Abstract
Epstein-Barr virus–induced lymphoproliferative disease (EBV-LPD) is a serious and potentially fatal complication after allogeneic stem cell transplantation (SCT). To evaluate levels of EBV DNA in SCT patients, a semiquantitative polymerase chain reaction (PCR) assay was developed. DNA was extracted from peripheral blood leukocytes and diluted, and PCR was performed by using a primer set specific for a well-conserved sequence of the internal repeat 1 region of the EBV genome. Forty-one SCT patients were screened with this method. Thirty-seven patients received allogeneic transplants, of which 18 were T-cell–depleted marrow. Four additional patients received autologous SCT, one of which was T-cell depleted. The mean time of follow-up by EBV PCR was 147 days (range, 47 to 328 days) posttransplant. The range of EBV copies/μg DNA from normal EBV sero-positive donors was 40 to 4,000. Seven patients had ≥40,000 copies of EBV DNA/μg DNA, all of whom were recipients of T-cell–depleted SCT. Five of the seven patients with elevated levels of EBV DNA developed EBV-LPD. Four of these five patients with EBV-LPD had elevated levels of EBV DNA from 1 to 8 weeks before diagnosis. Two patients with EBV-LPD had normal levels of EBV DNA, and two patients with ≥40,000 copies EBV/μg DNA did not develop EBV-LPD. In one patient, clinical resolution of disease correlated with a decrease in EBV DNA and an increase in the level of EBV-specific cytotoxic T-cell precursors. These data indicate that the measurement of EBV viral load with semiquantitative PCR is useful in detecting EBV-LPD in high-risk patients before the onset of clinical symptoms. Because not all patients with elevated levels of EBV DNA develop EBV-LPD, semiquantitative PCR results cannot substitute for clinical, radiographic, and pathological confirmation of this diagnosis.
EPSTEIN-BARR VIRUS–induced lymphoproliferative disease (EBV-LPD) is a serious complication because of the profound immune deficiency that occurs after allogeneic stem cell transplantation (SCT).1-5 EBV infects most individuals by adulthood and latently infects B lymphocytes.6 In the absence of adequate cellular immunity to protect against EBV, latently infected B cells undergo transformation into lymphoblastoid cells. After SCT, these lymphoproliferations are generally of donor B-cell origin.2-4,7,8 The clinical spectrum of EBV-LPD varies from polyclonal lymphoproliferations to clonal malignancies; the latter being frequently fatal.3,4,8 Among recipients of allogeneic SCT, the incidence of EBV-LPD is highest for those receiving grafts that have been manipulated to reduce the number of T cells or who have received aggressive immunosuppressive regimens to facilitate engraftment.3-5,7,8 Attempts to treat these patients with interferon, acyclovir, and anti–B-cell monoclonal antibodies are generally not successful.3,4,9-11 However, adoptive immunotherapy with unprimed peripheral blood mononuclear cells (PBMCs) or donor-derived, EBV-specific cytotoxic T cells (EBV-CTLs) has been shown to be curative for SCT patients with EBV-LPD.2 12
Rooney et al12 have shown that patients receiving T-cell–depleted (TCD) SCTs can be prevented from developing EBV-LPD by using prophylactic EBV-CTL infusions. Institutions performing TCD transplants are more frequently using strategies to prevent EBV-LPD in high-risk patients by using either donor PBMCs or EBV-CTLs. The differential susceptibility to EBV-LPD among recipients of TCD marrow grafts is not understood and may represent a combination of factors, such as viral burden and level of cellular immunity to EBV. Differences in levels of EBV DNA found in the peripheral blood posttransplant may distinguish which patients are at highest risk to develop EBV-LPD and, therefore, could permit earlier intervention in high-risk patients. Previous studies have shown a correlation between levels of EBV DNA and the occurrence of EBV-LPD in organ transplant patients13-15and in recipients of SCTs.16 However, it is unclear to what extent levels of EBV DNA correlate with the presence of EBV-LPD, disease outcome, and cellular immunity to EBV in SCT recipients. The purpose of this study was to examine the correlation between levels of EBV DNA in the peripheral blood of SCT patients posttransplant with (1) the type of transplant received, (2) the development of EBV-LPD, (3) clinical outcome of adoptive immunotherapy, and (4) the cellular immunity to EBV in recipients of donor lymphocytes.
MATERIALS AND METHODS
Patients.
Patient characteristics are summarized in Table 1. Both adult and pediatric stem cell transplant patients had 5 mL of whole blood collected starting at approximately 6 to 8 weeks posttransplant. This test was performed every 2 to 4 weeks thereafter. Forty-one patients had levels of EBV DNA measured. The mean time of follow-up was 147 days posttransplant (range, 47 to 328 days). Some patients were followed for a shorter time than others because of relapse, EBV-LPD, death, or distance from the hospital precluding frequent follow-up. Thirty-seven patients (90%) received allogeneic SCT, and 4 (10%) received autologous transplants. One of the autologous SCT patients received a TCD graft as treatment for multiple sclerosis. Of the 37 allogeneic transplant recipients, 16 patients received unmodified marrow grafts from a related donor, 3 patients received unrelated cord blood transplants, and 18 patients received TCD transplants. Three of the TCD SCT patients received CD34-selected marrow grafts (Baxter Healthcare, Santa Ana, CA) and 15 received marrow that was TCD with sheep red blood cells and soybean agglutination as previously described.17 These studies were performed under protocols approved by the Institutional Review Board of the Indiana University School of Medicine, Indianapolis, IN.
Patient Characteristics
| Mean age (yrs) | 24 (1-56) |
| Sex (male:female) | 20:21 |
| Pretransplant diagnosis | |
| Acute lymphoblastic leukemia | 11 |
| Acute myelogenous leukemia | 9 |
| Chronic myelogenous leukemia | 6 |
| Myelodysplastic syndrome | 7 |
| Other-150 | 8 |
| Allogeneic T-cell–depleted marrow grafts-151 | 18 |
| Unmodified marrow | 16 |
| Unrelated cord blood | 3 |
| Autologous, T-cell–depleted transplant | 1 |
| Autologous peripheral blood | 3 |
| Mean age (yrs) | 24 (1-56) |
| Sex (male:female) | 20:21 |
| Pretransplant diagnosis | |
| Acute lymphoblastic leukemia | 11 |
| Acute myelogenous leukemia | 9 |
| Chronic myelogenous leukemia | 6 |
| Myelodysplastic syndrome | 7 |
| Other-150 | 8 |
| Allogeneic T-cell–depleted marrow grafts-151 | 18 |
| Unmodified marrow | 16 |
| Unrelated cord blood | 3 |
| Autologous, T-cell–depleted transplant | 1 |
| Autologous peripheral blood | 3 |
The clinical characteristics of patients evaluated by semiquantitative EBV PCR are summarized above.
Includes 2 patients with non-Hodgkin's lymphoma, 3 patients with Ewing's sarcoma, 1 patient with neuroblastoma, 1 patient with juvenile myelomonocytic leukemia, and 1 patient with multiple sclerosis.
T-cell depletion by sheep erythrocyte and soybean agglutination (15) or CD34 selection device.3
Transplant regimens.
The preparative regimens varied depending on the clinical protocol. Recipients of TCD marrow grafts received hyperfractionated total body irradiation (TBI; 1,375 cGy) with 60 mg/kg cyclophosphamide over 2 days and 5 mg/kg/d thiotepa for 2 days. These patients received antithymocyte globulin (ATG) at total cumulative doses of 75 mg/kg posttransplant (6 patients) or 120 mg/kg pretransplant (12 patients). The patient receiving an autologous TCD bone marrow transplant as treatment for multiple sclerosis received a total of 90 mg/kg ATG pretransplant with 120 mg/kg cyclophosphamide and 1,200 cGy hyperfractionated TBI. Recipients of unmodified marrow grafts received TBI (1,375 cGy), 500 mg/m2/d etoposide for 2 days, and 60 mg/kg/d cyclophosphamide for 2 days. Autologous transplant recipients received 1,800 mg/m2/d etoposide for 1 day, 90 mg/m2/d melphalan for 2 days, and hyperfractionated TBI (1,375 cGy). The cord blood transplant patients received hyperfractionated TBI (1,375 cGy), 60 mg/kg/d cyclophosphamide for 2 days, and 30 mg/d ATG for 2 days. Recipients of unmodified allogeneic stem cell transplants received cyclosporin A and methotrexate for graft-versus-host disease (GVHD) prophylaxis.18
Diagnosis of EBV-LPD.
Biopsy specimens were evaluated morphologically, and immunostains were used against CD19, CD20, and latent membrane protein 1 (LMP-1). Clonality was determined by κ and λ immunoglobulin light chain restriction or by immunoglobulin gene rearrangement by polymerase chain reaction (PCR).
Semiquantitative PCR.
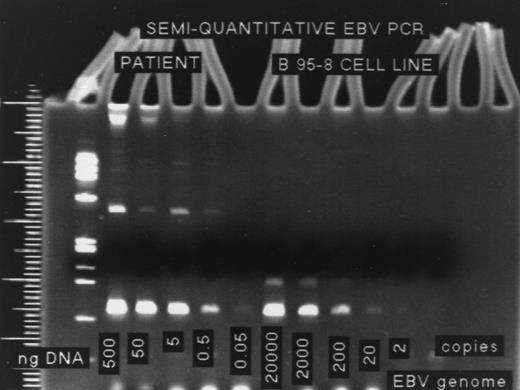
DNA from the B95-8 cell line, which contains two copies of the EBV genome per cell, was used as the positive control and to determine the sensitivity of the assay. DNA was isolated from a known number of B95-8 cells by phenol/chloroform extraction followed by alcohol precipitation. The DNA was quantified on a spectrophotometer at OD260, and then a 10-fold dilution series was prepared, ranging from 500 ng to 0.05 ng. These titrations were performed for all patient studies. These template concentrations were used in a PCR containing 10 μL DNA template, 4 μL of 10 μmol/L each of deoxynucleoside triphosphate (Oncor, Gaithersburg, MD), 2.5 units Taq polymerase (Oncor), and 20 pmol each of the primers EBV-1 and EBV-2.19 The final volume of 60 μL was placed in a thermal cycler (Perkin Elmer, Foster City, CA) for a 35-cycle PCR. The formation of a PCR product was detected by gel electrophoresis followed by ethidium bromide staining of the gel. The lowest dilution at which a band was visible corresponded to 10 cells. Because there are two copies of the EBV genome per cell, the sensitivity of this assay was determined to be 20 copies of the EBV genome (Fig 1).
The five lanes on the right, labeled “B 95-8 Cell Line”, represent the electrophoresis of DNA from this cell line, which is used as a control to determine the sensitivity of the semiquantitative EBV PCR assay. DNA from a known number of cells is run in each lane, and the lowest dilution at which a band is still visible corresponds to 10 cells. Because each B 95-8 cell has two copies of the EBV genome, the sensitivity of this assay is 20 copies EBV DNA. The first five lanes represent different amounts of DNA per lane from a patient. The last visible band corresponds to the 0.05-ng dilution. The number of copies of EBV genome per microgram DNA (in this case 400,000 copies/μg DNA) is calculated by dividing the sensitivity of the assay (20 copies/μg DNA) by the concentration of DNA for the last visible band (0.05 × 10−3 μg).
The five lanes on the right, labeled “B 95-8 Cell Line”, represent the electrophoresis of DNA from this cell line, which is used as a control to determine the sensitivity of the semiquantitative EBV PCR assay. DNA from a known number of cells is run in each lane, and the lowest dilution at which a band is still visible corresponds to 10 cells. Because each B 95-8 cell has two copies of the EBV genome, the sensitivity of this assay is 20 copies EBV DNA. The first five lanes represent different amounts of DNA per lane from a patient. The last visible band corresponds to the 0.05-ng dilution. The number of copies of EBV genome per microgram DNA (in this case 400,000 copies/μg DNA) is calculated by dividing the sensitivity of the assay (20 copies/μg DNA) by the concentration of DNA for the last visible band (0.05 × 10−3 μg).
Patient DNA was isolated from the white blood cells of whole blood using the Puregene DNA isolation kit (Gentra, Minneapolis, MN) according to the manufacturer's directions. PCR was performed on patient specimens as outlined previously, with known amounts of DNA per lane, ranging from 0.05 to 500 ng. The following formula was used to calculate the number of EBV copies per microgram DNA: sensitivity of the assay/DNA concentration of last visible band = number of copies EBV genome/μg DNA.
For the example in Fig 1, the lowest visible band was from the 0.05 ng dilution. Therefore, 20 copies (sensitivity of the assay)/0.05 × 10−3 μg DNA = 400,000 copies EBV genome/μg DNA.
Cell culture and chromium-51 release assays.
Blood was obtained on patients at study intervals and PBMCs were isolated by using Ficoll-Hypaque (Accurate Chemical Co, Westbury, NY) density gradient centrifugation. Bulk and limiting dilution cultures with patient PBMCs and irradiated donor B lymphoblastoid cell lines (BLCLs) were set up as previously described,5 and chromium release assays were performed on day 12 of culture by using an effector:target ratio of 12.5:1. Donor phytohemagglutinin blasts (EBV negative, autologous control), donor EBV BLCLs, and allogeneic BLCLs were used as targets cells. Spontaneous and total release for each target was used to calculate percent-specific release by using the following formula: % specific release = (experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm). Limiting dilution wells were scored as positive if release exceeded 10%. EBV-specific cytotoxic T-cell precursor (CTLp) frequencies were calculated by the method of Taswell20 by using a computer program provided by Dr Y. Kawanishi, Medical College of Wisconsin (Milwaukee, WI).
RESULTS
The clinical characteristics of the patients who developed EBV-LPD during this study period are presented in Table 2. Six of the seven patients with EBV-LPD were recipients of TCD unrelated-donor marrow grafts, and one patient (unique patient number [UPN] 361) received a TCD related-donor transplant. Five of these patients had ≥40,000 copies EBV genome/μg DNA. The time from transplant to the development of EBV-LPD ranged from 8 to 26 weeks. All patients had a diagnosis of EBV-LPD confirmed immunohistologically. Clonality was determined in five cases of EBV-LPD and all were monoclonal.
Characteristics of Patients With EBV-LPD
| Patient No. . | Age/ Sex . | Pretransplant Diagnosis . | Weeks Post-transplant . | Clonality . | Peak EBV DNA (copies/μg) . | Days With >40,000 Copies Before Dx. . | Treatment (CD3+ cells/kg) . | Response . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 361 | 48/F | MDS | 26 | ND | 4,000 | — | 1 × 106 | NR | D-LPD/ARDS |
| 379 | 6/M | MDS | 9 | ND | 40,000 | 12 | 1 × 106 | NR | D-LPD |
| 380 | 23/F | CML | 8 | Monoclonal | 400,000 | 7 | 1.2 × 105 | NR | D-LPD/GVH |
| 381 | 9/F | MDS | 11 | Monoclonal | 400,000 | 8 | 1.0 × 106 | NR | D-LPD/ARDS |
| 426 | 45/M | AML | 26 | Monoclonal | 400 | — | excision | CR | A-NED |
| 429* | 44/F | ALL | 25 | Monoclonal | 400,000 | 58 | 2 × 105 | CR | A-NED |
| 440 | 46/F | AML | 9 | Monoclonal | 40,000 | 3 | 7 × 105 | CR | D-GVH |
| Patient No. . | Age/ Sex . | Pretransplant Diagnosis . | Weeks Post-transplant . | Clonality . | Peak EBV DNA (copies/μg) . | Days With >40,000 Copies Before Dx. . | Treatment (CD3+ cells/kg) . | Response . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 361 | 48/F | MDS | 26 | ND | 4,000 | — | 1 × 106 | NR | D-LPD/ARDS |
| 379 | 6/M | MDS | 9 | ND | 40,000 | 12 | 1 × 106 | NR | D-LPD |
| 380 | 23/F | CML | 8 | Monoclonal | 400,000 | 7 | 1.2 × 105 | NR | D-LPD/GVH |
| 381 | 9/F | MDS | 11 | Monoclonal | 400,000 | 8 | 1.0 × 106 | NR | D-LPD/ARDS |
| 426 | 45/M | AML | 26 | Monoclonal | 400 | — | excision | CR | A-NED |
| 429* | 44/F | ALL | 25 | Monoclonal | 400,000 | 58 | 2 × 105 | CR | A-NED |
| 440 | 46/F | AML | 9 | Monoclonal | 40,000 | 3 | 7 × 105 | CR | D-GVH |
The clinical features of the 7 patients with EBV-LPD are summarized above.
Abbreviations: AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; ND, not done; GVHD, graft versus host disease; ARDS, adult respiratory distress syndrome; LPD, lymphoproliferative disease; Dx, diagnosis; NED, no evidence of disease; CR, complete remission; NR, no response; A, alive; D, dead.
UPN 429 received donor-derived EBV-specific T cells.
Table 3 presents the peak levels of EBV DNA for all patients. Ten EBV sero-positive normal adult donors were evaluated for levels of EBV DNA, and all had ≤4,000 copies EBV/μg DNA (data not shown). Patients receiving TCD transplants had a much higher prevalence of elevated levels of EBV DNA compared with recipients of unmodified transplants (P < .001, Fisher's exact test). Five of the seven patients with EBV-LPD had levels of EBV DNA ≥40,000 copies/μg DNA. Two of the 34 patients not developing EBV-LPD had levels of EBV DNA ≥40,000 copies/μg DNA. The positive predictive value of this test for developing EBV-LPD based on having ≥40,000 copies EBV genome/μg DNA was 71%, and the negative predictive value was 94%.
Levels of EBV DNA in Study Patients
| . | EBV DNA Level (copies/ μg DNA) . | Fisher's Exact Test . | |
|---|---|---|---|
| Type of transplant | ≥40,000 | ≤4,000 | |
| T-cell depleted* | 7 | 12 | P < .001 |
| Unmanipulated | 0 | 22 | |
| EBV-LPD | |||
| Present | 5 | 2 | P < .001 |
| Absent | 2 | 32 | |
| . | EBV DNA Level (copies/ μg DNA) . | Fisher's Exact Test . | |
|---|---|---|---|
| Type of transplant | ≥40,000 | ≤4,000 | |
| T-cell depleted* | 7 | 12 | P < .001 |
| Unmanipulated | 0 | 22 | |
| EBV-LPD | |||
| Present | 5 | 2 | P < .001 |
| Absent | 2 | 32 | |
Patient levels of EBV DNA in relation to type of transplant (T-cell depleted or unmanipulated) and the presence or absence of EBV-LPD.
T-cell depletion by soybean agglutinin and sheep erythrocyte rosette formation or by a CD34 selection device.
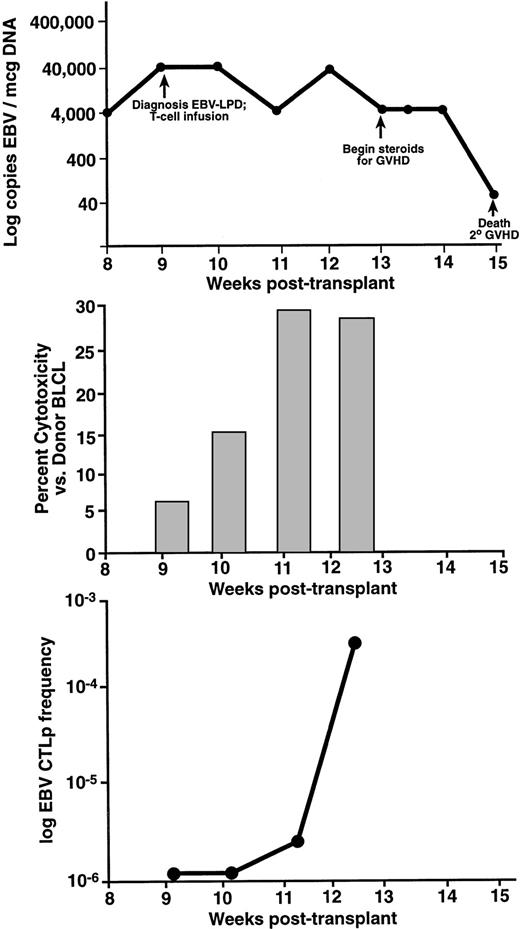
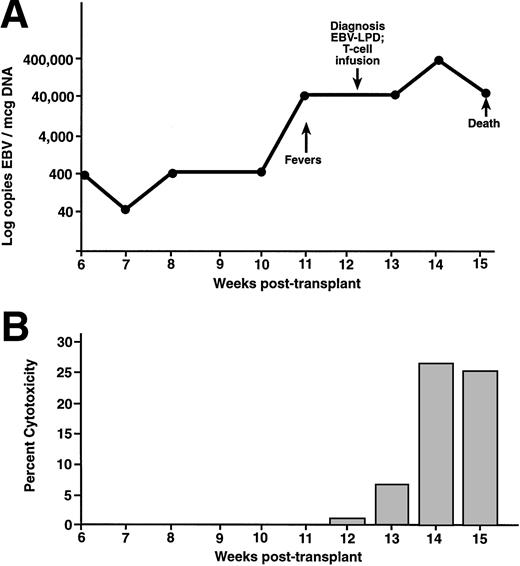
The immunologic and viral response to donor leukocyte infusions are presented for two patients in Figs 2 and3. UPN 440 (Fig 2) received a donor leukocyte infusion for EBV-LPD 9 weeks posttransplant. This patient had elevated levels of EBV DNA concomitant with the development of lymphadenopathy and pulmonary infiltrates. After biopsy confirmation of EBV-LPD, this patient received a donor leukocyte infusion. This patient had resolution of fevers and lymphadenopathy 3 weeks postinfusion with a persistent decrease in the level of EBV DNA by 4 weeks postinfusion. There was a progressive increase in EBV-specific CTLp frequencies (Fig2C) ranging from undetectable at the time of the infusion and 1 week postinfusion, 1/267,980 2 weeks postinfusion, to 1/5,160 at 24 days postinfusion (13 weeks posttransplant). There was also an increase in cytotoxicity from bulk cultures 2 weeks after the donor leukocyte infusion (Fig 2B). This patient developed grade-4 GVHD involving the liver and skin, required systemic corticosteroids, and died because of complications of GVHD. UPN 381 (Fig 3) developed fever and hepatomegaly 11 weeks after an unrelated-donor TCD transplant. One week before developing clinical symptoms there was an elevation in the level of EBV DNA. A liver biopsy specimen showed a diffuse, monoclonal, immunoblastic B-cell lymphoma. After a donor leukocyte infusion, this patient's level of EBV DNA continued to be elevated, and at week 14 she died. This patient had an increase in the level of cytotoxicity against the donor BLCLs 2 weeks after the donor leukocyte infusion (Fig3B). Postmortem examination showed a diffuse infiltration of the lungs, liver, and kidneys with CD19+ lymphoblastoid cells consistent with disseminated EBV-LPD. A CD3+ cellular infiltrate was present among the lymphoblastoid cells.
UPN 440 developed EBV-LPD 9 weeks after an unrelated, T-cell–depleted SCT. Levels of EBV DNA determined by semiquantitative PCR are presented on the top panel (A). Two weeks after receiving a donor leukocyte infusion, this patient had a decrease in levels of EBV DNA (B). There was a progressive increase in the amount of EBV-specific cytotoxicity against the donor BLCL, which had reached normal levels at 2 weeks postinfusion (C). This correlated with the development of normal levels of EBV-specific, cytotoxic T-cell precursor frequencies. This patient died from graft-versus-host disease.
UPN 440 developed EBV-LPD 9 weeks after an unrelated, T-cell–depleted SCT. Levels of EBV DNA determined by semiquantitative PCR are presented on the top panel (A). Two weeks after receiving a donor leukocyte infusion, this patient had a decrease in levels of EBV DNA (B). There was a progressive increase in the amount of EBV-specific cytotoxicity against the donor BLCL, which had reached normal levels at 2 weeks postinfusion (C). This correlated with the development of normal levels of EBV-specific, cytotoxic T-cell precursor frequencies. This patient died from graft-versus-host disease.
UPN 381 developed fevers and hepatosplenomegaly 11 weeks after an unrelated T-cell–depleted SCT (A). This patient received a donor leukocyte infusion at the time of diagnosis, at which time there were 40,000 copies of EBV/μg DNA in the peripheral blood. After the infusion, there was a progressive increase in the amount of EBV-specific cytotoxicity against the donor BLCL (B). This patient died secondary to pulmonary failure, complicated by persistent EBV-LPD in the liver and lungs.
UPN 381 developed fevers and hepatosplenomegaly 11 weeks after an unrelated T-cell–depleted SCT (A). This patient received a donor leukocyte infusion at the time of diagnosis, at which time there were 40,000 copies of EBV/μg DNA in the peripheral blood. After the infusion, there was a progressive increase in the amount of EBV-specific cytotoxicity against the donor BLCL (B). This patient died secondary to pulmonary failure, complicated by persistent EBV-LPD in the liver and lungs.
Two patients in this study with EBV-LPD (UPN 361 and 426) did not have elevations in their levels of EBV DNA at the time of diagnosis. UPN 361 developed a diffuse CD20+, LMP-1+, B-cell lymphoma of the lungs 26 weeks after a TCD related-donor SCT. The patient's levels of EBV DNA were persistently ≤4,000 copies EBV/μg DNA. This patient received 1 × 106 donor CD3+ cells/kg but developed worsening respiratory insufficiency. Two weeks postinfusion, pleural fluid showed persistence of lymphoblastoid cells and the patient died of progressive EBV-LPD. UPN 426 developed a monoclonal, polymorphic B-cell lymphoma of a cervical lymph node 26 weeks posttransplant, which was CD20+ and LMP-1+ and was strongly positive for EBV DNA by PCR. UPN 426 underwent an excisional biopsy of the involved node, did not receive donor leukocytes, and remained in remission 4 months after diagnosis without any evidence of recurrent EBV-LPD. Levels of EBV DNA in the peripheral blood did not exceed 400 copies/μg DNA. This patient had no significant EBV-specific cytotoxicity against the donor BLCLs at the time of diagnosis or 3 months after diagnosis and continues to be in remission with 400 copies EBV/μg DNA.
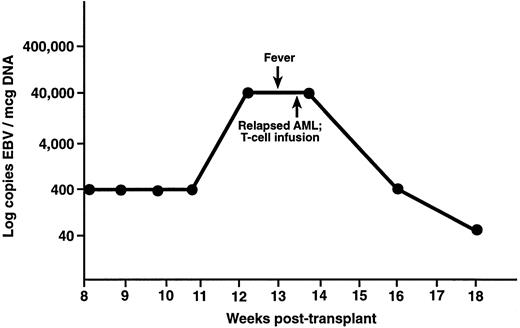
Two patients in this study had elevated levels of EBV DNA but did not develop EBV-LPD. UPN 386 was noted to have fever of undetermined origin and elevated levels of EBV DNA 3 months after an unrelated-donor TCD transplant (Fig 4). This patient had computerized tomography scans performed of the head, chest, abdomen, and pelvis, which showed no evidence of lymphoma. A bone marrow aspirate showed recurrent acute myelogenous leukemia, and this patient received 1 × 106 CD3+ donor leukocytes/kg as treatment for relapsed leukemia. Two weeks after the donor leukocyte infusion, UPN 386 had a 2-log decrease in the level of EBV DNA. Another patient had 40,000 copies EBV/μg DNA 6 months after a TCD unrelated-donor SCT, which decreased to 400 copies 1 month later. This patient did not have any symptoms of EBV-LPD and did not receive donor leukocytes.
UPN 386 received a T-cell–depleted, unrelated donor bone marrow transplant for acute myelogenous leukemia and developed fever 12 weeks posttransplant. There was no evidence of EBV-LPD on computerized tomography, but levels of EBV DNA were 40,000 copies/μg DNA. This patient was noted to have relapsed leukemia at this time, received a donor leukocyte infusion, and had a decrease in EBV DNA to the normal range.
UPN 386 received a T-cell–depleted, unrelated donor bone marrow transplant for acute myelogenous leukemia and developed fever 12 weeks posttransplant. There was no evidence of EBV-LPD on computerized tomography, but levels of EBV DNA were 40,000 copies/μg DNA. This patient was noted to have relapsed leukemia at this time, received a donor leukocyte infusion, and had a decrease in EBV DNA to the normal range.
To assess whether the stem cell donors of patients with EBV-LPD had deficient EBV-specific cytotoxicity, donor PBMCs were assayed for lysis of autologous BLCLs. Table 4 presents cytotoxicity data from bulk culture PBMCs and EBV-CTLp frequencies for four stem cell donors of patients who developed EBV-LPD. Three of four donors (UPN 361, 380, and 429) had ≥25% specific lysis of autologous EBV BLCLs at day 12 of culture, and the donor for UPN 426 (who did not receive a donor leukocyte infusion) had 15% EBV-specific cytotoxicity at this time, which increased to 50% at day 21 of culture. Analysis of EBV-CTLp frequencies for these donors shows that three of the four had low levels of EBV-specific CTLs (1/41,000 to 1/129,000).
EBV-Specific Cytotoxicity of Four Stem Cell Donors of Patients With EBV-LPD
| UPN Donor . | Percent Specific Lysis of Autologous BLCL3-150 . | EBV CTLp Frequency . |
|---|---|---|
| 361 | 31 | 1/129,000 |
| 426 | 15 | 1/81,000 |
| 429 | 41 | 1/25,000 |
| 440 | 25 | 1/41,000 |
| UPN Donor . | Percent Specific Lysis of Autologous BLCL3-150 . | EBV CTLp Frequency . |
|---|---|---|
| 361 | 31 | 1/129,000 |
| 426 | 15 | 1/81,000 |
| 429 | 41 | 1/25,000 |
| 440 | 25 | 1/41,000 |
EBV-specific cytotoxicity from bulk culture peripheral blood mononuclear cells and EBV-CTL precursor frequency analysis from donors of patients who developed EBV-induced lymphoproliferative disease.
Abbreviations: BLCL, B lymphoblastoid cell line; CTLp, cytotoxic T cell precursor.
From bulk culture peripheral blood mononuclear cells.
Table 5 summarizes all cases of EBV-LPD in stem cell transplant patients at Indiana University from January 1991 to June 1997. The preparative regimens varied during this time period, and patients received TCD transplants with sheep red blood cell and soybean agglutination17 or a device to selectively deplete T-cells (Applied Immunoscience, Menlo Park, CA) or enrich for CD34+ stem cells (Baxter Healthcare, Santa Ana, CA). Of the 165 patients receiving TCD transplants during this time period, 23 (13.9%) developed EBV-LPD. Of the 89 patients receiving TCD SCT from a related donor, there were 3 (3.4%) cases of EBV-LPD. Twenty of 76 patients (26.3%) who received unrelated TCD SCT during this time developed EBV-LPD. T-cell doses for the patients receiving donor leukocytes ranged from 1 × 105 to 1 × 106 CD3+ cells/kg. Of the 13 patients treated with donor leukocytes, 4 patients had a complete response; however, 1 of these patients succumbed from GVHD and another patient died from aspergillosis. The patients receiving no specific therapy and those receiving immunoglobulin, interferon, or chemotherapy did not have a clinical response. Five of the 13 patients receiving donor leukocytes who did not have a clinical response died from progressive EBV-LPD 2 to 10 days after the donor leukocyte infusion.
Past Cases of EBV-LPD in Stem Cell Transplant Patients at Indiana University
| Treatment . | Total Patients . | Complete Remission (%) . | Disease Progression (%) . | GVHD (%) . | Patients Surviving (%) . |
|---|---|---|---|---|---|
| Observation | 6 | 0 (0) | 6 (100) | 0 (0) | 0 (0) |
| Interferon, IVIg4-150 | 2 | 0 (0) | 2 (100) | 0 (0) | 0 (0) |
| Chemotherapy | 1 | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Excision | 1 | 1 (100) | 0 (0) | 0 (0) | 1 (100) |
| Donor leukocytes | 13 | 4 (31) | 9 (69)4-151 | 4 (31) | 2 (15)‡ |
| Treatment . | Total Patients . | Complete Remission (%) . | Disease Progression (%) . | GVHD (%) . | Patients Surviving (%) . |
|---|---|---|---|---|---|
| Observation | 6 | 0 (0) | 6 (100) | 0 (0) | 0 (0) |
| Interferon, IVIg4-150 | 2 | 0 (0) | 2 (100) | 0 (0) | 0 (0) |
| Chemotherapy | 1 | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Excision | 1 | 1 (100) | 0 (0) | 0 (0) | 1 (100) |
| Donor leukocytes | 13 | 4 (31) | 9 (69)4-151 | 4 (31) | 2 (15)‡ |
Treatment outcomes in 23 stem cell transplant patients with EBV-LPD at Indiana University.
Intravenous immunoglobulin.
Five of these nine patients died 2 to 10 days after receiving donor leukocytes and had advanced EBV-LPD at the time of diagnosis and treatment.
One of these patients received EBV-specific CTL and one patient unprimed donor leukocytes.
DISCUSSION
There are several risk factors for the development of EBV-LPD after SCT, the most significant of which are T-cell depletion, the use of unrelated or mismatched transplants, and the use of antithymocyte globulin.2-5,7 8 Differences in Epstein-Barr viral load and cellular immunity to EBV may account for the differential susceptibility to EBV-LPD in these patients. A reliable method of predicting which patients are likely to develop EBV-LPD would permit intervention at a time point earlier in lymphomagenesis and could possibly reduce the morbidity associated with this complication. From the present study there is a strong correlation between developing EBV-LPD and having an elevated level of EBV DNA after TCD SCT, with some patients noted to have high levels of EBV DNA weeks before the onset of symptoms. However, despite this correlation two of seven patients with EBV-LPD had levels of EBV DNA within the normal range, and two patients without EBV-LPD had ≥40,000 copies EBV/μg DNA. Elevated levels of EBV were only seen in recipients of TCD transplants, and none of the “low-risk” patients had elevated levels of EBV DNA or developed EBV-LPD.
Other groups have examined levels of EBV DNA to determine risk for developing EBV-LPD in both solid organ and SCT patients.12,14-16 Previous studies in SCT patients indicate a strong correlation between levels of EBV DNA and the occurrence of EBV-LPD.12,16 Rooney et al16 reported elevated levels of EBV DNA (≥20,000 copies EBV/μg DNA) in four SCT patients with EBV-LPD with normal levels (2 to 2,000 copies EBV/ μg DNA) in all unaffected patients. Although there was also a strong correlation between EBV-LPD and elevated levels of EBV DNA in the present study, some patients with ≥40,000 copies/μg DNA did not develop this complication. UPN 386, for example, had 40,000 copies EBV/μg DNA concurrently with fevers and leukemic relapse, with no evidence of EBV-LPD by computerized tomography. However, there was a sharp decline in this patient's level of EBV DNA after the donor leukocyte infusion, raising the possibility that this patient may have developed EBV-LPD if donor leukocytes were not administered. Also of interest is UPN 426, who had a monoclonal B-cell lymphoma but did not have elevated levels of EBV DNA at the time of diagnosis. UPN 361 in the present study had normal levels of EBV DNA, despite a clinically aggressive, disseminated form of EBV-LPD, which caused this patient's death. Rooney et al12 also reported one patient with EBV-LPD without elevated levels of EBV DNA. It is known that some BLCLs do not produce EBV, whereas others are associated with virus production.21-23 Katz et al24showed that only 40% of BLCLs grown from EBV-LPD lesions contain replicating EBV DNA. Therefore, it is possible that in vivo some transformed B cells do not produce virus, resulting in normal levels of EBV DNA in some patients with EBV-LPD.
A review of past cases of EBV-LPD at Indiana University shows a high incidence in recipients of TCD stem cell transplants (13.9%) with a striking difference in the incidence of EBV-LPD between recipients of related and unrelated TCD transplants (3.4% v 26.3%). Other groups have noted a similar distribution in cases of EBV-LPD among recipients of TCD transplants.4,5 Differences in levels of cellular immunity to EBV among recipients of TCD SCT patients may account for the fact that some patients with viral reactivation do not develop EBV-LPD. EBV-specific CTL precursor frequency analysis of SCT peripheral blood shows that many of these patients have deficient or emerging cellular immunity to EBV during the first 3 to 6 months posttransplant,5,25 the time period during which the majority of these disorders occur. T-cell depletion of marrow from a patient with a low level of EBV-CTL could result in a lower dose of these cells given at the time of transplant. However, it is unclear whether these differentiated precursors in the stem cell inoculum given at the time of transplant are the cells that result in the restoration of EBV-specific cellular immunity several months later. PBMCs in bulk culture from four stem cell donors of patients with EBV-LPD had in vitro capacity to lyse autologous BLCLs. However, three of these four stem cell donors had low levels of EBV-specific CTL precursors. Three of the four PBMCs assayed from these donors were used in leukocyte infusions, which resulted in resolution of disease in two patients with EBV-LPD (UPN 429 and 440). UPN 361, who had progressive EBV-LPD despite a donor leukocyte infusion, received PBMCs from a donor whose CTLp frequency was within the range previously reported in EBV sero-negative donors.5
In acute EBV infection in the normal host, the cellular immune response to EBV is mediated by human leukocyte antigen (HLA)-unrestricted and HLA-restricted cytotoxic lymphocytes.26,27 Protection against recurrent EBV-induced illness is largely mediated by CD8+, major histocompatibility complex class I–restricted, EBV-specific cytotoxic T cells.28-31 In the present study, donor leukocyte infusions resulted in the development of EBV-specific cytotoxicity in two patients studied. UPN 440 had a dramatic increase in levels of EBV-specific CTL precursors, which correlated with an increase in EBV-specific cytotoxicity against the donor BLCLs from bulk culture lymphocytes. There was a gradual decrease in levels of EBV DNA after the T-cell infusion. However, in one patient, increasing EBV-specific cytotoxicity after donor leukocytes was not accompanied by a clinical response. Despite an increase in EBV-specific cytotoxicity after a donor leukocyte infusion, UPN 381 continued to have elevated levels of EBV DNA, and at autopsy 3 weeks postinfusion had disseminated EBV-LPD. At the time of death, this patient had a CD3+infiltrate in the lymphomatous lesions, which may have been a result of the donor leukocyte infusions. Papadopoulos et al2 also reported a T-cell infiltrate in the lymphomatous lesions of SCT patients treated with donor leukocytes; however the patients in this latter study had complete resolution of their EBV-LPD. Although donor T-cell infusions generally result in resolution of these disorders,2 13 there may be a subset of patients with disease that is more aggressive and does not respond as quickly to this form of therapy. UPN 381's elevated levels of EBV DNA 3 weeks postinfusion may be indicative of failure of adoptive immunotherapy, and monitoring levels of EBV DNA may serve as a sensitive measure of response to therapy.
Overall, the patients reported here had a substantially higher morbidity compared with patients in other series who received donor leukocytes2 or EBV-CTLs.12 Of the five patients who died, one death was a result of severe GVHD, most likely caused by the donor leukocyte infusion. Another patient (UPN 379) had disseminated EBV-LPD 9 weeks posttransplant and had a rapidly progressive course, dying 2 days after the donor leukocyte infusion. These data contrasted with the report from Papadopoulos et al2 in which all five patients with EBV-LPD receiving donor leukocyte infusions had a complete response. Three of these patients developed chronic GVHD, and two patients, although with resolution of EBV-LPD on autopsy, expired from respiratory insufficiency possibly related to the T-cell infusion. Of interest is that two patients in the present study who received donor leukocytes developed GVHD, one patient with grade 2 GVHD of the skin (UPN 361) and another with grade 3 GVHD of the skin, gut, and liver (UPN 380), but neither patient had resolution of their EBV-LPD. Recently, Imashuku et al32have also reported an SCT patient with EBV-LPD who failed to respond to infusions of donor-derived EBV-CTLs (albeit at low doses of 9.2 × 106 total cells). In applying adoptive immunotherapy with donor leukocytes for relapsed leukemia, there is a direct correlation between the development of GVHD and disease response.33-36The relationship between GVHD and a graft-versus-leukemia effect is especially poignant in chronic myelogenous leukemia,35,36with a 91% response rate to donor leukocyte infusions if there was evidence of GVHD or myelosuppression and a 42% response rate in patients without these complications.36 Although GVHD and graft-versus-leukemia appear to be related in adoptive immunotherapy for relapsed leukemia, GVHD- and EBV-specific cytotoxicity are likely mediated by different effector cell populations. Therefore, the presence of GVHD in patients without a clinical response to donor leukocytes for EBV-LPD would be possible, especially if these donors have low levels of EBV-CTL precursors.
In comparing clinical features of patients in the present series with that reported by Papadopoulos et al,2 of note is the fact that the majority of patients in the latter study had nodular multiorgan involvement. Three of the four patients who died with EBV-LPD in the present series (Table 2) had diffuse infiltration of affected organs without distinct nodules identified radiographically. Three of the four patients with nodal disease had a complete response to donor leukocytes (UPN 429 and 440) or surgical excision (UPN 426). Another possible explanation for the clinically aggressive nature of some cases of EBV-LPD is that they are genetically different from other lymphoproliferations. The cases in the present study in which adoptive immunotherapy failed to induce remission of disease histopathologically resemble the more aggressive variant of EBV-LPD reported by Knowles et al,37 with an immunoblastic morphology and presenting with widely disseminated disease. Another possible explanation for poor response to donor leukocytes may be a lack of tumor cell immunogenicity as has been noted in a subset of EBV-related lymphomas in human immunodeficiency virus–infected patients lacking expression of EBV peptides important in immune recognition.38-40
Although the overall survival and success of therapy for the 13 patients receiving donor leukocytes is less than that previously reported,2 five of the nine “nonresponders” in the present series presented with advanced disease or were moribund at the time of diagnosis, surviving less than 2 weeks after the T-cell infusion. Therefore, this poor response rate may be in part caused by late detection of EBV-LPD, at a point when these patients were unable to survive the time period necessary to achieve an immunologic response, as well as by GVHD as a result of unprimed donor leukocyte infusions. These findings emphasize the importance of early detection of EBV-LPD in high-risk patients. Additionally, the high incidence of severe GVHD after infusion of unprimed donor leukocytes to treat either EBV-LPD or relapsed leukemia highlights a potential advantage of using EBV or leukemia-specific CTLs. Several patients in the present study had elevated levels of EBV DNA before the development of symptoms or signs of EBV-LPD. Semiquantitative EBV PCR offers the possibility for screening high-risk patients at a time before the development of overt disease. Patients who are found to have elevated levels on screening would be candidates for a more thorough clinical evaluation, such as computerized tomography of potentially affected areas and biopsies. With this assay, patients may be identified and treated at an earlier time point, and response to therapy can be assessed by measuring viral burden. This may be especially advantageous in patients with diffuse aggressive lymphomas that, from our series, have a poor outcome.
Supported by Grant No. 97-043-01-EDT from the American Cancer Society.
Address reprint requests to David J. Emanuel, MD, Stem Cell Transplantation Program, Riley Hospital for Children, 702 Barnhill Drive, Indianapolis, IN 46202-5225.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal