Abstract
Pretransplantation donor-specific transfusion (DST) can enhance allograft survival in man and animals. However, due to the lack of a specific marker to identify donor-reactive cells in vivo in man and normal (nontransgenic) animals, the underlying mechanism remains unknown. In this study, we use 2CF1 transgenic mice expressing a transgenic T-cell receptor (TCR) specifically recognizing Ld, a major histocompatibility complex (MHC) class I molecule, to delineate the role of DST in long-term skin allograft survival and its underlying mechanisms. Our main findings include: (1) in the absence of any other immunosuppressive treatment, a single dose pretransplantation infusion of viable splenocytes from an Ld+ donor is sufficient to induce permanent donor-specific skin allograft survival in 2CF1anti-Ld TCR transgenic mice; (2) DST leads to a deletion of the majority (>60%) of donor-reactive T cells in the periphery of the recipient. However, deletion does not necessarily result in tolerance; (3) remaining donor-reactive T cells from DST-treated mice are fully responsive to Ld in vitro, and can suppress the antidonor response of naive T cells in vitro only when exogenous interleukin (IL)-4 is provided; and (4) the sera level of IL-4 in DST-treated tolerant mice is significantly increased. These results suggest that the generation of a subset of T cells with the potential to specifically inhibit antidonor responses, together with promotion of IL-4 production in recipients, may be important mechanisms for the induction and maintenance of antigen-specific tolerance.
THE ULTIMATE goal of transplantation is to induce long-term tolerance to a specific allograft without continuing need for expensive, toxic and nonspecific immunosuppressive drugs. Numerous studies have shown that pretransplantation infusion of allogeneic donor lymphocytes, especially when some major histocompatibility complex (MHC) antigens are shared between donor and recipient, has the potential to fulfill such a goal.1-10The underlying mechanisms, as suggested by previous studies, might include clonal deletion, induction of anergy, generation of regulatory cells, regulation of cytokine production, promotion of microchimerism, provision of soluble MHC antigen (Ag), or a combination of these.7 Although experiments to address these issues have provided useful information, no definitive conclusions have been reached. The major obstacle to delineating the mechanisms involved in donor-specific transfusion (DST)-induced tolerance is the lack of a specific marker to directly follow the fate of donor-reactive cells in the recipient after DST. Therefore, it is difficult to view the precise mechanisms involved in DST-induced tolerance in vivo. With the development of transgenic mice that carry transgenic T-cell receptor (TCR) specifically recognizing transplantation Ags, it became possible to decipher this long-standing enigma in transplantation.
In our previous studies, anti-HY TCR transgenic mice were infused with male HY+ lymphocytes and the fate of male-reactive T cells was followed in vivo. It was found that the majority of male-reactive T cells were deleted from the periphery of the recipients after encountering male Ag, and about 20% to 30% of donor-reactive T cells persisted in the periphery for the period of our study (up to 4 months after DST).11-13 Because for unknown reasons, the anti-HY TCR transgenic mice can neither generate functional cytotoxic T cells (CTL) in vivo nor reject a male graft, it is not possible to study the effect of DST on graft survival in this particular system.
In the present study, we use 2C transgenic mice that carry a transgenic TCR that specifically recognizes an Ld (MHC class I molecule) as a model to delineate the mechanisms involved in DST-induced long-term allograft survival. We found that, in the absence of any other immunosuppression, a single dose infusion of viable lymphocytes from the graft donor before transplantation was sufficient to induce long-term donor-specific skin allograft survival in 2C transgenic mice. Because the tolerance was induced in transgenic mice, we were able to directly monitor the fate of donor-reactive cells in vivo and to explore the mechanisms of DST-induced long-term Ag-specific tolerance.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6, H-2b), SJL(H-2s), C3H(H-2k), (B6×BALB/c)F1- (BYJ, H-2b/d Ld+) and BALB/c H-2-dm2 (dm2, a BALB/c Ld loss mutant, H-2 Dd+, Kd+, Ld-) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Breeding stock of 2C transgenic mice (on B6 background) were kindly provided by Dr Dennis Y. Loh (Nippon Roche Research Center, Kamakura-shi, Japan).14 2C (H-2b/b) transgenic mice carry functionally rearranged TCR α-(one copy) and β-chain (eight copies) transgenes from a cytotoxic T-cell clone 2C, which is specific for Ld MHC class I Ag. The specificity for Ld requires both transgenic α and β chains.14,15. The 2C clonotypic TCR is recognized by monoclonal antibody (MoAb) 1B2.16 B62C mice (H-2b/b) were bred with dm2 mice. The subsequent 1B2+ 2CF1 mice (H-2b/d, Ld-, anti-Ld TCR+) were maintained in the animal colony at the Ontario Cancer Institute and used as recipients. The BYJ mice were used as lymphocytes and skin graft donors.
DST.
Spleen and lymph nodes were harvested from BYJ mice that mismatch only for Ld with the recipients. Single cell suspension was prepared by gently pressing the spleen and lymph nodes against a fine metal screen. The red blood cells were lyzed by lysing solution. Cells were then washed three times with 1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS). The viability was examined by eosin exclusion and was greater than 95%. One group of recipients was injected intravenously (IV) with 3 × 107/mouse sex-matched viable cells either on the day of skin grafting or 18 days before transplantation. Another group of recipients was injected with same number of syngeneic cells from (B6×dm2)F1 mice at the same time points as controls.
Cell surface marker staining.
To follow the fate of donor-reactive T cells after encountering donor Ag in vivo, lymphocytes from lymph nodes and spleen of recipient mice were collected and analyzed at various time points after DST. Lymphocytes were stained with fluorescein isothiocyanate (FITC)-labeled clonotypic MoAb 1B2 (which recognizes the transgenic TCR, this hybridoma was kindly provided by Dr Herman Eisen, Massachusetts Institute of Technology, Cambridge, MA) and phycoerythrin (PE)-conjugated anti-CD8 MoAb (Pharmingen, San Diego, CA). Data were acquired and analyzed on an EPICS XL-MCL flow cytometry machine (Coulter Corp, Miami, FL).
Tail skin grafting.
Skin grafting was performed as described previously.17Briefly, a piece of donor tail skin about 1 × 0.5 cm2and a thickness including the epidermis and most of the dermis was removed with a sharp scalpel and transferred to the sides on the recipient tail from which an equivalent amount of skin had been removed. The grafts were covered with a clear spray bandage (NEW-Skin, Dedtech Labs, Jackson, WY) and further protected with a light, loosely fitting transparent glass tube. Grafts were visually monitored daily and scored as rejected when greater than 90% dead.
Mixed lymphocyte responses (MLR).
At different time points after DST, splenocytes were collected from recipient mice and used as responder cells in an MLR. Varying numbers of responder cells (from 1 × 103 to 1 × 104 cells/well) were cocultured in 96-well plates with irradiated (20 Gy) sex-matched splenocytes (3 × 105 cells/well) from BYJ mice in α-minimal essential medium (MEM) supplemented with 10% fetal calf serum (FCS) and 30 U/mL of recombinant interleukin-2 (rIL-2) as a source of growth factor. After 3 days of incubation, 1 μCi of [3H] thymidine deoxy-ribonucleic (TdR) was added to each well. Fifteen hours later, cultures were procured and counted in a beta counter.
Cytotoxicity assay.
Varying numbers of donor-reactive T cells (1B2+CD8+), from 30 to 3,000 cells/well, were obtained from DST-treated and naive 2CF1 mice and cocultured with irradiated (20 Gy) sex-matched splenocytes (3 × 105 cells/well) from BYJ mice in α-MEM supplemented with 10% FCS and 30 U/mL of rIL-2 as a source of growth factor. After 5 days of incubation, percentage lysis of specific target cells (Ld+ P815 cells, 3,000 cells/well) was measured by a 4-hour 51Cr release assay and specific killing was calculated by the following formula: % Specific Lysis = (cpm Experimental − cpm Background 51Cr release)/(cpm Total 51Cr release in 2% Acetic Acid − cpm Background 51Cr release). All samples were performed in five replicates.
Detection of interleukin-4 (IL-4) by enzyme-linked immunosorbent assay (ELISA).
Serum samples were collected from recipient mice before, 4, and 18 days after DST, as well as from the mice with donor skin allografts surviving for 120 days. The concentration of IL-4 was measured using an IL-4 ELISA kit (Genzyme Inc, Cambridge, MA). Samples were tested in duplicates and the amounts of IL-4 were calculated from a standard curve and expressed in pg/mL. The sensitivity of the assay is 4 to 6 pg/mL according to the manufacturer.
RESULTS
Induction of long-term donor-specific skin allograft survival in 2C transgenic mice by pretransplantation DST.
To establish a system in which the response of recipient to a defined donor MHC allo Ag can be monitored in vivo, B62C mice were bred with dm2 mice. The subsequent 2CF1 mice were screened by staining the blood samples using the clonotypic MoAb 1B2 and the 1B2+ 2CF1 mice were used as recipients. Because sharing MHC class II Ag between donor and recipient has been suggested to be important for the induction of donor-specific tolerance,2-4 we chose sex-matched BYJ mice that are mismatched only for Ld Ag with the 2CF1 mice as lymphocyte and skin graft donors. As a result, the immune response that took place in this system would be 1B2+CD8+cells from the recipient reacting to Ld Ag expressed on the donor.
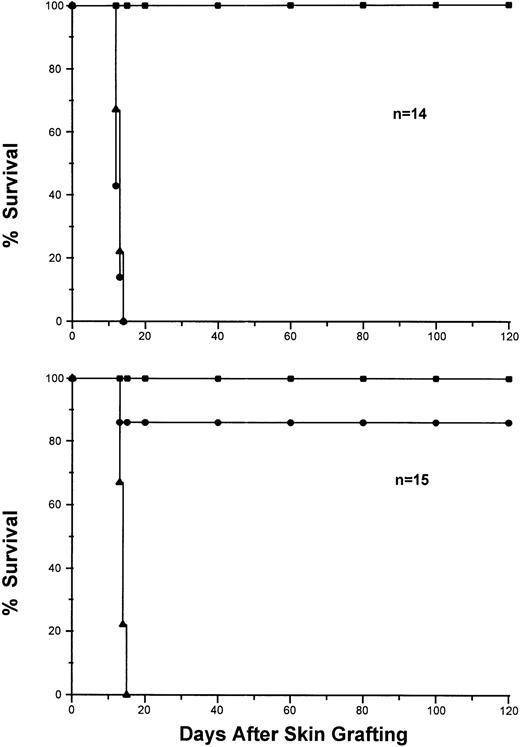
Based on our studies on anti-HY TCR transgenic mice,11-132CF1 mice were injected IV with 3 × 107viable lymphocytes from either sex-matched syngeneic 2CF1transgene negative littermates (control group, Fig1, top panel) or Ld+ cells from BYJ mice (experimental group, Fig 1, bottom panel). Eighteen days after injection, each recipient 2CF1 transgenic mouse from both groups received three sex-matched skin grafts from each of BYJ (donor-specific), (B6×dm2)F1 (syngeneic control) and SJL or C3H (H-2S and H-2k, respectively, as third party controls) mice. As shown in Fig 1, all syngeneic skin grafts were accepted permanently and third party allogeneic ones were rejected between 11 to 13 days by both groups of mice, indicating that 2CF1 transgenic mice are just as able to mediate allogeneic skin graft rejection as nontransgenic mice. Interestingly, the BYJ skin grafts survived permanently (over 120 days) in 13 of 15 mice that received DST, whereas they were rejected within 2 weeks by all mice in the control group. These results clearly indicate that rejection of a single class I Ag mismatched skin allografts mounted by 2CF1 transgenic mice can be abolished by a single dose pretransplant DST in the absence of any other immunosuppressive agent. Moreover, because the fate of donor-reactive T cells can be directly monitored in vivo after DST and transplantation, it provides a good model for delineating the cellular and molecular mechanisms involved in the induction and maintenance of Ag-specific tolerance in vivo.
Induction of long-term donor-specific skin graft survival by pretransplantation infusion of donor splenocytes. 2CF1transgenic mice were injected IV with splenocytes from Ld-syngeneic mice (top, n = 14) or Ld+ mice (bottom, n = 15). Eighteen days after injection, each mouse was given skin grafts from SJL (▴), BYJ (•) and (B6×dm2)F1 (▪). The grafts were monitored and scored after skin transplantation for more than 120 days.
Induction of long-term donor-specific skin graft survival by pretransplantation infusion of donor splenocytes. 2CF1transgenic mice were injected IV with splenocytes from Ld-syngeneic mice (top, n = 14) or Ld+ mice (bottom, n = 15). Eighteen days after injection, each mouse was given skin grafts from SJL (▴), BYJ (•) and (B6×dm2)F1 (▪). The grafts were monitored and scored after skin transplantation for more than 120 days.
Reduction of the number of donor-reactive T cells in the periphery of DST-treated recipient mice.
Clonal deletion has been considered as an important mechanism for both negative selection in the thymus and induction of peripheral tolerance to super Ags.18-20 Our previous studies have demonstrated that encountering allo Ag in vivo results in a deletion of specific allogeneic T cells in the periphery via activation-induced cell death.11,12,21,22 However, it was not known whether deletion of donor-reactive T cells, either centrally and/or peripherally, was involved in DST-induced long-term allograft acceptance. Theoretically, it is possible that the infused donor cells or peptides derived from them might enter the thymus and induce a native selection of 1B2+CD8+ cells in the thymus. To see whether this was the case in our system, thymocytes were collected from 2CF1 mice that were treated with DST and accepted the donor-skin graft. Cells were triple stained with MoAbs that specifically recognize 1B2, CD8, and CD4. The data were analyzed using three-color flow cytometry. The thymocytes obtained from naive 2CF1 mice were stained in the same way and used as controls. When the percentages of 1B2+CD8+, 1B2+CD4+, CD8+CD4+, 1B2+, CD8+ and CD4+ cells in the thymus of DST-treated mice were compared with those in the naive 2CF1 mice, no significant difference was found (data not shown), which is consistent with our previous findings.11 12 These experiments suggest that no thymic deletion of donor-reactive cells was involved in DST-induced long-term allo skin graft survival.
Next, we followed the physiologic fate of anti-Ld T cells in the periphery after encountering donor lymphocytes. As shown in Fig2, 4 days after DST, the number of 1B2+CD8+ cells remained similar to that in uninjected mice. By 18 days, however, the number of donor-reactive T cells was significantly reduced in the periphery of the recipients. This low number of 1B2+CD8+ cells remained for at least 35 days after a single DST treatment. In contrast, no significant changes were seen in the number of 1B2+CD8− cells after DST. The same results were obtained in the lymph nodes (data not shown). Together, these expriments show that DST can reduce the number of donor-reactive T cells in the periphery of recipients.
Decrease of 1B2+CD8+ cells in 2CF1 transgenic mice after DST. 2CF1 mice were injected IV with splenocytes from BYJ mice. The percentages of 1B2+CD8+ cells (A) and 1B2+CD8- cells (B) in spleen are shown from untreated 2CF1 mice (day 0, □), day 4 (▪), day 18 (▧), and day 35 (▩) after DST. *P < .0001.
Decrease of 1B2+CD8+ cells in 2CF1 transgenic mice after DST. 2CF1 mice were injected IV with splenocytes from BYJ mice. The percentages of 1B2+CD8+ cells (A) and 1B2+CD8- cells (B) in spleen are shown from untreated 2CF1 mice (day 0, □), day 4 (▪), day 18 (▧), and day 35 (▩) after DST. *P < .0001.
Decrease in the number of donor-reactive T cells did not correlate with enhancement of donor-specific skin allograft survival.
To investigate further if the reduction of donor reactive T cells before transplantation was essential for donor-specific skin graft acceptance, 2CF1 mice were given DST and skin grafts on the same day when no reduction of donor reactive T cells could be detected in the recipient. As shown in Fig 3, skin grafts from the lymphocyte donor strain survived over 120 days in five of six mice, whereas the third party allografts were rejected within 2 weeks by all of the 2CF1 mice that received DST. These findings were similar to those observed in mice that received skin grafts 18 days after DST, at which time about 60% of donor-reactive T cells had disappeared from the periphery of the recipients. Therefore, reduction of donor-reactive T cells before transplantation seems not to be essential for the induction of donor-specific skin graft survival. Furthermore, the number of 1B2+CD8+ cells in recipient mice treated with or without DST, 120 days after transplantation, was compared with those in naive mice. Interestingly, although a significantly (P < .01) lower number of 1B2+CD8+ cells was observed in both lymph nodes and spleen of mice that had DST and accepted donor skin grafts for more than 120 days compared with those in naive mice, the number of 1B2+CD8+ cells in the 2CF1 mice that did not receive DST and rejected the Ld+ skin grafts was also significantly reduced (Fig 4). This finding suggests that deletion of donor-reactive T cells may be a consequence of encountering allo Ag in vivo, either through infused donor lymphocytes or donor skin graft. However, reduction in the number of donor-reactive T cells in recipients may not necessarily lead to allograft acceptance. Together, our data indicate that although DST can induce deletion of donor-reactive T cells, this deletion appears not to be essential for the induction of skin allograft survival.
Survival of donor-specific skin grafts in 2CF1 transgenic mice given DST on the day of transplantation. 2CF1 transgenic mice (n = 6) were injected IV with splenocytes from BYJ (Ld+). On the same day of the injection, each mouse was given skin grafts from BYJ (▪) and SJL (•). The grafts were monitored and scored after skin transplantation for more than 120 days.
Survival of donor-specific skin grafts in 2CF1 transgenic mice given DST on the day of transplantation. 2CF1 transgenic mice (n = 6) were injected IV with splenocytes from BYJ (Ld+). On the same day of the injection, each mouse was given skin grafts from BYJ (▪) and SJL (•). The grafts were monitored and scored after skin transplantation for more than 120 days.
Deletion of 1B2+ CD8+ cells after transplantation in both DST-treated and non-DST–treated mice. The spleens (left panel) and lymph nodes (right panel) from naive (▪), DST-treated (□), and non-DST–treated (□) 2CF1mice 120 days after transplantation were collected and the percentages of 1B2+ CD8+ cells were compared. The percentages of 1B2+ CD8+ cells in both DST-treated and non-DST–treated mice were significantly reduced compared with that in the naive mice (*P < .001).
Deletion of 1B2+ CD8+ cells after transplantation in both DST-treated and non-DST–treated mice. The spleens (left panel) and lymph nodes (right panel) from naive (▪), DST-treated (□), and non-DST–treated (□) 2CF1mice 120 days after transplantation were collected and the percentages of 1B2+ CD8+ cells were compared. The percentages of 1B2+ CD8+ cells in both DST-treated and non-DST–treated mice were significantly reduced compared with that in the naive mice (*P < .001).
1B2+CD8+ cells remaining in DST-treated mice are fully responsive.
Although DST can induce deletion of the majority of donor-reactive T cells in the recipients, it was interesting to note that the deletion was never complete. About 30% to 40% of donor-reactive 1B2+CD8+ cells were always found to persist in the periphery of the recipients. To study if DST has any influence on the function of those remaining donor-reactive T cells, the remaining 1B2+CD8+ cells from 2CF1 mice 18 days after DST were isolated and stimulated by irradiated Ld+ lymphocytes from BYJ mice in vitro. Cell proliferation was measured by 3H-thymidine incorporation and compared with that of naive 2CF1 mice. As shown in the top panel of Fig 5, when the same numbers of 1B2+CD8+ cells were seeded in the culture, the remaining 1B2+CD8+ cells proliferated in the same way as those from naive 2CF1 mice. The same results were obtained from another four independent experiments.
Full responsiveness of remaining donor-reactive T cells to donor Ag 18 days after DST. Splenocytes from 2CF1 mice either untreated (◊) or 18 days after DST (□) were stimulated by irradiated (20 Gy) splenocytes from BYJ mice in α-MEM supplemented with 10% FCS and 30 U/mL rIL-2. In the top panel, proliferation ([3H] TdR incorporation) was measured after a 3.5-day culture. Data are plotted as cpm versus number of 1B2+CD8+/well. Each data point represents five replicates. In the bottom panel, percentage lysis of specific Ld target cells (open symbols) was measured by51Cr release assay after a 5-day culture. Data are plotted as percentage specific killing versus number of 1B2+CD8+ cells/well. These results represent four independent experiments. Killing of third-party control (H-2K) target cells by the highest number of effector cells is shown in solid symbols. Each data represents five replicates. Similar results were obtained at 35 days after DST.
Full responsiveness of remaining donor-reactive T cells to donor Ag 18 days after DST. Splenocytes from 2CF1 mice either untreated (◊) or 18 days after DST (□) were stimulated by irradiated (20 Gy) splenocytes from BYJ mice in α-MEM supplemented with 10% FCS and 30 U/mL rIL-2. In the top panel, proliferation ([3H] TdR incorporation) was measured after a 3.5-day culture. Data are plotted as cpm versus number of 1B2+CD8+/well. Each data point represents five replicates. In the bottom panel, percentage lysis of specific Ld target cells (open symbols) was measured by51Cr release assay after a 5-day culture. Data are plotted as percentage specific killing versus number of 1B2+CD8+ cells/well. These results represent four independent experiments. Killing of third-party control (H-2K) target cells by the highest number of effector cells is shown in solid symbols. Each data represents five replicates. Similar results were obtained at 35 days after DST.
To further study if the remaining donor-reactive T cells can kill Ld+ target cells, 1B2+CD8+ cells were isolated from 2CF1 mice 35 days after DST. Their capacity of lysing donor-specific, as well as third-party (H-2K) target cells, was compared with 1B2+CD8+ cells from naive mice. The bottom panel of Fig 5 shows identical killing of Ld+ target cells by remaining and naive 1B2+CD8+ cells. No difference was seen in killing of third-party targets. These data indicate that remaining 1B2+CD8+ cells in DST-treated mice are fully reactive to the donor antigen in vitro.
Suppression of naive donor-reactive T cells by residual 1B2+CD8+ cells from tolerant mice in the presence of IL-4.
Because none of our recipient mice were thymectomized and no deletion of 1B2+CD8+ cells was seen in the thymus after DST, it was possible that new naive 1B2+CD8+cells were constantly produced by the thymus. Given the fact that remaining 1B2+CD8+ cells in the periphery of the DST recipients were fully capable of killing donor cells, yet did not reject donor skin grafts, we reasoned that there must be an active mechanism by which the function of 1B2+CD8+cells could be inactivated in the donor graft accepted mice. In our previous studies in which 1B2+CD8+ cells were adoptively transferred into Ld-expressing severe combined immunodeficiency (Scid) mice, the residual 1B2+CD8+ cells in the recipients could inhibit proliferation of newly injected naive 1B2+CD8+cells.21 It is possible that the remaining 1B2+CD8+ cells might themselves function as regulatory/suppressor cells in the maintenance of donor-specific graft survival. To test this possibility, 120 days after transplantation, splenocytes were collected from 2CF1 mice that had received DST and accepted skin allografts, as well as non-DST–treated 2CF1 mice that rejected skin allografts. These cells were used as putative suppressor cells. Naive 2CF1 cells were stimulated by irradiated Ld+ cells in an MLR to which different ratios of suppressor cells were added. When the proliferation of 1B2+CD8+ cells from naive 2CF1mice was measured, a dose-dependent inhibition of3H-incorporation by the 1B2+CD8+cells from mice that accepted Ld skin allograft was observed only when exogenous IL-4 was provided (Fig6, bottom right panel). No inhibition was observed in cells from mice that have rejected skin allografts regardless of the presence or absence of IL-4 (Fig 6, top and bottom, left panels), or by cells from graft tolerant mice in the absence of IL-4 (Fig 6, top right panel). These findings suggest that DST could promote the induction of suppressor cells, which have the potential to inhibit the activation of naive donor-reactive T cells. However, this suppression seems to be dependent on the presence of IL-4.
Suppression of naive 1B2+CD8+T cells response to Ld in vitro by cells from tolerant mice in the presence of exogenous IL-4. Naive1B2+CD8+ cells (1 × 103cells/well) from 2CF1 mice were used as responder cells and stimulated by 3 × 105 cells/well of irradiated (20 Gy) splenocytes from BYJ mice. 1B2+CD8+ cells from 2CF1 mice that either rejected (left panels) or accepted (right panels) Ld+ skin allografts 120 days after transplantation were added into the MLR cultures as putative suppressor cells. The suppressor to responder cell ratios were 0:1, 10:1, 5:1, 2:1, and 1:1 as indicated. Cells were cultured in α-MEM supplemented with 10% FCS, 30 U/mL rIL-2 in the presence (top panels) or absence (bottom panels) of 50 U/mL rIL-4. Cell proliferation was measured by [3H] TdR incorporation after a 3.5-day culture. (□), S/R = 0:1; (▪), S/R = 10:1; (▤), S/R = 5:1; (▥), S/R = 2:1; (□), S/R = 1:1.
Suppression of naive 1B2+CD8+T cells response to Ld in vitro by cells from tolerant mice in the presence of exogenous IL-4. Naive1B2+CD8+ cells (1 × 103cells/well) from 2CF1 mice were used as responder cells and stimulated by 3 × 105 cells/well of irradiated (20 Gy) splenocytes from BYJ mice. 1B2+CD8+ cells from 2CF1 mice that either rejected (left panels) or accepted (right panels) Ld+ skin allografts 120 days after transplantation were added into the MLR cultures as putative suppressor cells. The suppressor to responder cell ratios were 0:1, 10:1, 5:1, 2:1, and 1:1 as indicated. Cells were cultured in α-MEM supplemented with 10% FCS, 30 U/mL rIL-2 in the presence (top panels) or absence (bottom panels) of 50 U/mL rIL-4. Cell proliferation was measured by [3H] TdR incorporation after a 3.5-day culture. (□), S/R = 0:1; (▪), S/R = 10:1; (▤), S/R = 5:1; (▥), S/R = 2:1; (□), S/R = 1:1.
Increased IL-4 expression in sera of DST-treated skin allograft tolerant mice.
Since Mosmann et al23 first proposed the Th1/Th2 paradigm, numerous studies have been performed to determine if Th1 and Th2 type cytokine profiles are associated with rejection and tolerance, respectively.24,25 Although some studies suggested the presence of Th2 and absence of Th1-associated cytokines in graft tolerant animals,26,27 the overall results are controversial. Our previous work showed a significant increase in the expression of IL-4 and decrease in IL-2 mRNA in the remaining 1B2+CD8+ cells after encountering Ld+ Ag in vivo.21 As shown in Fig 6, only when exogenous IL-4 was provided could the non-DST–depleted cells in skin graft tolerant mice inhibit anti-Ld responses of naive 1B2+CD8+ cells. Based on these findings, we wondered if the serum IL-4 level was increased in vivo after DST. Serum samples were collected from 2CF1 mice before, 4, and 18 days after DST, as well as 120 days after skin grafting, and the concentration of IL-4 was measured by ELISA. As shown in Fig7, the sera level of IL-4 was increased with time after DST and most significantly increased in mice that permanently accepted donor skin allografts. These results suggest that DST can promote IL-4 production, which may assist regulatory T cells in the induction and maintenance of Ag-specific tolerance.
Increase of IL-4 in the sera of DST-treated mice. Serum samples were collected from 2CF1 mice before (□), 4 (▪), and 18 (□) days after DST, as well as more than 120 days (▩) after skin grafting. The concentration of IL-4 was measured by ELISA.
Increase of IL-4 in the sera of DST-treated mice. Serum samples were collected from 2CF1 mice before (□), 4 (▪), and 18 (□) days after DST, as well as more than 120 days (▩) after skin grafting. The concentration of IL-4 was measured by ELISA.
DISCUSSION
We have shown in this study that long-term donor-specific skin allograft survival can be induced in 2C anti-Ld TCR transgenic mice by a single dose infusion of donor lymphocytes before transplantation. This donor-specific tolerance is induced and maintained in the absence of any other immunosuppressive agent. Moreover, the fate of donor-reactive T cells in the recipients was directly monitored in vivo after DST and transplantation to further delineate the mechanisms involved in the induction and maintenance of tolerance.
Clonal deletion has been shown to be an important mechanism for the induction of tolerance to self Ags and bacteria super Ags.18-20 Its role in the induction of transplantation tolerance, however, was not known. In this study, we found that after DST, no significant change in the percentage of 1B2+CD8+ cells in the thymus of the recipients, implying that the thymus may not be involved in DST-induced long-term donor-skin graft survival. In contrast, there was a profound reduction of donor-reactive T cells both in the lymph nodes and spleen (Fig 2), suggesting that DST can induce deletion of donor-reactive T cells in the periphery of the recipients. However, several lines of evidence from our further studies suggested that clonal deletion may be an epiphenomenon rather than a major mechanism involved in DST-induced tolerance to allo Ags. First, long-term graft survival could also be induced when DST was given on the day of transplantation, at which time a reduction of donor reactive T cells had not occurred. Second, significant deletion of donor-reactive T cells was also observed in non-DST–treated mice that rejected their allografts. Third, deletion of donor reactive T cells is never complete. In the lymph nodes and spleen of all 2CF1 mice 18 or 35 days after DST, as well as those with long-term surviving donor-specific skin grafts, about 30% to 40% of the initial number of donor-reactive T cells was detected (Figs 2 and 4).
There are three possible sources for the donor-reactive T cells seen in the periphery after DST. (1) These cells have not encountered donor Ag; (2) they are new emigrants from the thymus because the recipients were not thymectomized; and (3) these cells are resistant to clonal deletion and remain to serve as regulatory cells in the maintenance of tolerance. Generally, a high dose of Ag and repeated stimulation are in favor of deletion of Ag specific T cells. In this study, we gave only one dose infusion of 3 × 107 donor lymphocytes and the infused donor cells could be removed from within a few days to a few weeks by CD8+ T cells in the recipient28(and our unpublished data). It is thus possible that not every potential donor-reactive T cell has encountered Ag. However, in our previous studies in which 2CF1 cells were adoptively transferred into scid mice that express Ld+, otherwise completely matched for major and minor histocompatibility Ags with the donor, about 20% of the initial 1B2+CD8+ cells were also found to persist in the periphery of the recipients.22 It seems unlikely that some anti-Ld cells persisted in the periphery due to failure to encounter Ag in the Scid system because the Ag was constitutively expressed on all of the nucleated cells of the recipients. This finding also argues against the possibility that the residual 1B2+CD8+ cells are new thymic emigrants because the thymus of scid mice cannot produce 1B2+CD8+ cells.
In fact, the persistence of a small population of antigen-reactive T cells in the periphery after encounter with Ags in vivo has been observed in most of the systems where peripheral tolerance has been induced by administration of Ags.11-13 20-22 Therefore, it is unlikely to be a coincidence. Rather, it is possible that the persistence of antigen-reactive T cells may represent an intrinsic feature of the mechanism of peripheral tolerance, eg, some antigen-reactive T cells may persist to function as regulatory/suppressor cells in the maintenance of tolerance. This possibility might prove to be true based on the following observations: (1) mice made tolerant to allografts by injection of nondepleting MoAbs to transiently block CD4 and CD8 molecules or anti-CD4 Ab plus DST-generated lymphocytes, which could suppress naive syngeneic T cells and also guide them to tolerance to the same Ags29,30; (2) the T-cell clones generated from the remaining cells in mice that have been made orally tolerant to myelin basic protein were able to protect naive mice from developing encephalomyelitis induced by the same protein31,32; (3) some T-cell clones from long-term donor-specific skin graft tolerant 2CF1 mice could inhibit the function of naive 1B2+CD8+ cells in an Ag-specific, dose-dependent manner (Yang, et al, manuscript in preparation). Together, these findings strongly suggest that the generation of a population of T cells, which can specifically suppress the function of naive syngeneic T cells in responding to the same Ag, is one of the mechanisms of peripheral tolerance.
Our observation that the concentration of IL-4 in the serum of tolerant mice was significantly increased supports the view that DST may promote IL-4 production. It is not clear what the source of IL-4 is. We have shown in the previous studies using an adoptive transfer system inScid mice that after encountering Ld Ag in vivo, the persisting 1B2+CD8+ cells expressed increased mRNA levels of IL-4 and IL-10 and decreased levels of IL-2.21 In this study, when the mRNA level of IL-4 was measured using reverse transcriptase-polymerase chain reaction (RT-PCR), an increased expression of IL-4 mRNA was also observed in T cells obtained from tolerant mice (data not shown). It is possible that a high level of IL-4 was secreted by the non-DST–depleted T cells. However, when cultured in vitro, these T cells seemed unable to produce sufficient IL-4 and their suppressive function was dependent on exogenous IL-4. These findings suggest that non-DST–depleted donor-reactive T cells have the potential to produce IL-4, but the production may be regulated by some other cells in vivo, such as mast cells,33 γδ T cells34 or a subset of αβ+ T cells expressing natural killer (NK) cell markers.35
It is not known how IL-4 exerts its role in the induction and/or maintenance of donor-specific allograft survival. It has been suggested that IL-4 could induce immune deviation and tolerance to autoimmune disease.36 In our system, when IL-4 was added to an MLR without putative suppressor T cells, no inhibition of proliferation of naive 1B2+CD8+ cells on Ld+ cell stimulation was detected, suggesting that IL-4 alone is not able to produce tolerance. Furthermore, we found that only when both IL-4 and regulatory cells were present at the same time could a suppression of naive 1B2+CD8+ cells be observed. This suggests that IL-4 may exert its effect indirectly through regulatory T cells. These findings open a new window for our understanding of the Th1/Th2 paradigm, as well as Ag-specific suppression in peripheral tolerance.
ACKNOWLEDGMENT
We thank Drs D.Y. Loh and H.M. Eisen for kindly providing breeding stock of 2C transgenic mouse and hybridoma 1B2, respectively. The authors also thank Drs D.H. Sachs, R.G. Miller, R. Gorczynski, and S. Albert for critically reading the manuscript and giving useful comments.
Supported by Medical Research Council of Canada Grant No. MT 12639 (to L.Z.).
Address reprint requests to Li Zhang, MD, PhD, Department of Cellular and Molecular Pathology, CCRW 2-852, 101 College St, Toronto, Canada, M5G 2C4.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 5. Full responsiveness of remaining donor-reactive T cells to donor Ag 18 days after DST. Splenocytes from 2CF1 mice either untreated (◊) or 18 days after DST (□) were stimulated by irradiated (20 Gy) splenocytes from BYJ mice in α-MEM supplemented with 10% FCS and 30 U/mL rIL-2. In the top panel, proliferation ([3H] TdR incorporation) was measured after a 3.5-day culture. Data are plotted as cpm versus number of 1B2+CD8+/well. Each data point represents five replicates. In the bottom panel, percentage lysis of specific Ld target cells (open symbols) was measured by51Cr release assay after a 5-day culture. Data are plotted as percentage specific killing versus number of 1B2+CD8+ cells/well. These results represent four independent experiments. Killing of third-party control (H-2K) target cells by the highest number of effector cells is shown in solid symbols. Each data represents five replicates. Similar results were obtained at 35 days after DST.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.324/3/m_blod4010805.jpeg?Expires=1765945887&Signature=uyxZ2cL7UjvGGuUaX9HkkNLARZulYc5q95QHTrIaoibWDB9aiVNbiTBgytYxKhp9enKeDxJNtfqwwzeCqU8ATEdMLgqPpBhnFVhVSJCnm9gieEhvs7NxytIy8V4~W0~5k5yeW2pPG-fbWoRaholT3I2UT23d0lHZrb7Q-mzHUD4zd6is8Wl4y6V5hdYSMPYNFB4yIqYXBE4vY3T4II-lc2z5YzJJkjxhKoANPn3UTfdlg6NsiQrR1JeGj0pYJz8rxjz6fXX4wEtnzq-pHVNNmYzRir1Mm0wm7qm4Aul-mtYz1tZfAhvQk3SQfgO3FMCK402Tkjv~auGwC2oue3a60w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Suppression of naive 1B2+CD8+T cells response to Ld in vitro by cells from tolerant mice in the presence of exogenous IL-4. Naive1B2+CD8+ cells (1 × 103cells/well) from 2CF1 mice were used as responder cells and stimulated by 3 × 105 cells/well of irradiated (20 Gy) splenocytes from BYJ mice. 1B2+CD8+ cells from 2CF1 mice that either rejected (left panels) or accepted (right panels) Ld+ skin allografts 120 days after transplantation were added into the MLR cultures as putative suppressor cells. The suppressor to responder cell ratios were 0:1, 10:1, 5:1, 2:1, and 1:1 as indicated. Cells were cultured in α-MEM supplemented with 10% FCS, 30 U/mL rIL-2 in the presence (top panels) or absence (bottom panels) of 50 U/mL rIL-4. Cell proliferation was measured by [3H] TdR incorporation after a 3.5-day culture. (□), S/R = 0:1; (▪), S/R = 10:1; (▤), S/R = 5:1; (▥), S/R = 2:1; (□), S/R = 1:1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.324/3/m_blod4010806.jpeg?Expires=1765945887&Signature=4ycoZzeFlRsUTqgALdKmtmLm-sLB0uJ0RCOnxC4TtjRzYnUvBJttbIStBENleSBgEOvaPg9GgOyvnYYzoxcTP2piSplo0fT1NV5pU9Ps7ihXOKbvLCqZNFpT46PdNQzNtpLlT8Q~optpZGcE02pYZaC65hrR2G7xmHeRsDjd578XBroamH~nEHus7FwsCg4TP~IaU~X6SIPW38kOupNkrdjoxDnwLP7Li~SSCxjrfe~Wa7Gw3GXa0uc-bawENZTz2bQP91IQOiPRBUkH17LEKM-YpZ7aIlrErE5bBY751ZajSMsOzo5niJ-ONl1NU6c30FJoSsiOvppAv1c6RMVNyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal