Abstract
The molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome is the presence of a mutation in the iron-responsive element (IRE) of the L ferritin gene, located on chromosome 19q13.3-13.4. Two mutations have been reported so far, altering adjacent nucleotides in the IRE loop, in a region that has been extensively studied in vitro and shown to mediate high affinity interaction with the iron-responsive protein. In this report, we describe two families with a new mutation in the bulge of the IRE stem, and we show that this mutation alters the protein-binding affinity of the IRE in vitro to the same extent as the loop mutation. In addition, we present evidence that some variability in the age of onset of cataract can be associated with this genetic syndrome, probably because of additional genetic or environmental factors that modulate the penetrance of the L ferritin defect in the lens. We confirm that the patients do not have increased iron stores despite the persistence of elevated serum ferritin levels and that, accordingly, they do not tolerate well venesection therapy. Further studies will be necessary to elucidate the mechanism responsible for the onset of cataract.
THE HEREDITARY hyperferritinemia-cataract syndrome is a new syndrome recently discovered simultaneously in France1 and in Italy.2 In each of the three families described so far, several members presented with an elevated level of serum ferritin and a bilateral cataract of early onset. Genetic studies were performed in two of the three families,3,4 and a mutation was found in the iron-responsive element (IRE) of the L ferritin gene, located on chromosome 19q13.3-13.4. Intracellular iron homeostasis is normally achieved through an iron-mediated posttranscriptional regulation. This regulation operates through the interaction between a cytoplasmic iron-regulatory protein (IRP) and a conserved stem-loop motif that is known as IRE and that is present in the 5′ untranslated region of all ferritin mRNAs5,6 of the erythroid 5-aminolevulinate synthase (ALA-S) mRNA7 and in the 3′ untranslated region of the transferrin receptor (TfR) mRNA.8 Under conditions of limited iron supplies, the IRE-binding affinity of the cytoplasmic IRP is high, leading to repression of ferritin synthesis and stabilization of the TfR mRNA.9,10 The overall structure of the various IRE is highly conserved with a six-nucleotide loop of consensus sequence 5′-CAGUGN-3′ and a paired stem with a small asymmetrical bulge. The two mutations identified so far in the hyperferritinemia-cataract syndrome affect adjacent nucleotides in the IRE loop, with either an A to G change at the second position of the loop3 or a G to C change in the third position.4 The A to G change has been shown to reduce the protein-binding affinity of the IRE in vitro and to affect L ferritin synthesis in vivo. Functional studies performed on lymphoblastoid cell lines established from the patients showed that the presence of a mutated IRE in the L ferritin mRNA impaired the negative feedback regulation that normally operates on ferritin synthesis in conditions of limited iron supplies.3 Identification of a mutated IRE in the patients with a hereditary hyperferritinemia-cataract syndrome was the first evidence that a constitutive nonregulated L ferritin synthesis can be responsible for a pathological condition, but the mechanism responsible for the onset of cataract is still not elucidated. Hereditary cataracts are clinically and genetically heterogeneous, and the genes responsible have only been found in the hyperferritinemia-cataract syndrome and in the Coppock-like cataract, which is caused by the activation of the γE-crystallin pseudogene.11 The genes for other distinct cataracts have been mapped to chromosome regions 1q21-q25, 2q33-q36, 16q22.1, and 17q24.12-15
The hyperferritinemia-cataract syndrome had remained undetected until recently, probably because serum ferritin determination is not a regular test in ophthalmology departments. Therefore, the proportion of hereditary cataract as well as the heterogeneity of the mutations that are caused by the mutated L ferritin IRE are still largely unknown.
Here we describe a new mutation that affects the bulge of the iron responsive element in two new families, with the first hint that some phenotypic variability is possible within this syndrome.
MATERIALS AND METHODS
Case Report
Family 1.
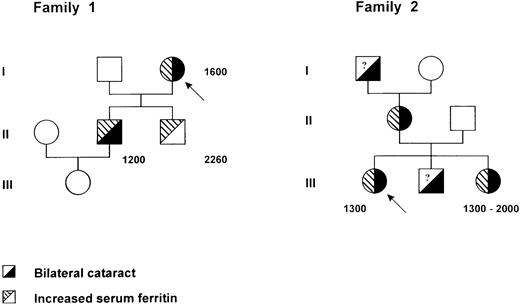
The proband (I1) (Fig 1), a 77-year-old woman, was hospitalized for a sudden episode of generalized edema accompanied by reduced serum albumin levels. In her past clinical history a bilateral cataract was reported. Hemoglobin, leukocyte, and platelet counts were in the normal range, serum iron was 12.8 μmol/L, transferrin 3.14 g/L, and ferritin 1,600 μg/L. Sedimentation rate was elevated, whereas the other acute phase reactants were within normal range. Because a kidney and hepatic origin of the edema was ruled out, as well as malnutrition and gastrointestinal protein loss, the patient was subjected to a liver biopsy, which showed a normal liver structure and a scant amount of iron. A monoclonal IgGλ gammapathy was present, but the bone marrow biopsy was normal with no evidence of iron overload. After health recovery, the patient was discharged from the hospital with normal blood tests, with the exception of serum ferritin, which was persistently elevated. In an attempt to reduce the hyperferritinemia, the patient was started on a program of phlebotomies, but after only three phlebotomies of 350 mL each, she developed anemia (hemoglobin [Hb] decreased from 11.7 to 8.4), transferrin saturation decreased from 20% to 5%, but serum ferritin did not change. A family study was performed, and two sons and a grandchild were tested.
Pedigree of two families with the hereditary hyperferritinemia-cataract syndrome. Within each symbol, the left part indicates serum ferritin level and the right part the lens status. Serum ferritin levels are indicated in micrograms per liter. A question mark denotes an unknown serum ferritin level. Probands are identified by an arrow.
Pedigree of two families with the hereditary hyperferritinemia-cataract syndrome. Within each symbol, the left part indicates serum ferritin level and the right part the lens status. Serum ferritin levels are indicated in micrograms per liter. A question mark denotes an unknown serum ferritin level. Probands are identified by an arrow.
The elder son (II1), 52 years old, did not have any pathological report but did have a bilateral cataract that had been operated on 10 years before. All his tests were normal (serum iron 21.4 μmol/L and transferrin 2.1 g/L), but his ferritin was 1,200 μg/L. He was phlebotomized, and after six phlebotomies Hb turned from 15.6 to 13, transferrin saturation decreased, and serum ferritin did not vary. Later, the patient moved to another city and he was again subjected to several phlebotomies for the high values of ferritin and consequently developed a severe anemia without any modification of ferritin values. He was also subjected to liver biopsy, which showed a completely normal liver without evidence of iron or inflammatory cells. However, some granular, yellowish pigment of unknown composition was also observed. His 16-year-old daughter was examined; blood cell counts and iron indices were all in the normal range. The younger son (II2), 45 years old, had increased serum ferritin (2,264 μg/L) and a normal transferrin saturation. He was subjected to 14 phlebotomies, once a week, with a decrease of Hb from 15 to 12.5 g/dL, a decrease of transferrin saturation, but no modification of ferritin levels. He had no history of cataract, and a recent control ruled out the presence of cataract.
Family 2.
The proband (III1) (Fig 1), a 24-year-old woman whose sole previous medical problem was a bilateral cataract, presented in 1995 with constant hyperferritinemia. This biochemical abnormality was detected in the frame of a family study initiated from her sister who had been suspected from having genetic hemochromatosis. She was otherwise clinically asymptomatic. Serum ferritin levels ranged between 1,250 and 1,400 μg/L (upper normal limit 250). Serum iron was normal (16 to 19 μmol/L), and transferrin saturation (19% to 28%) slightly decreased. Blood cell counts, sedimentation rate, serum hepatic enzyme activities (aspartate transaminase [AST], alanine transaminase [ALT], γ-glutamyl transferase [GGT]) were normal. Liver biopsy showed no abnormalities, and particularly Perl's staining was negative.
Her family members presented as follows: (1) her mother (II1) had a bilateral congenital cataract and hyperferritinemia; (2) her maternal grandfather had a bilateral cataract; (3) her brother (III2) had a bilateral cataract, no serum ferritin could be checked; and (4) her sister (III3), born in 1953, had been explored in 1993 to 1994 for chronic hyperferritinemia discovered after a malaise in 1992. Serum ferritin levels were comprised between 1,025 and 1,330 μg/L, with erythrocyte ferritin at 182 attograms (ag)/cell (normal range = 4 to 28 ag/cell). Serum iron was 22.8 μmol/L, and transferrin saturation was 35%. Hb was 14.2 g/dL, and MCV 93.8 fL. The liver biopsy showed no iron deposition. A few venesections were performed, but they were poorly tolerated with rapid appearance of microcytic anemia (Hb, 10.5 g/dL; MCV, 84 fL). The patient had been operated on for a left cataract and presented a mild right cataract.
Genomic DNA Analysis
Genomic DNA was extracted from blood samples collected on citrate-EDTA and subjected to polymerase chain reaction (PCR) amplification. A first round of amplification was performed using Lprom and Lex2 (see below) as 5′ and 3′ primers, and 30 cycles with 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute. The resulting 610-bp fragment was then purified on agarose gel and further amplified using seminested primers (Lex1 and Lex2), mapping to exon 1 and 2. The resulting 570-bp fragment was purified and sequenced.
Primers used for PCR amplification were (see Fig2A): Lprom: 5′ CGGCGCACCATAAAAGAAGCC (upper primer); Lex2: 5′ GCTGGTTTTGCATCTTCAG (lower primer); and Lex1: 5′ AGTTCGGCGGTCCCGCGGGTC (upper primer).
Identification of the mutation in the IRE of the L ferritin gene. (A) Schematic representation of the functional L ferritin gene on chromosome 19q13.3-q13.4 and position of the primers used for the PCR amplification. (B) Sequence analysis of genomic DNA from a normal individual (left) and from patient III1 in Family 2 (right). Comparison of the sequence of the reverse strand reveals a C to A mutation at the heterozygous state in the patient DNA. (C) Predicted secondary structure of the IRE in the L ferritin mRNA and position of the mutation in the 5′ UGC bulge. Numbering is from the first nucleotide in exon 1.
Identification of the mutation in the IRE of the L ferritin gene. (A) Schematic representation of the functional L ferritin gene on chromosome 19q13.3-q13.4 and position of the primers used for the PCR amplification. (B) Sequence analysis of genomic DNA from a normal individual (left) and from patient III1 in Family 2 (right). Comparison of the sequence of the reverse strand reveals a C to A mutation at the heterozygous state in the patient DNA. (C) Predicted secondary structure of the IRE in the L ferritin mRNA and position of the mutation in the 5′ UGC bulge. Numbering is from the first nucleotide in exon 1.
Gel Retardation Assay
Labeled RNAs were transcribed in vitro from oligonucleotide template using T7 RNA polymerase, as previously described.3 The oligonucleotide used to synthesize the normal L ferritin IRE probe was: 5′ TGTTCCGTCCAAACACTGTTGAAGCAAGAGACA-TCGCCCTATAGTGAGTCGTATTAGCC. The sequence of the oligonucleotide used to synthesize the mutated IRE probes differed by substituting a C to a T at position 17 (mut1) and an A to a C at position 25 (mut2) from the 5′ end of the oligonucleotide. The underlined sequence represents the T7 RNA polymerase promoter and was double-stranded in the transcription reaction with an oligonucleotide complementary to this region. The RNA used as a probe was labeled with 32P-UTP to a specific activity of approximately 106 cpm/ng. The RNA used as a competitor was trace-labeled with 35S-UTP to a specific activity of 500 to 200 cpm/ng. Cytoplasmic extracts were prepared from the human K562 erythroleukemia cell line.
Gel retardation assays were performed by incubating 5 μg of K562 cytoplasmic extracts treated with β-mercaptoethanol, with 50,000 cpm of 32P-labeled IRE at room temperature for 30 minutes. Low affinity interactions were removed by a further incubation with 5 mg of heparin per mL for 10 minutes. Electrophoresis of RNA-protein complexes was performed on 6% nondenaturing polyacrylamide gel in 0.5 × TBE (acrylamide/Bis ratio 60:1) for 2 hours.
RESULTS
We performed PCR amplification of genomic DNA from affected members of both families. Two rounds of PCR with a different primer pair were necessary to avoid the amplification of the ferritin L subunit intronless pseudogenes.16 A first round was performed using a sense primer mapping to the proximal promoter region of the L ferritin gene and an antisense primer mapping to exon 2. The resulting 610-bp fragment was gel-purified, and a nested PCR was performed using a combination of oligonucleotides mapping to the beginning of exon 1 and to exon 2. Sequencing of the PCR fragment revealed that affected members were heterozygous for a point mutation, consisting in a G to T transition in the bulge of the IRE (Fig 2B and C).
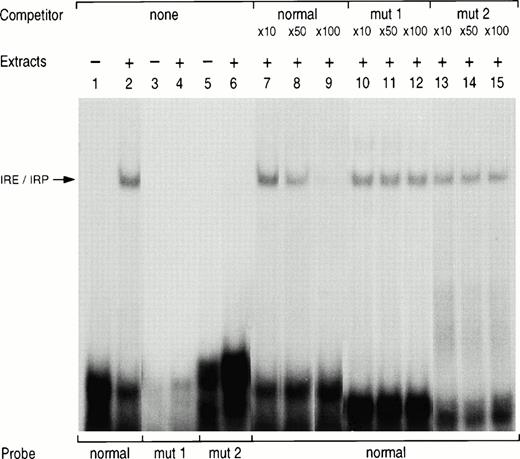
To confirm that the mutation was responsible for a reduced binding affinity of the IRE to the IRP, we performed gel retardation assay. Normal and mutated sense RNA probes were generated by in vitro transcription in the presence of 32P-UTP. In the presence of K562 cytoplasmic extracts, the normal probe generated a high-affinity protein RNA complex (Fig 3,lane 2). Mutated probes corresponding to the previously described loop mutation (mut1, lane 4) or to the new bulge mutation (mut2, lane 6) did not generate any specific complex. In addition, the IRE/IRP complex generated by the normal IRE probe was partially displaced by a 50-fold molar excess of the cold probe (lane 8) and almost entirely by a 100-fold excess (lane 9), whereas similar excess of the cold probe bearing the loop mutation (mut1, lanes 10 to 12) or the bulge mutation (mut2, lanes 13 to 15) did not compete for binding to the normal IRE probe.
Gel retardation assay with normal and mutated L ferritin IRE probes. 32P-labeled L ferritin IRE probe (5 × 104 cpm) of either normal sequence (lanes 1, 2, and 7 to 15) or carrying the loop mutation (mut1, lanes 3 and 4) or the bulge mutation (mut2, lane 5 and 6) was incubated with 5 μg K562 cytoplasmic extracts. The products were analyzed by electrophoresis on nondenaturing polyacrylamide gels either as free probe (lanes 1, 3, and 5) or after incubation with the extract only (lanes 2, 4, and 6). For the competition studies, the normal radiolabeled IRE probe was incubated with the extracts in the presence of a 10-, 50- or 100-fold molar excess of a cold normal IRE probe (lanes 7 to 9) or of a cold mutated IRE probe with a loop mutation (mut1, lanes 10 to 12) or a bulge mutation (mut2, lanes 13 to 15).
Gel retardation assay with normal and mutated L ferritin IRE probes. 32P-labeled L ferritin IRE probe (5 × 104 cpm) of either normal sequence (lanes 1, 2, and 7 to 15) or carrying the loop mutation (mut1, lanes 3 and 4) or the bulge mutation (mut2, lane 5 and 6) was incubated with 5 μg K562 cytoplasmic extracts. The products were analyzed by electrophoresis on nondenaturing polyacrylamide gels either as free probe (lanes 1, 3, and 5) or after incubation with the extract only (lanes 2, 4, and 6). For the competition studies, the normal radiolabeled IRE probe was incubated with the extracts in the presence of a 10-, 50- or 100-fold molar excess of a cold normal IRE probe (lanes 7 to 9) or of a cold mutated IRE probe with a loop mutation (mut1, lanes 10 to 12) or a bulge mutation (mut2, lanes 13 to 15).
The results confirm that the G to T mutation in the bulge of the L ferritin IRE fully impairs the protein-binding affinity of the IRE and is likely to be responsible for the increased serum ferritin levels in patients of the two families and probably for the associated cataract.
DISCUSSION
In this report, we describe a new mutation in the IRE of the L ferritin mRNA in two families with a hereditary hyperferritinemia-cataract syndrome, one from Italy and one from France. The first two mutations previously reported affected adjacent nucleotides from the IRE loop, whereas the two families described here have the same G to T change in the three unpaired nucleotide bulge of the IRE. This finding suggests that this syndrome is likely to be more frequent than was expected at first and reinforces the idea that the presence of a mutated IRE in the L ferritin gene, located on chromosome 19q13.3-13.4, is responsible for the association of cataract and hyperferritinemia. In addition, the position of the mutation is the first in vivo evidence for the key role of the bulge in the binding affinity of the IRE, although it has been shown that the tertiary structure of the stem-bulge region of the IRE is a critical determinant of translational regulation by iron.17 The structure of the stem varies between the different IREs,6 with a three-base UGC bulge in both H and L ferritin IRE, whereas the stem of the five IREs in the transferrin receptor mRNA and of the single IRE in the erythroid ALA-S mRNA have a single cytosine bulge. An extensive in vitro study performed by Henderson et al has shown the importance for the IRE function of this highly conserved cytosine present in the bulge of all known IREs.18 Our results present evidence that the G from the UGC bulge is important for the IRE function in vivo and for the high-affinity RNA protein interaction in vitro. This mutation abrogates the base pairing between G32 and C50 (Fig 2C), which might be necessary for the proper conformation of the IRE, as shown by studies based on nuclear magnetic resonance (NMR) spectroscopy19 or on identification of ligands with the highest affinity for the IRP.20 Alternately, the mutation might impair a specific point of protein-RNA interaction, because studies using UV radiation–induced cross-links have shown that nucleotides 31 to 43, including the UGC bulge and most of the loop, interact with the IRP and are likely to be buried within the active site of the native, iron-free protein.21
In the two families reported here, seven individuals had a known history of cataract, and whenever serum ferritin levels were assayed, they were found to be elevated (six patients), ranging from 1,200 to 2,200 μg/L. These values are very similar to those reported in the family with an A to G mutation in the loop,3 suggesting that mutations in both the loop and the bulge affect the in vivo function of the IRE to the same extent. This is in agreement with our in vitro studies, which show that both mutated IREs have lost IRP-binding activity and have no ability to compete for IRP binding to the wild-type sequence. However, we observed a marked phenotypic variability in the age of onset of cataract between the two families reported here, despite the presence of the same mutation. In fact, whereas in Family 2 affected members developed cataract during the late childhood, the patients from Family 1 showed signs of cataract around the age of 40; surprisingly, patient II2, age 45 years, still does not show any evidence of lens opacification, although he carries the mutation and his level of serum ferritin is even higher than the other members of the family (2,260 v 1,200 to 1,600). The mechanism leading to the onset of cataract is still unknown, but it is possible to speculate that abnormal deposits of ferritin molecules in the successive layers of lens epithelial cells lead progressively to lens opacification. In the case of patient II2, it is possible that additional factors, either genetic or environmental, modulate the penetrance of the L ferritin defect in the lens. Further follow-up will show whether he develops cataract or not.
Altogether, five families with the hyperferritinemia-cataract have now been described, and it becomes clear that the elevated serum ferritin levels do not reflect the presence of increased iron stores. Whenever a liver biopsy or a bone marrow biopsy (this report, Family 1, patient I1) was performed, there was no increased staining for iron. Accordingly, these patients did not tolerate well venesection therapy. It is also important to emphasize that recently a new syndrome has been described where patients who most often present metabolic disorders, such as increased body mass index, hyperlipidemia, glucose intolerance, and hypertension, have hyperferritinemia and normal transferrin saturation.22 However, contrary to the hereditary hyperferritinemia-cataract syndrome, these patients have iron overload and need phlebotomies. It becomes mandatory to be aware of the existence of these two syndromes because it appears from the few cases described so far that patients with the hyperferritinemia-cataract syndrome may rapidly developed severe anemia when they are phlebotomized, even if serum ferritin does not decrease. On the contrary, the phlebotomies will lead to a progressive reduction in the serum ferritin level in patients with iron overload. Therefore, a strict follow-up of these patients should be performed, including regular hemoglobin determination together with serum ferritin assay.
ACKNOWLEDGMENT
We are grateful to Dr Devidas (Service de Médecine Interne, Centre Hospitalier de Corbeil, Corbeil-Essones, France) for kindly providing clinical information on his patients.
Address reprint requests to C. Beaumont, PhD, INSERM U409, Faculté Xavier Bichat, 16 rue Henri Huchard, BP 416, 75870 Paris cedex 18, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal