after BMT (B), and cGVHD status 6 months after BMT (C). See criteria of definition of T-cell functions in Table 2.
Probability of survival according to T-cell function status 6 months after BMT (A), T-cell function status 1 year after BMT (B), and cGVHD status 6 months after BMT (C). See criteria of definition of T-cell functions in Table 2.
The SCID diagnosis also influenced the outcome, because, among patients alive 6 months after BMT, 9 of the 24 B− SCID patients (37%) later died, compared with only 9 of the 71 B+ SCID patients (13%;P < .01, Mantel Cox). No difference in long-term survival was detected in the patients alive 6 months after BMT who had or had not received a conditioning regimen (data not shown).
In a multivariate analysis including type of SCID, the presence of cGVHD 6 months after BMT, the absence of T-cell function 6 months after BMT, the presence of a pulmonary infection before BMT, and the type of CR, only two factors were found to impair the survival among patients alive 6 months after BMT: cGVHD and absence of T-cell function 6 months after BMT (OR = 6.6 [3.59 to 12.3] and OR = 4.2 [2.55 to 7.02], respectively).
Analysis of the factors influencing kinetics of T- and B-cell function development.
Development of immune function varied according to the type of SCID. Among the patients alive with evidence of engraftment 6 months after BMT, the number of patients with normal T- and B-cell function were both significantly higher among the B+ SCID patients compared with the B− SCID patients (Fig 4A and B). Conversely, the number of patients with absent T- and B-cell function were both significantly higher in the B− SCID patients compared with the B+ SCID patients (Fig 4A and B). There were too few patients with ADA deficiency, reticular dysgenesis, and other forms of SCID to draw any conclusions for these groups, but the results appeared to be comparable with those of B+ SCID group (data not shown). In both B+ SCID and B− SCID patient groups, the number of patients with normal T- and B-cell functions increased with time (Fig 4A and B).
Evolution of T-cell (A) and B-cell (B) functions among the transplanted patients with B+ SCID and B− SCID diagnosis. Last follow-up, 3 to 13 years. At 6 months, the number of patients with normal T- and B-cell function was higher among B+ SCID than among B− SCID patients (51% v 14% [P < .01] and 29%v 9% [P < .05], respectively). Correspondingly, the number of patients with absent T- and B-cell function was higher among B− SCID than among B+ SCID patients (41% v 11% [P < .01] and 61% v 37% [P = .05], respectively). See criteria of definition of T- and B-cell functions in Table 2.
Evolution of T-cell (A) and B-cell (B) functions among the transplanted patients with B+ SCID and B− SCID diagnosis. Last follow-up, 3 to 13 years. At 6 months, the number of patients with normal T- and B-cell function was higher among B+ SCID than among B− SCID patients (51% v 14% [P < .01] and 29%v 9% [P < .05], respectively). Correspondingly, the number of patients with absent T- and B-cell function was higher among B− SCID than among B+ SCID patients (41% v 11% [P < .01] and 61% v 37% [P = .05], respectively). See criteria of definition of T- and B-cell functions in Table 2.
aGVHD had no influence on B-cell function development, but influenced T-cell function at 6 months after BMT, because, among the 77 patients with no or grade I aGVHD, 38 (49%) had normal T-cell function 6 months after BMT, whereas, among the 34 patients with grade II to IV aGVHD, only 10 (29%) had normal T-cell function 6 months after BMT (P= .05), irrespective of the type of SCID. However, aGVHD had no influence on T-cell function development when the analysis was performed from 1 year after BMT to the last follow-up (data not shown).
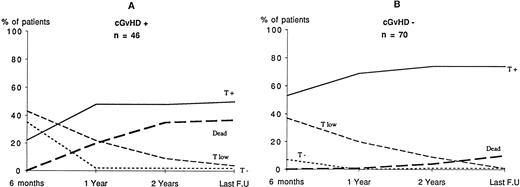
The prevalence of cGVHD was 40% 6 months after BMT, 26% 1 year after BMT, 18% 2 years after BMT, and 16% at last follow-up. The frequency of cGVHD 6 months after BMT was 54% in the B− SCID patients and 34% in the B+ SCID patients, but this difference did not reach significance (P = .07). As shown in Fig 5, the occurrence of cGVHD 6 months after BMT had a significant negative influence on the development of T-cell function. However, the occurrence of cGVHD did not significantly influence the development of B-cell function (data not shown).
Influence of cGVHD 6 months after BMT on T-cell function development. (A) Patients with cGVHD (n = 46). (B) Patients without cGVHD (n = 70). y axis, number of patients; x axis, time lapsed post-BMT. The number of patients with normal T-cell function 6 months after BMT was higher among patients without GVHD than with GVHD (55%v 24%; P < .01). See criteria of definition of T-cell functions in Table 2.
Influence of cGVHD 6 months after BMT on T-cell function development. (A) Patients with cGVHD (n = 46). (B) Patients without cGVHD (n = 70). y axis, number of patients; x axis, time lapsed post-BMT. The number of patients with normal T-cell function 6 months after BMT was higher among patients without GVHD than with GVHD (55%v 24%; P < .01). See criteria of definition of T-cell functions in Table 2.
In vivo infusion of MoAbs (ie, anti-LFA1 antibody alone or anti-LFA1 and anti-CD2 antibodies) led to a slower development of T-cell function, because, at 6 months after BMT, 41 of the 85 patients (48%) who did not receive MoAb had normal T-cell function, whereas only 7 of the 29 patients (24%) who received MoAbs had normal T-cell function (P = .02). However, from 1 year after BMT to the last follow-up, this difference was no longer significant. The use of MoAbs led to a lower rate of B-cell function development, because, among the patients who did not receive MoAb, 28 of 84 (33%) had normal B-cell function 6 months after BMT, whereas, among patients who did receive MoAbs, 1 of 28 (3%) had normal B-cell function (P < .05). Moreover, at last follow-up, among the patients who did not receive MoAb, 49 of 61 (80%) had normal B-cell function, whereas, among patients who did receive MoAbs, 8 of 21 (38%) had normal B-cell function (P < .001).
Once engraftment occurred, whether patients had received CR or not did not influence the kinetics and/or quality of T-cell function (data not shown). The use of CR was associated with a trend towards a better B-cell function development at last follow-up, because 64% of the patients who received Bu and/or immunosuppressive agents had normal B-cell function, whereas 45% of the patients who did not receive any CR had normal B-cell function. However, this difference was not significant. Interestingly, at last follow-up, among B+ SCID patients, 47% of those who did not receive any CR had normal B-cell function, whereas, among B− SCID patients, the only patients who developed normal B-cell function were those who had received myeloablative CR that included Bu.
The method of TCD (E-rosetting with or without albumin gradient separation v soybean agglutination and E-rosetting or MoAbs with complement lysis) did not influence the reconstitution of T- or B-cell function, regardless of the type of SCID (data not shown).
In a multivariate analysis including type of SCID (B+ SCID or B-SCID), aGVHD (no or grade I aGVHD v grade II to IV aGVHD), cGVHD (the presence of cGVHD v absence of cGVHD), the use of anti-LFA1 MoAb, the method of TCD, CR, and the presence of pulmonary infection before BMT, two factors were found to impair the T-cell function 6 months after BMT: B− SCID and grade II to IV aGVHD (OR = 6 [3.0 to 12.1] and OR = 3.6 [2.1 to 6.1]), respectively. Using the same variables, the only factor that impaired the B-cell function 6 months after BMT was the use of anti-LFA1 MoAb (OR = 10.7 [3.7 to 31]).
DISCUSSION
This retrospective analysis of 116 patients with SCID who had received a TCD HLA-nonidentical BMT in European centers and who were alive at 6 months post-BMT shows that, overall, the immune development was good, because at last follow-up (median, 6 years), 93% had normal T-cell function and 68% had normal B-cell function. T-cell function developed before B-cell function and was more often present at last follow-up. These results in a large series of patients confirm previously published data on the kinetics of immune reconstitution after TCD HLA-nonidentical BMT for SCID patients. Buckley et al7showed in a series of 17 patients that normal T-cell function was reached between 4 and 7 months, whereas B-cell function took 2 to 2.5 years to fully develop. Dror et al12 showed in 14 patients surviving more than 1 year after BMT that, for most of the patients, T-cell numbers and subsets were normal by 10 to 12 months after BMT, whereas B-cell function became normal in 10 of 14 patients 2 to 8 years after BMT (7 continued to receive gammaglobulin therapy). Also, Wijnaendts et al13 showed in 15 patients that the median time to achieve normal T-cell function was 8 months (1 month to 4 years) and to achieve normal B-cell function was 12 months (3 months to 4 years), with 6 patients still showing poor antibody responses to immunization. The similarity of the results observed in those studies as well as the present one confirm that the definitions of T- and B-cell functional parameters we chose to define T+, T low, and T− and B+, B low, and B− were appropriate.
In this retrospective study, the type of SCID was shown to be the most important factor influencing the development of T-cell function post-BMT. One of the major differences between B+ SCID and B− SCID patients is the presence of natural killer (NK) cells with normal NK activity in the latter subset.15,21 NK cells are known to mediate marrow graft rejection in murine models, including murine SCID.22,23 The presence of these cells may underscore the requirement for pre-BMT immunosuppression to achieve sustained engraftment in B− SCID patients,21 as shown by Bertrand et al (manuscript submitted). Also, evidence for increased cell radiosensitivity has been provided in some of these patients.24,25 It is therefore possible that use of a CR in B− SCID patients could damage the medullary and thymic stroma and therefore disturb the development of T and B cells. Such damage might also favor the development of GVHD, which, as shown in this study, is also more frequently associated with poorer and delayed immune function.26 It might be also possible that host NK cells may persist despite CR (mostly Bu 8 Cy 200) and could disturb the development of lymphoid precursors and delay the development of a normal immune system. Competition between normal and abnormal precursors of lymphoid lineage could also impair lymphocyte development in B− SCID. Indeed, in patients with B+ SCID, deficiency of γc or of JAK-3 leads to a very early block in the development of T-cell precursors,27,28 whereas, in B− SCID, the block occurs slightly later in the T-cell development, at the time of V(D)J recombination processes.29 Therefore, putative T-cell precursors may still be present after BMT in patients with B− SCID and could delay the development of donor T cells in the host thymus because of potential reduced accessibility of precursors to environmental factors.
In this survey, we also show that the use of in vivo anti-LFA1 antibody leads to a poorer development of B-cell function at the last follow-up. Anti-LFA1 antibody infusion alone or in association with anti-CD2 antibodies was found to lead to a better engraftment of HLA-nonidentical TCD BMT in other congenital immunodeficiency settings.16,17 In addition, Bertrand et al (manuscript submitted) have shown that the use of anti-LFA1 antibody led to better engraftment in B− SCID patients, possibly because of its profound inhibition of NK cell function.30 Why infusion of nondepleting anti-LFA1 antibody appeared to impair B-cell function development is presently unclear. The results suggest that anti-LFA1 should not be used in HLA-nonidentical TCD BMT at least in B+ SCID patients.
The use of preBMT chemotherapy did not enhance development of T-cell function, whatever the type of SCID. There is only a trend towards better B-cell function at last follow-up in B+ SCID patients. However, of the B− SCID patients who did not receive any CR, none developed B-cell function. Bertrand et al (manuscript submitted) have shown that the use of CR significantly improved engraftment in B− SCID patients. It is therefore likely that use of a CR in B− SCID patients leads more frequently to myeloid and B-cell engraftment. In this retrospective analysis, half of patients with B+ SCID who did not receive CR have nevertheless developed normal B-cell function. These results confirm those of Buckley et al7 and Van Leeuwen et al31 and suggest that γc(−) or JAK-3(−) B cells might be somewhat functional in vivo. Further detailed analysis of chimerism still have to be performed in B+ SCID patients post-BMT to determine the exact influence of CR on B-cells, NK cells, and monocyte chimerism and the possible influence of the genetic defect on the development of B-cell function.
The kinetics and quality of the development of immune function influenced outcome, because survival was significantly lower for the patients with absent T-cell function 6 months after BMT and for the patients with low or absent T-cell function 1 year after BMT. These results raise the question of retransplantation of such patients and when it should be performed. In this study, 4 patients underwent a second BMT after 3 years in 1 case, 4 years in 2 cases, and 5 years after the first BMT in another, because of the absence of T-cell function despite sustained T-cell engraftment. Three of these patients now have normal T-cell function, whereas one still has poor T-cell function 8 months after the second BMT. Considering the results of kinetics of immune development and its influence on outcome, one can propose that the absence of normal T-cell function 2 years after BMT could be an indication for a second BMT. On the other hand, several patients with poor T-cell reconstitution have died during the first 2 years after BMT, suggesting that a second BMT should be performed earlier. The type of SCID should influence such a decision, because most of the B+ SCID patients with poor T-cell functions 6 months or 1 year after BMT survived and developed T- and B-cell function later, whereas most of the B− SCID patients in the same situation died. The presence of cGVHD should also influence the decision, because, in the multivariate analysis of the factors influencing the late outcome, cGVHD was found to increase the risk of death sixfold. The fact that B-cell function does not affect survival may be due to the IVIg replacement.
This retrospective analysis sheds light on the disease-related as well as BMT-related parameters that influence SCID disease correction and thereby long-term outcome after HLA nonidentical TCD BMT. It should lead to careful prospective assessment of protocols adapted for the treatment of the different forms of SCID.
ACKNOWLEDGMENT
In addition to the authors, the following persons and institutions participated in this study: P. Bordigoni, Hôpital d'Enfants, Vandœuvre, France; R. Seger, Kinderspital, Zürich, Switzerland; O. Schofer, University Hospital of Mainz, Mainz, Germany; A. Ferster, Hôpital Universitaire Des Enfants De La Reine Fabiola, Bruxelles, Belgium; F. Locatelli, University of Pavia, Pavia, Italy; B.R. De Graeff-Meeder and N.M. Wulfraat, University Hospital for Children, Utrecht, The Netherlands; M. Abinum, Belgrade, Yugoslavia; and G. Souillet, Hôpital Debrousse, Lyon, France.
Supported by EBMT, ESID, and concerted action Biomed I PL 9321.
Address reprint requests to Elie Haddad, MD, Unité d'Immunologie et d'Hématologie Pédiatriques, Hôpital Necker Enfants-Malades, 149 Rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: ehaddad@igr.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Evolution of T-cell (A) and B-cell (B) functions after BMT. Last follow-up, 3 to 13 years. The number of patients with normal T-cell function was higher than the number of patients with normal B-cell function (41% v 26% at 6 months [P < .0001] and 94% v 69% at last follow-up [P < .001]). See criteria of definition of T- and B-cell functions in Table2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/10/10.1182_blood.v91.10.3646/3/m_blod41030001x.jpeg?Expires=1769091840&Signature=HRPCcrNuyWw6P3lt1VBoVSHHoKc3PNKXXazJHc9OjXHd8HsfTKZcePhfdzina5hXIyu7rrJLP9Xw-AeD0m3vXD54zXazWJ9LEz3rDDNd4YH3DPkfJHGpkjnIgYW5N06xItwYlkyJqBxomQBkcchWE8kCBuctjtVmefTmrctvQqjxdofpdYnACTnHlAOeQOdJySFD-z-h0aKkaVzeW9SAdNxgoVOxcIiQM4dHnV5~3QqKQsLLRHwWmaPFMcWA0d502DzfuW3RtilpCsF4EGbBFKSsp1NMqYioKTXEyWRYIkityuP4G9egcrZZq6tKWLOe2PRjqklZ1jjoJehvMDpDQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Evolution of T-cell (A) and B-cell (B) functions among the transplanted patients with B+ SCID and B− SCID diagnosis. Last follow-up, 3 to 13 years. At 6 months, the number of patients with normal T- and B-cell function was higher among B+ SCID than among B− SCID patients (51% v 14% [P < .01] and 29%v 9% [P < .05], respectively). Correspondingly, the number of patients with absent T- and B-cell function was higher among B− SCID than among B+ SCID patients (41% v 11% [P < .01] and 61% v 37% [P = .05], respectively). See criteria of definition of T- and B-cell functions in Table 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/10/10.1182_blood.v91.10.3646/3/m_blod41030004ax.jpeg?Expires=1769091840&Signature=qKQWPQEwetnulncgRObvZFsAL1MKG0JBaeUnJAnJHtYRxs4nW85JSoQQ2dAawTAu1Ugpp2SlDZDR316IUCDIO4ghXarlTq7xyKvMIJxStjUpNDqFbB9IssodHD1eZX6fAfe2HxxphA8WrSkHLjyRKyQjnPajVwrgLvgmAeuzBbtrxzPCO4e9tFWlpBPyAkFgll-w7ovA8U2xQg-Hsb2BeeoFZA9hLToNECJPN~HouxiFdmykBAbLOv7ayYGUDFS64ciPVI~c1K4DF8jTBUxvd8acVrONDP9n8VE~e8CN6QOa2bAbS9NuBm3DYX0GdanW0cOydt98JY5paHJx~L919w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Evolution of T-cell (A) and B-cell (B) functions among the transplanted patients with B+ SCID and B− SCID diagnosis. Last follow-up, 3 to 13 years. At 6 months, the number of patients with normal T- and B-cell function was higher among B+ SCID than among B− SCID patients (51% v 14% [P < .01] and 29%v 9% [P < .05], respectively). Correspondingly, the number of patients with absent T- and B-cell function was higher among B− SCID than among B+ SCID patients (41% v 11% [P < .01] and 61% v 37% [P = .05], respectively). See criteria of definition of T- and B-cell functions in Table 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/10/10.1182_blood.v91.10.3646/3/m_blod41030004bx.jpeg?Expires=1769091840&Signature=eR53S2ytMRPwNYHExxt3KepvJNJtlXUeuA9krjmDz4k2eYaRCnzMwfGOMdb5WBUFgTKVM7V6f9-3yX1~jI1VeCqQ7OsnrCIh~CY680DcexG8QesgB9MyjsJQ6ZALUPEBX0JjXc0BI5pTjVwCgUlZ-Ceg64H6XkuevdOOn-qkKIsxkVqRPm9fCzCaK6h0yTuAnNNKbmtiKcxuLn7HBItX2mUiJz-oeIkjr6FCVvxW4y~HI3wLM218tuA8EIwbpp5tw3Ufb64A2jrg0Useaj0QxPgsp379zb3ASteTVxRFJ40e7pm06-mMw37SYFHlgs4MOg7JnkMRIgRMKgjRQnlIZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal