Abstract
Cells from individuals with Fanconi anemia (FA) arrest excessively in the G2/M cell cycle compartment after exposure to low doses of DNA cross-linking agents. The relationship of this abnormality to the fundamental genetic defect in such cells is unknown, but many investigators have speculated that the various FA genes directly regulate cell cycle checkpoints. We tested the hypothesis that the protein encoded by the FA group C complementing gene (FAC) functions to control a cell cycle checkpoint and that cells from group C patients (FA[C]) have abnormalities of cell cycle regulation directly related to the genetic mutation. We found that retroviral transduction of FA(C) lymphoblasts with wild-type FAC cDNA resulted in normalization of the cell cycle response to low-dose mitomycin C (MMC). However, when DNA damage was quantified in terms of cytogenetic damage or cellular cytotoxicity, we found similar degrees of G2/M arrest in response to equitoxic amounts of MMC in FA(C) cells as well as in normal lymphoblasts. Similar results were obtained using isogenic pairs of uncorrected, FAC- or mock-corrected (neo only) FA(C) cell lines. To test the function of other checkpoints we examined the effects of hydroxyurea (HU) and ionizing radiation on cell cycle kinetics of FA(C) and normal lymphoblasts as well as with isogenic pairs of uncorrected, FAC-corrected, or mock-corrected FA(C) cell lines. In all cases the cell cycle response of FA(C) and normal lymphoblasts to these two agents were identical. Based on these studies we conclude that the aberrant G2/M arrest that typifies the response of FA(C) cells to low doses of cross-linking agents does not represent an abnormal cell cycle response but instead represents a normal cellular response to the excessive DNA damage that results in FA(C) cells following exposure to low doses of cross-linking agents.
FANCONI ANEMIA (FA) is an autosomal recessive disorder characterized by progressive pancytopenia; a high risk of malignancies, especially acute myelogenous leukemia (AML)1; and in some cases by congenital malformations including skin pigmentation abnormalities, skeletal deformities, and renal anomalies.2-5 Most patients are diagnosed during the first 10 years of life and die as young adults of bone marrow failure or AML. Standard medical therapy is directed at supporting bone marrow function and may include the use of androgens, hematopoietic growth factors, antibiotics, and transfusion of blood products.6Although the National Heart Lung and Blood Institute has recently instituted a limited trial of gene therapy for individuals with FA(C),7 currently the only known definitive therapy for bone marrow failure or AML in FA patients is allogeneic bone marrow transplantation from a histocompatible donor.6 8
The cellular hallmark of FA is a unique hypersensitivity to DNA cross-linking agents. Treatment of FA cells with agents such as mitomycin C (MMC) or diepoxybutane (DEB) at doses that have little impact on normal cells results in chromosomal instability and cellular death.6,8-10 Using cell-cell fusion techniques, it has been established that there are at least five FA complementation groups (FA[A] to FA[E]).11
The gene encoding the defective or missing protein in patients with FA(C) has been cloned by Strathdee et al using a functional complementation strategy.12 The FA group C complementing (FAC) gene encodes a protein of 558 amino acids with a predicted molecular mass of ∼63 kD and has been localized to chromosome 9q22.3.12 The FAC protein contains numerous hydrophobic residues, consensus sites for Ser/Thr phosphorylation, and binding sites for the molecular chaperone immunoglobulin heavy-chain binding protein.13 However, the sequence contains no significant homologies with other known proteins in genetic data banks, and the exact biochemical function of theFAC protein remains unknown. Using polyclonal rabbit antiserum, a number of groups including ours have found theFAC protein localized primarily in the cytoplasm.13,14 Using immunoprecipitation or affinity purification techniques, a family of cytosolic proteins that bind ex vivo to FAC protein have been identified, but their function and in vivo significance are unknown.14 15
FAC mRNA has been found in every normal tissue tested to date, and several different types of mutations have been described in FA(C) patients.12,16,17FAC gene expression appears to be constitutive, and we have recently found that neither FAC mRNA or protein is induced in hematopoietic cells after cellular exposure to MMC, DEB, hydrogen peroxide, γ-irradiation, heat shock, transforming growth factor (TGF)-β1, or interferon-γ.18
Because of the universal occurrence of bone marrow failure in patients with FA, we and others postulated that the FAC protein may play some direct role in the regulation of hematopoietic cell proliferation. Gain- and loss-of-function strategies have confirmed this hypothesis. We have shown that suppression of FAC gene expression reduced clonal growth of normal erythroid and granulocyte-macrophage progenitor cells and that FA(C) progenitor cells are hypersensitive to the mitotic inhibitory effects of interferon-γ.19-21 Walsh et al have found that retroviral or adeno-associated viral transduction ofFAC cDNA into CD34+ cells from FA(C) patients results in increased clonal growth of colony forming units.22 23
Abnormalities in the cell cycle kinetics of cells derived from individuals with FA were first reported by Sasaki in 1975. In these studies it was noted that FA cells passed more slowly than did normal cells through the G2/M phase of the cell cycle. Treatment of FA cells with MMC was noted to further increase the delay in cell cycle transit.24 An increased percentage of cells in the G2/M compartment has been noted in untreated cells but is most marked in cells that have been exposed to DNA cross-linking agents such as MMC or DEB.25-27
Recent advances in the understanding of cellular proliferation has lead to the realization that there are certain restriction points for controlling cell cycle progression. Cell cycle checkpoints order events in the cell cycle so that initiation of later events is dependent on the completion of earlier events. For example, the initiation of mitosis is dependent on the completion of DNA synthesis.28By ordering the cell cycle in this way, checkpoints ensure that cells maintain genome integrity (remain euploid) and withstand episodic DNA damage and delays in DNA replication.29 Studies using yeast mutants have defined multiple checkpoints for the G1, S, and G2/M phases of the cell cycle, and homologues of these checkpoints have now been defined in mammalian cells.29-34 Each checkpoint may serve multiple functions. For example, the G2/M checkpoint serves (1) to delay mitosis or arrest cells in response to DNA damage; (2) to maintain the dependence of mitosis on chromosome replication; and (3) to make the exit from mitosis dependent on completion of spindle assembly and proper chromosome segregation.33
Many investigators have speculated that the observed G2/M accumulation of FA cells after low-dose MMC treatment is caused by defective cell cycle checkpoint control and that the FA genes may directly regulate one or more checkpoints.2,3,35-46 Such speculation has been fueled by the report that the protein product of a novel cyclin-related gene is able to correct the repression of DNA synthesis seen in FA(A) fibroblasts after treatment with psoralen and ultraviolet-A light.39 Others suggest that the observed cell cycle abnormalities are only secondary to DNA damage and that FA genes serve to prevent and/or repair DNA damage.6 To distinguish between these alternatives, we sought to determine whether the aberrant G2/M arrest in FA(C) cells is a manifestation of primary cell cycle checkpoint dysfunction. For the studies described here we have used lymphoblast cell lines derived from patients with various mutations of the FAC gene, retrovirally transduced isogenic cells, and cells from normal individuals. Based on these studies we argue that the aberrant G2/M arrest that characterizes the response of FA(C) cells to low doses of cross-linking agents is a secondary result of excessive DNA damage in these cells.
MATERIALS AND METHODS
Plasmids
pLXSN.
This retroviral vector was the generous gift of Dr A.D. Miller (Fred Hutchinson Cancer Research Center, Seattle, WA) and has been previously described.47
pLFACSN.
A 314-bp polymerase chain reaction (PCR) product (FAC ATG start to EcoRI site [bases 256-569 Genbank sequence X66893]) was cloned into the PCR Script SK(+) vector (Stratagene, LaJolla, CA) to form PCR-Script FAC ATG-RI. The sense primer was designed to add anXho I site just 5′ of the FAC start codon. A 1,452-bpHindIII to Xba I (stop codon) fragment of FACcDNA derived from pFAC3 was cloned into the HindIII andXba I sites of pUC18 to create pUC FAC-D1. The pFAC3 plasmid contains the entire human FAC cDNA sequence and was the generous gift of Dr Manuel Buchwald (The Hospital for Sick Children, Toronto, Ontario, Canada).12 pLFACSN was constructed by performing a three-way ligation of an XhoI/EcoRI fragment from PCR-Script FAC ATG-R1, anEcoRI/BamHI fragment from pUC FAC-DI, andXhoI/BamHI linearized pLXSN plasmid. pLFACSN contains the full-length coding sequence cDNA for human FAC.
Retroviral Vector Amplification
Purified pLFACSN or pLXSN plasmid DNA (10 μg) was transfected as a calcium phosphate precipitate, using protocol A as previously described, into culture dishes that contained a 1:1 mixture of Ψ-2 and PA12 cells.48 Supernatants from the ping-pong culture were obtained, filtered, and used for transduction of FA(C) lymphoblasts. During the transduction procedure, polybrene was added to achieve a final concentration of 8 μg/mL. Cell lines were exposed to supernatants 4 to 6 times before beginning selection in G418.
Cell Lines
Epstein-Barr virus (EBV)–transformed lymphoblast cell lines HSC536N, PD4L, and PD149L were provided by the Fanconi Anemia Cell Repository (Oregon Health Sciences University). The HSC536N cell line has an L554P mutation of one allele and a deletion of the second FAC allele. The PD4L cell line has a delta G322 mutation of one allele and an R185X mutation of the other allele. The PD149L cell line has the IVS4+4 A → T mutation on both alleles.49 HSC536N pLFACSN and HSC536N pLXSN were produced by transducing HSC536N cells with amphotropic retrovirus generated by transfecting packaging cell lines with the retroviral constructs pLFACSN and pLXSN, respectively (see above). PD4L pLFACSN and PD4L pLXSN were produced in the same way. The EBV-transformed cell line JY (gift of Dr Richard Maziarz, Oregon Health Sciences University) was derived from a normal individual and has been described in detail elsewhere.50 All lymphoblast cell lines were grown in RPMI-1640 (GIBCO-BRL, Grand Island, NY) supplemented with 15% heat-inactivated low-endotoxin fetal bovine serum (FBS; Hyclone, Logan, UT), 1% glutamine (GIBCO-BRL), and 50 μg/mL gentamicin (GIBCO-BRL). Ψ-2 ecotropic-packaging cells51and PA12 cells amphotrophic-packaging cells52 were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS.
MMC Cytotoxicity Assay
Cellular sensitivity to MMC was assayed by plating cells at a density of 2 × 105/mL in 96-well plates. Increasing doses of MMC were added to the cells, which were then incubated in the dark for 120 hours. Following treatment, cellular viability was determined using an XTT-based assay as described previously.45 Each sample was tested in replicates of 12.
Cytogenetic Studies
Lymphoblast cultures were treated with doses of MMC from 0 to 400 ng/mL and incubated in the dark for 48 hours. Cultures were obtained after a 1-hour exposure to 0.25 μg/mL colcemid. After a 10-minute treatment with 0.075 mol/L KCl, the cells were fixed with a 3:1 mixture of methanol:acetic acid. Wet-mount slides were prepared, air-dried, and stained with Wright's stain. Fifty metaphase figures from each culture were scored for DNA breaks (gaps greater than one chromatid width) and radial formation. A radial was defined as a chromatid interchange in which chromatids remained paired in mitosis giving rise to a structure with multiple arms.22
Cell Cycle Analysis
Cells were obtained and resuspended in 100 μL of Dulbecco's phosphate buffered saline and transferred to a polystyrene tube. Following transfer, 250 μL of pH 7.2 propidium iodide (PI) stain (50 μg/mL PI, 30 mg/mL polyethylene glycol 8000, 2 μg/mL RNase A, 0.1% Triton X-100, 36 mmol/L sodium citrate) was added to each tube, and the cells were incubated at 37°C for 20 minutes. Next, 250 μL of PI salt solution (50 μg/mL PI, 30 mg/mL polyethylene glycol 8000, 0.1% Triton X-100, 0.38 mol/L NaCl) was added, and the samples were incubated at 4°C for 10 minutes. Following staining, the samples were analyzed for DNA content using a Becton Dickinson FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Data were analyzed by the Multicycle software program, which uses the polynomial S-phase algorithm (Phoenix Flow Systems, San Diego, CA).53
RESULTS
Retroviral Transduction of FAC cDNA Normalizes FA(C) Lymphoblast Cell Cycle Response to MMC
We inserted the human FAC cDNA into the retroviral vector pLXSN to create pLFACSN. Amphotropic virus was generated by transfection of a coculture of ecotropic and amphotropic retroviral packaging cells (ping-pong amplification) with pLXSN or pLFACSN. FA(C) lymphoblasts were exposed to cell-free supernatants from a ping-pong culture, and stably transduced cells were selected in G418 (250 μg/mL). To confirm that retroviral transduction of FAC cDNA resulted in phenotypic correction of cell lines derived from FA patients, we measured the effect of MMC on cell viability. FA(C) cells transduced with pLFACSN amphotropic retrovirus had normal sensitivity to MMC, whereas FA(C) cells transduced with pLXSN amphotropic retrovirus remained as abnormally sensitive to MMC as the original nontransduced cell lines (data not shown). Expression of retrovirally derived FAC mRNA and protein was verified by Northern and Western blot analysis, respectively (data not shown).
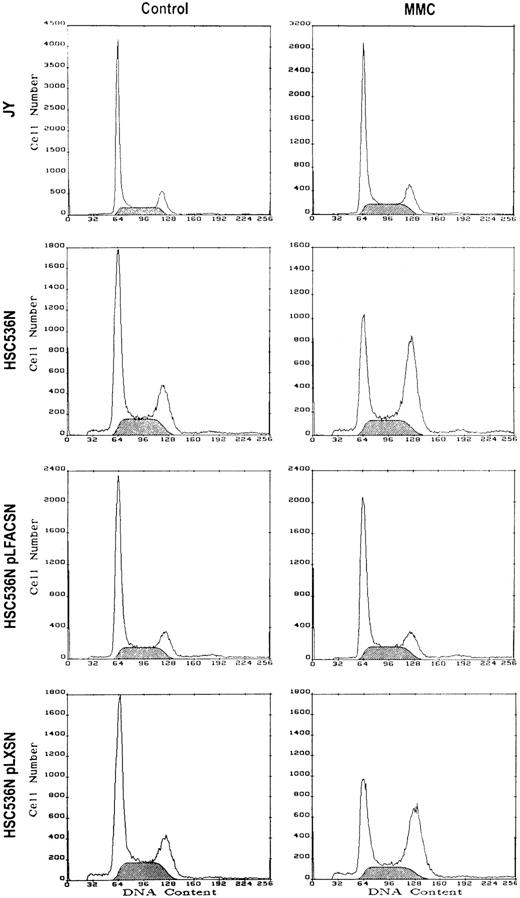
Exposure of lymphoblast cell lines derived from normal donors to low doses of MMC (eg, 30 to 40 ng/mL) for 2 hours had no significant effect on cell cycle kinetics (Fig 1, Table1). In particular, there was no increase in the percentage of cells in the G2/M compartment 24 hours after MMC treatment. In contrast, lymphoblast cell lines derived from FA(C) donors accumulate in the G2/M compartment after exposure to low-dose MMC. Transduction of FA(C) lymphoblasts with retrovirus encoding normalFAC cDNA resulted in normalization of cell cycle kinetics after MMC exposure. Similar degrees of correction were seen in two different FA(C) cell lines with different genotypes. Transduction of FA(C) lymphoblasts with retrovirus lacking the FAC cDNA sequence did not normalize the cell cycle response of these cells to low-dose MMC exposure.
FAC gene replacement normalizes the cell cycle response of FA(C) lymphoblasts to low-dose MMC. Cells were treated with control medium or medium containing 40 ng/mL MMC for 2 hours. Following treatment, the cells were washed three times and placed back in culture in complete medium. After 24 hours the cells were obtained and DNA content measured by PI staining and flow cytometry. Results of a representative experiment are shown. The shaded area represents cells in S phase.
FAC gene replacement normalizes the cell cycle response of FA(C) lymphoblasts to low-dose MMC. Cells were treated with control medium or medium containing 40 ng/mL MMC for 2 hours. Following treatment, the cells were washed three times and placed back in culture in complete medium. After 24 hours the cells were obtained and DNA content measured by PI staining and flow cytometry. Results of a representative experiment are shown. The shaded area represents cells in S phase.
FAC Gene Replacement Normalizes the Cell Cycle Response of FA(C) Lymphoblasts to Low-Dose MMC
| Cell Line . | MMC (ng/mL) . | Delta G2/M% (mean ± SD) . |
|---|---|---|
| JY | 30 | 1.9 ± 0.7 (n = 3) |
| HSC536N | 30 | 18.3 ± 6.1 (n = 3) |
| HSC536N pLFACSN | 30 | 0.8 ± 0.8 (n = 4) |
| HSC536N pLXSN | 30 | 26.9 ± 0.3 (n = 2) |
| JY | 40 | 2.8 ± 2.5 (n = 4) |
| PD4L | 40 | 11.4 ± 1.5 (n = 3) |
| PD4L pLFACSN | 40 | 0.8 ± 0.1 (n = 2) |
| PD4L pLXSN | 40 | 7.3 ± 0.1 (n = 2) |
| Cell Line . | MMC (ng/mL) . | Delta G2/M% (mean ± SD) . |
|---|---|---|
| JY | 30 | 1.9 ± 0.7 (n = 3) |
| HSC536N | 30 | 18.3 ± 6.1 (n = 3) |
| HSC536N pLFACSN | 30 | 0.8 ± 0.8 (n = 4) |
| HSC536N pLXSN | 30 | 26.9 ± 0.3 (n = 2) |
| JY | 40 | 2.8 ± 2.5 (n = 4) |
| PD4L | 40 | 11.4 ± 1.5 (n = 3) |
| PD4L pLFACSN | 40 | 0.8 ± 0.1 (n = 2) |
| PD4L pLXSN | 40 | 7.3 ± 0.1 (n = 2) |
Lymphoblast cells lines were treated with MMC for 2 hours, washed extensively, and cultured in complete medium. After 24 hours of culture cells were harvested and cell cycle parameters measured by PI staining of DNA content followed by flow cytometry. Delta G2/M% = G2/M% (MMC) − G2/M% (control). Cumulative results are shown as mean ± 1 SD.
Normal and FA(C) Lymphoblasts Have Similar G2/M Compartment Accumulations in Response to Equivalent Amounts of MMC-Induced Cytogenetic Damage
The above results in isogenic pairs are consistent with the notion that the FAC gene encodes a protein that helps regulate G2/M cell cycle progression. However, because FA(C) lymphoblasts are known to have cellular hypersensitivity to the DNA damaging effects of MMC, the prominent G2/M accumulation in FA(C) cells might be a secondary rather than a primary phenomenon. To test the hypothesis that FA(C) cells have a normal cell cycle response to DNA damage induced by MMC, we compared the cell cycle response of normal and FA(C) lymphoblasts with doses of MMC that induced equivalent amounts of DNA damage. Rao et al have previously reported that G2/M compartment accumulation in normal cells treated with BCNU, CCNU, or VM26 was directly related to the percentage of cells with cytogenetic abnormalities.54 55 Therefore, we sought to define experimental conditions that would induce cytogenetic abnormalities in normal cells that were quantitatively and qualitatively similar to those seen in FA(C) lymphoblasts treated with low doses of MMC.
We found that treatment of wild-type or mock-corrected FA(C) cells with 20 to 40 ng/mL of MMC for 48 hours induced the same quantitative and qualitative cytogenetic abnormalities as seen in normal orFAC-corrected FA(C) cells treated with 200 to 400 ng/mL of MMC for 48 hours. We analyzed cell cycle parameters for cells treated with these doses of MMC for 6 to 72 hours. G2/M accumulation was present by 18 to 24 hours and persisted throughout the treatment period. There was a gradual increase in G2/M accumulation over the last 48 hours of the time course. These data are consistent with previous reports of prolonged MMC treatment of cells in which the G2/M accumulation is caused not only by a prolongation of the G2/M phase but also by complete arrest of some cells in the G2/M during each passage through the cell cycle.37 56 The rate of G2/M accumulation during MMC treatment was equivalent in normal, FA(C), mock-corrected FA(C), and FAC-corrected FA(C) cells. Because the 48-hour treatment protocol yielded the most consistent cytogenetic results, this time period was selected for use in our subsequent experiments.
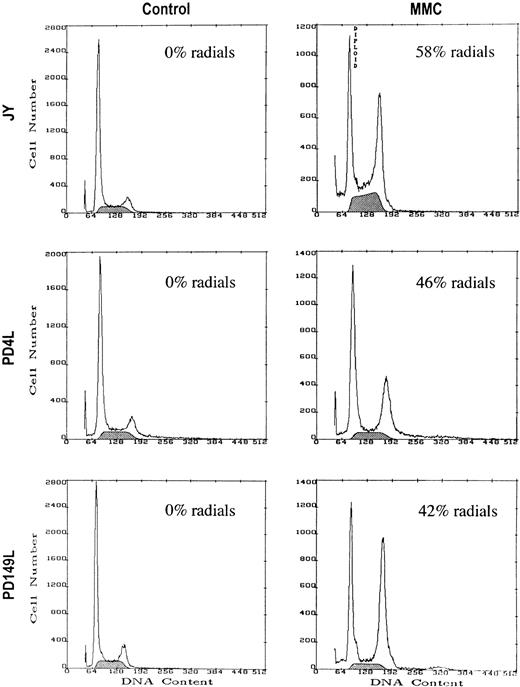
Using these defined conditions, we treated cells with control medium or medium supplemented with MMC for 48 hours and then harvested the cells for simultaneous cytogenetic and cell cycle analysis (Fig2, Table 2).Similar degrees of G2/M compartment accumulation were seen in normal and FA(C) lymphoblasts that had equivalent amounts of MMC-induced DNA damage; however, the S-phase compartment response differed dramatically. FA(C) cells treated with MMC had increases in G2/M compartment accumulation but substantial decreases in the percent of cells in S phase with either no change or a slight increase in the G1/S ratio. In contrast, normal or FAC-corrected cells treated with MMC had increased G2/M accumulation and an increased percent of cells in S phase associated with a significant decrease in the G1/S ratio. The appearance of a discrete peak of cells in the later half of S phase suggested that there was a cohort of cells that were synchronized in late S phase. This was confirmed by Chi-squared analysis of the cell cycle data to select the most appropriate mathematical cell cycle model (data not shown). Continuous exposure of normal orFAC-corrected cells to doses of MMC greater than 400 ng/mL resulted in S-phase arrest and a loss of G2/M accumulation (data not shown).
Normal and FA(C) cells with similar amounts of MMC-induced cytogenetic abnormalities have similar G2/M accumulation. Cells were treated with control medium or medium containing MMC for 48 hours. JY cells were treated with 300 ng/mL of MMC, and PD149L and PD4L cells were treated with 40 ng/mL of MMC. After 48 hours the cells were harvested and DNA content measured by PI staining and flow cytometry. Cytogenetic analysis was performed on an aliquot of each sample. Results of a representative experiment are shown. The shaded area represents cells in S phase.
Normal and FA(C) cells with similar amounts of MMC-induced cytogenetic abnormalities have similar G2/M accumulation. Cells were treated with control medium or medium containing MMC for 48 hours. JY cells were treated with 300 ng/mL of MMC, and PD149L and PD4L cells were treated with 40 ng/mL of MMC. After 48 hours the cells were harvested and DNA content measured by PI staining and flow cytometry. Cytogenetic analysis was performed on an aliquot of each sample. Results of a representative experiment are shown. The shaded area represents cells in S phase.
Normal and FA(C) Cells With Similar Amounts of MMC-Induced Cytogenetic Abnormalities Have Similar G2/M Accumulation
| Cell Line . | MMC Dose (ng/mL) . | Delta G2/M% . | Delta S% . | DNA Damage (breaks/cell) . | DNA Damage (% radials) . |
|---|---|---|---|---|---|
| JY | 200 | 25.6 ± 0.9 | 2.80 ± 1.8 | 0.89 | 24 |
| PD4L | 40 | 28.4 ± 1.1 | −6.10 ± 1.1 | 1.29 | 44 |
| JY | 200 | 20.0 ± 1.0 | 8.4 ± 2.0 | 1.38 | 36 |
| JY | 300 | 28.0 ± 1.2 | 8.9 ± 2.3 | 2.19 | 58 |
| PD149L | 40 | 40.1 ± 1.1 | −13.1 ± 1.6 | 1.86 | 42 |
| PD4L | 20 | 14.6 ± 1.2 | −9.1 ± 2.1 | 1.73 | 34 |
| PD4L | 40 | 19.4 ± 1.6 | −8.0 ± 2.6 | 2.89 | 46 |
| PD4L | 10 | 20.8 ± 1.9 | −11.6 ± 3.1 | 1.22 | 10 |
| PD4L | 40 | 30.2 ± 2.2 | −11.7 ± 3.3 | 3.14 | 30 |
| PD4L pLXSN | 10 | 14.3 ± 1.6 | −8.8 ± 3.2 | 1.07 | 10 |
| PD4L pLXSN | 40 | 21.3 ± 2.3 | −16.7 ± 2.7 | 2.41 | 32 |
| PD4L pLFACSN | 200 | 21.6 ± 1.7 | 8.6 ± 3.1 | 0.91 | 14 |
| PD4L pLFACSN | 400 | 30.4 ± 2.2 | 10.8 ± 4.0 | 1.85 | 34 |
| Cell Line . | MMC Dose (ng/mL) . | Delta G2/M% . | Delta S% . | DNA Damage (breaks/cell) . | DNA Damage (% radials) . |
|---|---|---|---|---|---|
| JY | 200 | 25.6 ± 0.9 | 2.80 ± 1.8 | 0.89 | 24 |
| PD4L | 40 | 28.4 ± 1.1 | −6.10 ± 1.1 | 1.29 | 44 |
| JY | 200 | 20.0 ± 1.0 | 8.4 ± 2.0 | 1.38 | 36 |
| JY | 300 | 28.0 ± 1.2 | 8.9 ± 2.3 | 2.19 | 58 |
| PD149L | 40 | 40.1 ± 1.1 | −13.1 ± 1.6 | 1.86 | 42 |
| PD4L | 20 | 14.6 ± 1.2 | −9.1 ± 2.1 | 1.73 | 34 |
| PD4L | 40 | 19.4 ± 1.6 | −8.0 ± 2.6 | 2.89 | 46 |
| PD4L | 10 | 20.8 ± 1.9 | −11.6 ± 3.1 | 1.22 | 10 |
| PD4L | 40 | 30.2 ± 2.2 | −11.7 ± 3.3 | 3.14 | 30 |
| PD4L pLXSN | 10 | 14.3 ± 1.6 | −8.8 ± 3.2 | 1.07 | 10 |
| PD4L pLXSN | 40 | 21.3 ± 2.3 | −16.7 ± 2.7 | 2.41 | 32 |
| PD4L pLFACSN | 200 | 21.6 ± 1.7 | 8.6 ± 3.1 | 0.91 | 14 |
| PD4L pLFACSN | 400 | 30.4 ± 2.2 | 10.8 ± 4.0 | 1.85 | 34 |
Cells were incubated with various doses of MMC for 48 hours. After treatment the cells were harvested and the samples divided for simultaneous cytogenetic and cell cycle analysis. The double lines divide independent experiments. Delta G2/M% = G2/M% (MMC) − G2/M% (control) and is expressed as the mean difference ± 95% confidence intervals. Delta S% = S% (MMC) − S% (control) and is expressed as the mean difference ± 95% confidence intervals. DNA breaks/cell = average number of DNA breaks per cell (for purposes of computation cells with radials were not included).
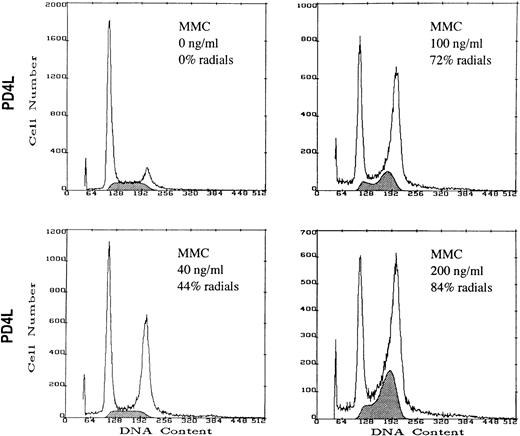
FA(C) Lymphoblasts Treated With High-Dose MMC Develop Both S Phase and G2/M Compartment Accumulation
The above results are consistent with the notion that regulation of the G2/M checkpoint is similar in both normal and FA(C) cells and that the degree of G2/M accumulation is directly related to the amount of DNA damage. To test whether an S-phase checkpoint was defective in FA(C) cells, we treated uncorrected FA(C) cells with the same doses of MMC that were required to induce cytogenetic abnormalities in normal or corrected FA(C) cells. In the presence of high-dose MMC (≥100 ng/mL continuous exposure for 48 hours) FA(C) cells develop both S-phase and G2/M-compartment accumulation. In normal or FAC-corrected FA(C) cells the S-phase accumulation always accompanied the G2/M-compartment accumulation and was only seen with high-dose MMC. In contrast, in uncorrected FA(C) cells the G2/M accumulation was seen with both low- and high-dose MMC, and the S-phase accumulation was seen only with high-dose MMC treatment.
Normal and FA(C) Lymphoblasts Have Similar Cell Cycle Responses to MMC Doses That Have Equivalent Cytotoxicity
We treated FAC-corrected and uncorrected FA(C) cell lines with a range of doses of MMC. Cell cycle parameters were measured after 24 and 48 hours of continuous exposure, and cytotoxicity was measured after 120 hours of continuous MMC exposure (the duration of our standard MMC cytotoxicity assay). Cell cycle responses of uncorrected, mock corrected, or FAC corrected HSC536N cells were similar when comparing doses of MMC that induced similar amounts of cytotoxicity (Table 3). Of note, G2/M accumulation occurred in both FA(C) and corrected FA(C) cells treated with doses of MMC that induced equivalent cytotoxicity. However, the G2/M accumulation was accompanied by either no significant change or a decrease in the percent of cells in S phase. The decrease in percent of cells in S phase was accompanied by a proportional decrease in the percent of cells in the G1 compartment so that the G1/S ratio did not change (data not shown). Thus, no abnormalities of G1/S or S-phase cell cycle checkpoints in response to these equitoxic doses of MMC was found in either mock- or FAC-corrected FA(C) lymphoblasts.
Treatment of FA(C) or FAC-Corrected FA(C) Cells With Equitoxic Doses of MMC Results in Similar G2/M Compartment Accumulation
| Cell Line . | MMC Dose (nmol/L) . | % Inhibition . | Delta G2/M% (24 h) . | Delta G2/M% (48 h) . | Delta S% (24 h) . | Delta S% (48 h) . |
|---|---|---|---|---|---|---|
| HSC536N | 4 | 73.3 ± 8.7 | 13.0 ± 2.4 | 7.2 ± 2.3 | −4.8 ± 4.5 | −4.9 ± 4.9 |
| HSC536N pLFACSN | 100 | 73.5 ± 4.1 | 11.9 ± 2.5 | 13.5 ± 2.5 | 3.7 ± 4.7 | 4.1 ± 3.7 |
| HSC536N | 8 | 89.4 ± 5.7 | 18.3 ± 1.5 | 16.9 ± 3.3 | −9.5 ± 3.6 | −9.8 ± 6.5 |
| HSC536N pLFACSN | 125 | 91.9 ± 1.7 | 13.7 ± 3.5 | 16.6 ± 1.8 | 4.3 ± 6.5 | −11.3 ± 4.1 |
| Cell Line . | MMC Dose (nmol/L) . | % Inhibition . | Delta G2/M% (24 h) . | Delta G2/M% (48 h) . | Delta S% (24 h) . | Delta S% (48 h) . |
|---|---|---|---|---|---|---|
| HSC536N | 4 | 73.3 ± 8.7 | 13.0 ± 2.4 | 7.2 ± 2.3 | −4.8 ± 4.5 | −4.9 ± 4.9 |
| HSC536N pLFACSN | 100 | 73.5 ± 4.1 | 11.9 ± 2.5 | 13.5 ± 2.5 | 3.7 ± 4.7 | 4.1 ± 3.7 |
| HSC536N | 8 | 89.4 ± 5.7 | 18.3 ± 1.5 | 16.9 ± 3.3 | −9.5 ± 3.6 | −9.8 ± 6.5 |
| HSC536N pLFACSN | 125 | 91.9 ± 1.7 | 13.7 ± 3.5 | 16.6 ± 1.8 | 4.3 ± 6.5 | −11.3 ± 4.1 |
Cells were treated with control media or media containing MMC, and cell cycle parameters were measured at 24 and 48 hours and cellular proliferation at 120 hours. Percent inhibition = (1 − MMC proliferation/control proliferation) × 100 ± 1 SD. Delta G2/M% = G2/M% (MMC) − G2/M% (control) and is expressed as the difference ± 95% confidence intervals. Delta S% = S% (MMC) − S% (control) and is expressed as the difference ± 95% confidence intervals. The results of a representative experiment are shown.
High-dose MMC induces S-phase accumulation in uncorrected FA(C) lymphoblasts. PD4L cells were cultured in control medium or medium containing MMC for 48 hours. After 48 hours the cells were harvested and DNA content measured by PI staining and flow cytometry. Cytogenetic analysis was performed on an aliquot of each sample. The shaded area represents cells in S phase.
High-dose MMC induces S-phase accumulation in uncorrected FA(C) lymphoblasts. PD4L cells were cultured in control medium or medium containing MMC for 48 hours. After 48 hours the cells were harvested and DNA content measured by PI staining and flow cytometry. Cytogenetic analysis was performed on an aliquot of each sample. The shaded area represents cells in S phase.
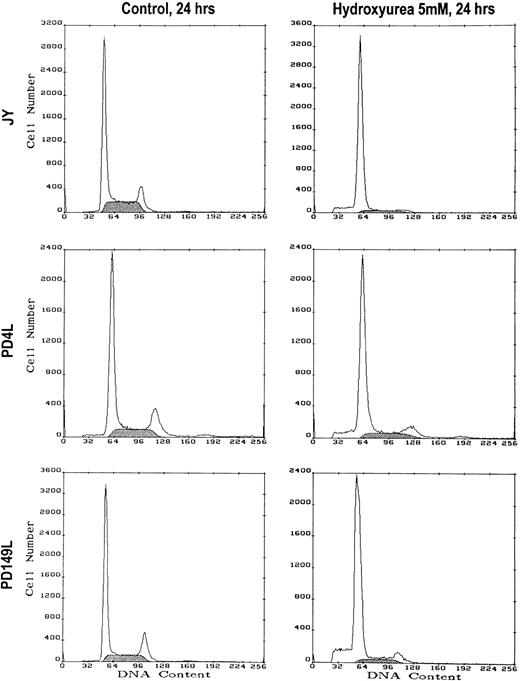
The S-Phase Checkpoint Response to Hydroxyurea (HU) Is Intact in FA(C) Lymphoblasts
S-phase checkpoints prevent cell cycle progression into the G2 compartment until DNA replication/repair is complete. To further test the function of these checkpoints in FA(C) cells, we treated cells with HU, which is known to inhibit the enzyme ribonucleotide reductase resulting in depletion of intracellular dNTP precursors and cell cycle arrest at the G1/S boundary.57,58 Yeast mutants have been described that lack normal S-phase checkpoint function and have abnormally high levels of cellular death after exposure to HU.29,32 34 We treated normal or FA(C) lymphoblasts with control media or media containing 1 or 5 mmol/L HU for 24 hours. At the end of the treatment period these cells were harvested for cell cycle analysis. Both normal and FA(C) lymphoblasts had similar degrees of cell synchronization at the G1/S boundary after treatment with HU (Table 4, Fig4).
HU Induces Identical Cell Cycle Responses in Normal and FA(C) Cells
| Cell Line . | HU Dose (mmol/L) . | %G1 . | %S . | G1/S Ratio . | %G2/M . |
|---|---|---|---|---|---|
| JY (n = 5) | 0 | 63.6 ± 7.2 | 28.6 ± 4.1 | 2.3 ± 0.7 | 7.7 ± 0.5 |
| JY (n = 5) | 5 | 87.2 ± 4.7 | 12.8 ± 4.7 | 7.8 ± 3.4 | 0.0 ± 0.0 |
| PD4L (n = 5) | 0 | 61.3 ± 2.4 | 25.5 ± 2.7 | 2.4 ± 0.3 | 13.3 ± 1.5 |
| PD4L (n = 5) | 5 | 80.5 ± 1.4 | 17.9 ± 1.6 | 4.5 ± 0.4 | 1.7 ± 1.7 |
| PD4L pLXSN (n = 3) | 0 | 62.9 ± 2.1 | 27.1 ± 2.5 | 2.3 ± 0.2 | 10.1 ± 2.7 |
| PD4L pLXSN (n = 3) | 5 | 85.4 ± 1.8 | 14.4 ± 2.0 | 6.0 ± 0.9 | 0.2 ± 0.3 |
| PD4L pLFACSN (n = 3) | 0 | 65.7 ± 3.7 | 28.4 ± 3.4 | 2.3 ± 0.4 | 5.8 ± 2.6 |
| PD4L pLFACSN (n = 3) | 5 | 81.9 ± 3.2 | 16.4 ± 2.2 | 5.1 ± 0.9 | 1.8 ± 2.2 |
| PD149L (n = 2) | 0 | 61.8 ± 1.2 | 24.4 ± 0.4 | 2.5 ± 0.1 | 13.8 ± 0.7 |
| PD149L (n = 2) | 5 | 83.4 ± 5.9 | 10.9 ± 2.7 | 8.0 ± 2.5 | 5.8 ± 3.2 |
| HSC536N (n = 4) | 0 | 62.3 ± 4.6 | 26.4 ± 4.2 | 2.4 ± 0.5 | 11.4 ± 0.6 |
| HSC536N (n = 4) | 5 | 76.4 ± 10.8 | 21.1 ± 10.5 | 4.8 ± 3.3 | 2.9 ± 1.5 |
| HSC536N pLXSN (n = 4) | 0 | 58.7 ± 4.2 | 30.3 ± 3.5 | 2.0 ± 0.3 | 11.1 ± 2.1 |
| HSC536N pLXSN (n = 4) | 5 | 74.8 ± 16.0 | 24.0 ± 14.8 | 4.8 ± 3.7 | 1.2 ± 1.7 |
| HSC536N pLFACSN (n = 3) | 0 | 55.6 ± 4.8 | 31.7 ± 3.3 | 1.8 ± 0.0 | 12.7 ± 8.1 |
| HSC536N pLFACSN (n = 3) | 5 | 73.4 ± 8.6 | 20.8 ± 11.4 | 4.3 ± 2.1 | 5.8 ± 5.8 |
| Cell Line . | HU Dose (mmol/L) . | %G1 . | %S . | G1/S Ratio . | %G2/M . |
|---|---|---|---|---|---|
| JY (n = 5) | 0 | 63.6 ± 7.2 | 28.6 ± 4.1 | 2.3 ± 0.7 | 7.7 ± 0.5 |
| JY (n = 5) | 5 | 87.2 ± 4.7 | 12.8 ± 4.7 | 7.8 ± 3.4 | 0.0 ± 0.0 |
| PD4L (n = 5) | 0 | 61.3 ± 2.4 | 25.5 ± 2.7 | 2.4 ± 0.3 | 13.3 ± 1.5 |
| PD4L (n = 5) | 5 | 80.5 ± 1.4 | 17.9 ± 1.6 | 4.5 ± 0.4 | 1.7 ± 1.7 |
| PD4L pLXSN (n = 3) | 0 | 62.9 ± 2.1 | 27.1 ± 2.5 | 2.3 ± 0.2 | 10.1 ± 2.7 |
| PD4L pLXSN (n = 3) | 5 | 85.4 ± 1.8 | 14.4 ± 2.0 | 6.0 ± 0.9 | 0.2 ± 0.3 |
| PD4L pLFACSN (n = 3) | 0 | 65.7 ± 3.7 | 28.4 ± 3.4 | 2.3 ± 0.4 | 5.8 ± 2.6 |
| PD4L pLFACSN (n = 3) | 5 | 81.9 ± 3.2 | 16.4 ± 2.2 | 5.1 ± 0.9 | 1.8 ± 2.2 |
| PD149L (n = 2) | 0 | 61.8 ± 1.2 | 24.4 ± 0.4 | 2.5 ± 0.1 | 13.8 ± 0.7 |
| PD149L (n = 2) | 5 | 83.4 ± 5.9 | 10.9 ± 2.7 | 8.0 ± 2.5 | 5.8 ± 3.2 |
| HSC536N (n = 4) | 0 | 62.3 ± 4.6 | 26.4 ± 4.2 | 2.4 ± 0.5 | 11.4 ± 0.6 |
| HSC536N (n = 4) | 5 | 76.4 ± 10.8 | 21.1 ± 10.5 | 4.8 ± 3.3 | 2.9 ± 1.5 |
| HSC536N pLXSN (n = 4) | 0 | 58.7 ± 4.2 | 30.3 ± 3.5 | 2.0 ± 0.3 | 11.1 ± 2.1 |
| HSC536N pLXSN (n = 4) | 5 | 74.8 ± 16.0 | 24.0 ± 14.8 | 4.8 ± 3.7 | 1.2 ± 1.7 |
| HSC536N pLFACSN (n = 3) | 0 | 55.6 ± 4.8 | 31.7 ± 3.3 | 1.8 ± 0.0 | 12.7 ± 8.1 |
| HSC536N pLFACSN (n = 3) | 5 | 73.4 ± 8.6 | 20.8 ± 11.4 | 4.3 ± 2.1 | 5.8 ± 5.8 |
Cells were treated with control media or media containing 5 mmol/L HU for 24 hours. Following treatment, cell cycle parameters were measured by propidium iodide staining and flow cytometry. G1/S ratio = G1%/S%. Data are represented as mean ± 1 SD. Similar results were obtained using 1 mmol/L HU (data not shown).
HU induces identical cell cycle responses in normal and FA(C) cells. Cells were cultured in control medium or medium containing 5 mmol/L HU. After 24 hours the cells were harvested and DNA content measured by PI staining and flow cytometry. Results of a representative experiment are shown. The shaded area represents cells in S phase.
HU induces identical cell cycle responses in normal and FA(C) cells. Cells were cultured in control medium or medium containing 5 mmol/L HU. After 24 hours the cells were harvested and DNA content measured by PI staining and flow cytometry. Results of a representative experiment are shown. The shaded area represents cells in S phase.
Radiation-Induced G1 and G2/M Checkpoints Function Normally in FA(C) Lymphoblasts
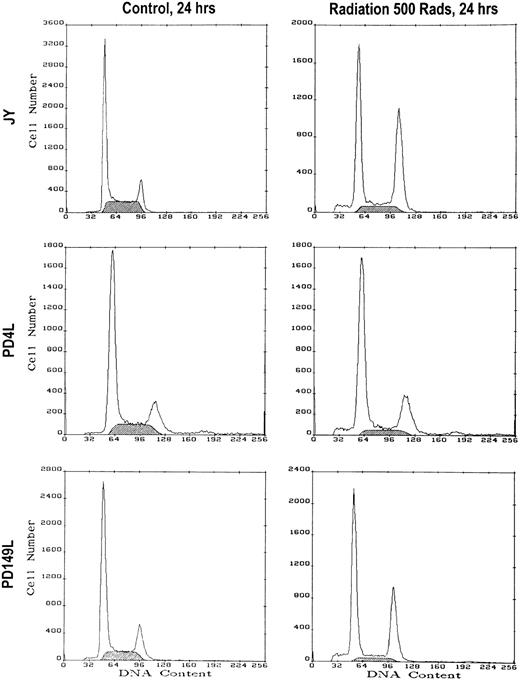
The above data strongly suggest that the G2/M accumulation seen in FA(C) lymphoblasts is secondary to appropriate cellular recognition of DNA damage and normal functioning of the G2 checkpoint. However, an alternative explanation is that FA cells have a defective checkpoint function in G1 or S phase such that cells with DNA damage abnormally progress through G1 and/or S phase and then appropriately arrest in G2/M. Therefore, we decided to test G1/S and G2/M checkpoint function in response to ionizing radiation, a genotoxic stressor known to induce both G1- and G2/M-compartment accumulation in normal cells.59-61 We treated normal and FA(C) lymphoblasts with 5 Gy of γ-irradiation and measured cell cycle parameters of control and irradiated cells after 6 and 24 hours (Fig5, Table 5).Both normal and FA(C) cells had similar cell cycle responses to 5 Gy of γ-irradiation.
γ-Irradiation induces identical cell cycle responses in normal and uncorrected FA(C) cell lines. Cells were treated with 500 rads of γ-irradiation. Following irradiation the cells were washed extensively and placed in culture in a 12-well plate. Twenty-four hours after irradiation the cells were harvested along with nonirradiated control cells. DNA content was measured by PI staining and flow cytometry. Results of a representative experiment are shown. The shaded area represents cells in S phase.
γ-Irradiation induces identical cell cycle responses in normal and uncorrected FA(C) cell lines. Cells were treated with 500 rads of γ-irradiation. Following irradiation the cells were washed extensively and placed in culture in a 12-well plate. Twenty-four hours after irradiation the cells were harvested along with nonirradiated control cells. DNA content was measured by PI staining and flow cytometry. Results of a representative experiment are shown. The shaded area represents cells in S phase.
γ-Irradiation Induces Identical Cell Cycle Responses in Normal, Uncorrected FA(C), FAC-Corrected FA(C) (pLFACSN), and Mock-Corrected FA(C) (pLXSN) Cell Lines
| Cell Line . | %G1 . | %S . | G1/S Ratio . | %G2/M . |
|---|---|---|---|---|
| JY control | 55.9 ± 6.5 | 36.7 ± 6.9 | 1.6 ± 0.6 | 7.4 ± 2.3 |
| JY radiation | 51.2 ± 4.9 | 14.0 ± 4.9 | 4.1 ± 1.6 | 34.9 ± 8.4 |
| PD149L control | 61.9 ± 1.0 | 26.7 ± 3.5 | 1.6 ± 0.6 | 11.3 ± 2.4 |
| PD149L radiation | 58.5 ± 1.2 | 4.6 ± 4.5 | 11.3 ± 4.2 | 37.0 ± 5.0 |
| PD4L control | 58.1 ± 4.0 | 32.4 ± 5.6 | 1.9 ± 0.5 | 9.5 ± 1.7 |
| PD4L radiation | 64.2 ± 3.0 | 16.1 ± 1.8 | 4.0 ± 0.7 | 19.7 ± 2.4 |
| PD4L pLXSN control | 61.4 ± 2.2 | 29.5 ± 2.1 | 2.1 ± 0.2 | 9.1 ± 1.0 |
| PD4L pLXSN radiation | 65.6 ± 3.1 | 20.1 ± 2.7 | 3.3 ± 0.6 | 14.4 ± 3.2 |
| PD4L pLFACSN control | 63.2 ± 3.8 | 28.2 ± 1.7 | 2.3 ± 0.3 | 8.6 ± 2.5 |
| PD4L pLFACSN radiation | 61.3 ± 1.8 | 19.5 ± 1.7 | 3.2 ± 0.3 | 19.3 ± 2.8 |
| HSC536N control | 53.6 ± 2.6 | 31.0 ± 4.0 | 1.7 ± 0.2 | 15.3 ± 6.2 |
| HSC536N radiation | 50.2 ± 5.4 | 12.4 ± 2.7 | 4.2 ± 0.7 | 37.3 ± 7.0 |
| HSC536N pLXSN control | 49.3 ± 1.4 | 37.0 ± 6.0 | 1.4 ± 0.2 | 11.3 ± 8.7 |
| HSC536N pLXSN radiation | 42.7 ± 4.9 | 13.4 ± 3.0 | 3.2 ± 0.4 | 43.9 ± 7.9 |
| HSC536N pLFACSN control | 44.8 ± 7.0 | 42.2 ± 13.8 | 1.2 ± 0.5 | 13.1 ± 8.9 |
| HSC536N pLFACSN radiation | 42.7 ± 8.0 | 16.3 ± 3.2 | 2.7 ± 0.4 | 41.0 ± 10.5 |
| Cell Line . | %G1 . | %S . | G1/S Ratio . | %G2/M . |
|---|---|---|---|---|
| JY control | 55.9 ± 6.5 | 36.7 ± 6.9 | 1.6 ± 0.6 | 7.4 ± 2.3 |
| JY radiation | 51.2 ± 4.9 | 14.0 ± 4.9 | 4.1 ± 1.6 | 34.9 ± 8.4 |
| PD149L control | 61.9 ± 1.0 | 26.7 ± 3.5 | 1.6 ± 0.6 | 11.3 ± 2.4 |
| PD149L radiation | 58.5 ± 1.2 | 4.6 ± 4.5 | 11.3 ± 4.2 | 37.0 ± 5.0 |
| PD4L control | 58.1 ± 4.0 | 32.4 ± 5.6 | 1.9 ± 0.5 | 9.5 ± 1.7 |
| PD4L radiation | 64.2 ± 3.0 | 16.1 ± 1.8 | 4.0 ± 0.7 | 19.7 ± 2.4 |
| PD4L pLXSN control | 61.4 ± 2.2 | 29.5 ± 2.1 | 2.1 ± 0.2 | 9.1 ± 1.0 |
| PD4L pLXSN radiation | 65.6 ± 3.1 | 20.1 ± 2.7 | 3.3 ± 0.6 | 14.4 ± 3.2 |
| PD4L pLFACSN control | 63.2 ± 3.8 | 28.2 ± 1.7 | 2.3 ± 0.3 | 8.6 ± 2.5 |
| PD4L pLFACSN radiation | 61.3 ± 1.8 | 19.5 ± 1.7 | 3.2 ± 0.3 | 19.3 ± 2.8 |
| HSC536N control | 53.6 ± 2.6 | 31.0 ± 4.0 | 1.7 ± 0.2 | 15.3 ± 6.2 |
| HSC536N radiation | 50.2 ± 5.4 | 12.4 ± 2.7 | 4.2 ± 0.7 | 37.3 ± 7.0 |
| HSC536N pLXSN control | 49.3 ± 1.4 | 37.0 ± 6.0 | 1.4 ± 0.2 | 11.3 ± 8.7 |
| HSC536N pLXSN radiation | 42.7 ± 4.9 | 13.4 ± 3.0 | 3.2 ± 0.4 | 43.9 ± 7.9 |
| HSC536N pLFACSN control | 44.8 ± 7.0 | 42.2 ± 13.8 | 1.2 ± 0.5 | 13.1 ± 8.9 |
| HSC536N pLFACSN radiation | 42.7 ± 8.0 | 16.3 ± 3.2 | 2.7 ± 0.4 | 41.0 ± 10.5 |
Cell cycle parameters were measured in untreated cells and cells that had been irradiated 24 hours previously. Irradiated cells received a dose of 500 rads. Similar results were obtained using cells that were analyzed 6 hours after radiation. G1/S ratio = G1%/S%. Values represent mean ± 1 SD. The cumulative results of four independent experiments are shown (except for JY [n = 5] and PD149L [n = 3]).
DISCUSSION
The excessive G2/M accumulation seen in FA cells, especially after DNA cross-linker exposure, is caused not only by a prolongation of the G2/M in each cell cycle but also by complete arrest of some cells in the G2/M phase.36,37,56 Hoehn et al noted that the minimum duration of the G2 phase during the first cell cycle of lymphocytes after phytohemagglutinin stimulation was 10.7 hours in FA patient samples and 5.2 hours in normal cells. Additionally, 19.5% of the cycling FA cells arrested after entering the first G2/M compartment as opposed to 2.7% of cycling lymphocytes isolated from normal subjects.56 FA lymphocytes also have delayed G2/M transit and increased arrest in the G2/M compartment of the second cell cycle compared with normal lymphocytes.36,37 Although initial reports suggested that FA cells had slowed transit through G1- and/or S-phase compartments, more recent analyses of FA cells using sensitive flow cytometry techniques have not found any evidence of such delays. Indeed, some investigators have noted that the G1 phase of the second cell cycle is abnormally short (3.7 hours v 6.1 hours in normal cells).37
We have recently described the phenotype of mice homozygous for a targeted deletion of exon 9 of the murine FAC gene. The cells from these mice have cellular hypersensitivity to DNA cross-linking agents and exhibit spontaneous and clastogen-induced chromosomal instability. Low-dose MMC treatment of splenocytes from homozygous mutant mice resulted in an G2/M-compartment accumulation similar to that seen in cells from FA(C) patients. No such accumulation was seen in cells from heterozygous mice treated with similar doses of MMC.20
In our studies we found that transduction of two different FA(C) lymphoblast cell lines that contained different types of inactivatingFAC mutations with a retroviral vector encoding wild-type humanFAC cDNA normalized the cell cycle response to low-dose MMC. In contrast, transduction of these cell lines with a control retroviral vector that lacked any FAC sequences did not alter the aberrant cell cycle response of these cells to low-dose MMC.
We sought, in the studies described here, to determine whether the G2/M arrest in FA cells reflects a secondary response to the increased amount of damaged DNA following exposure to DNA cross-linking agents. We compared the cell cycle response of control and FA(C) lymphoblastic cell lines with equivalent amounts of MMC-induced DNA damage. All cell types tested exhibited similar degrees of G2/M accumulation in response to equivalent amounts of cytogenetically quantified DNA damage. To further confirm these findings we also tested the cell cycle response of isogenic sets of uncorrected, mock- and FAC-corrected FA(C) lymphoblasts to doses of MMC that induced equivalent amounts of DNA damage. After exposure to doses of MMC that induced similar amounts of cytogenetically quantified DNA damage, all pairs of isogenic cell lines had equivalent accumulation of cells in the G2/M compartment. For each cell line there was a positive correlation between the degree of cytogenetic abnormalities and the amount of G2/M-compartment accumulation.
Normal and FA(C) cells did differ in the development of S-phase accumulation in response to equivalent amounts of MMC-induced cytogenetic damage. In FA(C) cells, significant cytogenetic and G2/M-compartment accumulation developed in response to low-dose MMC (40 ng/mL for 48 hours). With this dose of MMC the percent of cells in the S-phase compartment decreased significantly compared with untreated cells. High-dose MMC treatment of FA(C) cells resulted in both S-phase and G2/M-compartment accumulation. Treatment of normal or corrected FA(C) cells with high-dose MMC resulted in simultaneous development of cytogenetic abnormalities as well as both S-phase and G2/M-compartment accumulation. These data are consistent with defective S-phase checkpoint in FA(C) cells in response to MMC-induced DNA damage. However, it is also possible that high-dose MMC induces a different spectrum of DNA damage lesions than low-dose MMC and that the S-phase accumulation reflects an appropriate cell cycle response to these additional types of DNA damage.
To further test the cell cycle response of FA(C) cells to MMC-induced DNA damage, we exposed HSC536N and FAC-corrected HSC536N cells to doses of MMC that induced equivalent cytotoxicity. The dose of MMC used to treat FAC-corrected HSC536N cells (100 and 125 nmol/L = 33 and 41.67 ng/mL, respectively) was similar to the low-dose MMC doses used in our cytogenetic experiments. Under these conditions both wild-type and FAC-corrected HSC536N cells responded with similar increases in the G2/M compartment accompanied by decreases in the percent of cells in S phase. Thus, MMC-induced G2/M-phase arrest is not necessarily associated with S-phase arrest. Therefore, we conclude that the MMC induced G2/M accumulation seen in FA(C) cells is a normal response to excessive DNA damage and is not reflective of G2/M checkpoint dysfunction.
Our findings are largely in agreement with those of Kruyt et al, who investigated the effects of a 1-hour pulse of MMC on mock- andFAC-corrected HSC536N cells. Mock-corrected cells treated with a 1-hour pulse of 1 μmol/L MMC developed a marked G2/M accumulation.FAC-corrected cells treated with a 1-hour pulse of 10 μmol/L MMC developed similar amounts of apoptosis and G2/M accumulation as seen in mock-corrected HSC536N treated with a 1-hour pulse of 1 μmol/L MMC. In both conditions the G2/M accumulation was associated with increased phosphorylation of cdc2 and increases in cyclin B1 protein levels at 24 hours. In mock-corrected cells these protein changes were persistent over the time period of 24 to 48 hours, whereas in the FAC-corrected cells the cdc2 phosphorylation and cyclin B1 levels decreased somewhat after 24 hours. Treatment of mock- orFAC-corrected cells with higher doses of MMC (15 to 30 μmol/L 1-hour pulse exposure) resulted in S-phase arrest and decreased G2/M accumulation with associated induction of massive apoptosis, degradation of cyclin B1, and dephosphorylation of cdc2.42 62
Direct comparisons between the current study and the results of Kruyt et al are difficult because of differences in methodology. For example, in our cytotoxicity experiments the low-dose treatments consisted of 4 to 8 nmol/L continuous exposure as opposed to their 1 μmol/L 1-hour pulse exposure. In our cytotoxicity experiments the high-dose treatment consisted of 100 to 125 nmol/L continuous exposure as opposed to their 15 to 30 μmol/L 1-hour pulse exposure. The endpoints of the studies also varied as we used cytogenetic abnormalities and cytotoxicity to define equitoxic doses of MMC, whereas apoptosis was the endpoint used in their studies. However, in both the current study and the work of Kruyt et al equitoxic doses of MMC induced similar degrees of G2/M arrest in both mock- and FAC-corrected lymphoblasts. Additionally, both studies show that treatment of mock- orFAC-corrected FA(C) lymphoblasts with high doses of MMC result in S-phase arrest and loss of G2/M accumulation.42 62
To further test whether FA(C) cells have G1/S checkpoint dysfunction we used the ribonucleotide reductase inhibitor HU, which depletes the intracellular nucleotide pool resulting in cell cycle arrest at the G1/S boundary.57,58 The molecular mechanisms by which HU arrests cells are unknown but may be p53 dependent.63 Frias et al have recently reported that DNA synthesis in lymphocytes from patients with FA was hypersensitive to the inhibitory effects of HU. In their studies the complementation group of the patients was not specified.64 In our studies, treatment of normal,FAC-, or mock-corrected FA(C) lymphoblasts with HU resulted in equivalent degrees of cell cycle arrest at the G1/S boundary. Therefore, we conclude that the S-phase checkpoint that responds to incomplete replication of DNA is intact in FA cells and find no evidence of hypersensitivity of FA(C) lymphoblasts to HU.
To further test G1/S- and G2/M-checkpoint function in FA(C) cells we measured the effect of ionizing radiation (5 Gy) on cell cycle kinetics. γ-Irradiation of cells induces both G1 and G2/M accumulation, which is abrogated in p53 nullizygous cells. Cells nullizygous for p21 fail to arrest in G1 after γ-irradiation but have normal G2/M accumulation. Thus, p21 mediates in part the p53-dependent G1 arrest after γ-irradiation but is not essential for p53-dependent G2/M arrest.63 γ-Irradiation of normal and FA(C) lymphoblastic cell lines resulted in identical accumulation of cells in the G1 and G2/M compartment. To further confirm these findings we tested isogenic pairs of uncorrected, FAC-, or mock-corrected FA(C) cells. In all cases the cell cycle response to γ-irradiation was similar. Therefore, we conclude that the molecular mechanisms that comprise the cell cycle response to γ-irradiation, including induction of p53, are intact in FA(C) cells. Both radiation and MMC have been reported to induce p53 protein expression, which should lead to induction of p21 and G1 arrest.45 65 However, in our experiments the effect of MMC and radiation on G1/S transition was much less than that seen in response to HU.
These results with γ-irradiation are in agreement with those of Kruyt et al and Kupfer and D'Andrea, who found that FAC gene replacement did not affect γ-irradiation induction of p53 protein or G2/M arrest in FA(C) cell lines.42,45 Our results are also in agreement with those of Rosselli et al who found that γ-irradiation induced similar degrees of cell cycle disturbance in normal and FA(C) lymphoblasts. However, in their study no inductive effect of γ-irradiation on p53 protein expression was found, leading to the speculation that the observed G1 and G2/M cell cycle arrest in FA cells was p53 independent.66
Therefore, using low-dose MMC, γ-irradiation, or HU we are unable to show any evidence of primary G1/S or S-phase cell cycle checkpoint dysfunction in FA(C) cells. High-dose MMC induces S-phase arrest in both FA(C), normal, mock- and FAC-corrected FA(C) cells. This S-phase arrest may be caused by the induction of additional metabolic and/or DNA damage abnormalities beyond those induced by low-dose MMC. However, the S-phase checkpoint control mechanism activated by high-dose MMC is not directly related to FACfunction as it occurs in both FA(C), normal, mock-, andFAC-corrected FA(C) cells treated with high-dose MMC. These results are consistent with previous investigators who found G2/M delays and G2/M cell cycle arrest but relatively normal G1/S and S-phase progression in FA(C) cells.37,38 56
Based on our studies we propose the following model of MMC-induced cell cycle disturbance. Low-dose MMC (<100 ng/mL continuous exposure) induces G2/M delay and arrest. These results are seen in both normal, FA(C), mock-, and FAC-corrected FA(C) cells, but the magnitude of the G2/M arrest is greater in uncorrected FA(C) cells treated with equimolar doses of MMC. However, the magnitude of the G2/M arrest is identical in FA(C) and FAC-corrected FA(C) cells treated with equitoxic doses of MMC. High-dose MMC induces G2/M delay and S-phase accumulation in normal, FA(C), and FAC-corrected FA(C) cells. Still higher doses of MMC (>400 ng/mL continuous exposure) result in S-phase arrest and a loss of G2/M accumulation.
Recently, several reports have appeared concerning a possible role of the FAC polypeptide in regulating the function of the M-phase kinase cdc2. Kupfer and D'Andrea have reported that caffeine not only inhibited the G2/M accumulation seen in mock- or FAC-corrected FA(C) cells but also caused a FAC-corrected FA(C) cell line (HSC536N) to be come as sensitive to MMC as mock-corrected HSC536N cells. In contrast, caffeine did not further sensitize mock-corrected HSC536N to the cytotoxic effects of MMC.45 Because caffeine is known to stimulate the activity of the M-phase kinase cdc2,67 these authors argue that this differential response to caffeine is consistent with the notion that the FACpolypeptide regulates the G2/M checkpoint.
These results with caffeine are somewhat in conflict with previous studies concerning the effects of caffeine on FA cells. Pincheira et al found that caffeine dramatically increased the chromosomal breakage level in homozygote and heterozygote FA lymphocytes.68Seyschab et al found that caffeine completely resolved the G2 accumulation in FA cells but that the rescued cells arrested in G1 of the following cell cycle.38 Kruyt et al reported that caffeine released the low-dose pulse-MMC–treatment induced G2/M block in FA(C) lymphoblasts but did not protect these cells from undergoing apoptosis. FAC-corrected FA(C) cells treated with the same low-dose pulse-MMC treatment did not undergo apoptosis even in the presence of caffeine. Additionally, these investigators showed that caffeine regulation of cdc2 kinase activity was similar in both mock- and FAC-corrected HSC536N cells.62
Although caffeine is known to stimulate the kinase activity of cdc2, it also has other effects on cell biology, including alterations in calcium transport, inhibition of cAMP phosphodiesterase, possible inhibition of DNA repair mechanisms, and direction interactions of caffeine with DNA.69,70 Indeed, Kupfer and D'Andrea's data show that caffeine inhibits MMC-induced induction of p53 protein in both mock- and FAC-corrected HSC536N cells. These results suggest that caffeine might modulate either MMC-induced DNA damage and/or the signal transduction pathway by which DNA damage induces the expression of p53 protein.71 Because the effect of caffeine on cellular physiology is nonspecific, we feel that their studies with caffeine do not provide strong direct evidence that theFAC polypeptide regulates G2/M cell cycle checkpoint function.
More recently, Kupfer et al have reported that expression ofFAC protein is regulated during cell cycle progression with levels increasing during S phase to reach a maximum at the G2/M boundary and then declining during M phase. Additionally, wild-typeFAC polypeptide coimmunoprecipitated with cdc2 protein and could bind to cdc2 protein in vitro. In contrast, a mutant FACpolypeptide (L554P mutation) did not coimmunoprecipitate with cdc2 protein and did not bind to cdc2 protein in vitro.46However, the biological significance of these observations is unclear as no functional abnormality of the cdc2 protein in FA(C) cells has been shown. Even if FAC protein does associate with cdc2 in vivo it does not necessarily follow that FAC protein modulates the M-phase kinase activity of cdc2 (ie, to regulate cell cycle checkpoint function) as cdc2 might instead serve to regulateFAC protein activity. Another possibility is that FACprotein may modulate other activities of cdc2 such as participation in fas-mediated apoptosis.72,73 Such a model would be congruent with our recent observations that inactivation of theFAC gene augments interferon-γ–induced fas-mediated apoptosis in hematopoietic cells from FAC nullizygous mice and children with FA(C).21
Cell cycle abnormalities and hypersensitivity to DNA damaging agents can be linked through three distinct mechanisms. First, cells may be primarily defective in preventing and/or repairing DNA damage. In these cells the presence of unrepaired DNA will activate normal cell cycle checkpoints and result in secondary abnormal compartmental distribution of cells in the cell cycle, reversible or irreversible cell cycle arrest, and apoptosis. In the budding yeast Sacharomyces cerevesiae, temperature-sensitive mutants for DNA repair proteins, such as CDC9 DNA ligase, arrest in late S or G2 phase following culture at the restrictive temperature.29 Second, cells may have primary defects in the function of one or more checkpoints. In these cells, inappropriate cell cycle progression in the presence of unrepaired DNA damage leads to secondary hypersensitivity to genotoxic agents. Cell cycle distribution may be primarily abnormal or may subsequently become abnormal as cells arrest when they encounter a normally functioning checkpoint. For example, ataxia telangiectasia (AT) cells have defective S-phase checkpoint control and continue to replicate their DNA in the presence of DNA damage. As a consequence, cells as well as individuals with AT are hypersensitive to radiation and radiomimetic agents.74 Finally, cells may be defective in both DNA repair and checkpoint function. Such cells are hypersensitive to DNA damage because their DNA repair defect is exacerbated by inappropriate cell cycle progression, which may shorten the time available for DNA repair. S cerevesiae strains with temperature-sensitive mutations of a DNA repair/replication gene and a temperature insensitive mutation of checkpoint control function have been characterized. Cells with only the DNA repair/replication defect arrest in G2 and survive at the restrictive temperature, but the double mutant cells fail to arrest in G2 and rapidly die at the restrictive temperature.29
In our studies we have found no evidence of a primary cell cycle defect and believe that the aberrant G2/M arrest in FA(C) lymphoblasts after low-dose MMC treatment represents a normal cellular response to the excessive DNA damage incurred in such cells (ie, mechanism one as described above). Youssoufian has found that FA(C) lymphoblasts are hypersensitive to induction of DNA cross-links but have normal rates of DNA repair.75 Based on his and our studies we suggest that FA(C) lymphoblasts have a prerepair defect and that both the cell cycle and the DNA repair response to induced interstrand DNA cross-links is normal. Additional studies will be required to see if this is also true in other complementation groups. The gene that is mutated in FA(A) has recently been cloned by two groups,76,77 and the gene for FA(D) has been localized to chromosome 3p22-26.78 The cloning of FA genes from the other four known complementation groups will allow the testing of cell cycle control in uncorrected and corrected isogenic cell lines.
Supported in part by NHLBI Program Project Grant No. 1PO1HL48546 and grants from the NIH (CA36306 and HL48546), Fanconi Anemia Research Foundation, and Merit Review Grants from the Department of Veterans Affairs (M.C.H. and G.C.B.). M.C.H. is the recipient of a Research Associate Career Development Award from the Department of Veterans Affairs.
Address reprint requests to Michael C. Heinrich, MD, 151B 3710 SW US Veterans Hospital Road, Portland, OR 97207.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal