Abstract
Cerebrovascular accident (CVA) is a major complication of sickle cell disease. The incidence and mortality of and risk factors for CVA in sickle cell disease patients in the United States have been reported only in small patient samples. The Cooperative Study of Sickle Cell Disease collected clinical data on 4,082 sickle cell disease patients enrolled from 1978 to 1988. Patients were followed for an average of 5.2 ± 2.0 years. Age-specific prevalence and incidence rates of CVA in patients with the common genotypes of sickle cell disease were determined, and the effects of hematologic and clinical events on the risk of CVA were analyzed. The highest rates of prevalence of CVA (4.01%) and incidence (0.61 per 100 patient-years) were in sickle cell anemia (SS) patients, but CVA occurred in all common genotypes. The incidence of infarctive CVA was lowest in SS patients 20 to 29 years of age and higher in children and older patients. Conversely, the incidence of hemorrhagic stroke in SS patients was highest among patients aged 20 to 29 years. Across all ages the mortality rate was 26% in the 2 weeks after hemorrhagic stroke. No deaths occurred after infarctive stroke. Risk factors for infarctive stroke included prior transient ischemic attack, low steady-state hemoglobin concentration and rate of and recent episode of acute chest syndrome, and elevated systolic blood pressure. Hemorrhagic stroke was associated with low steady-state hemoglobin and high leukocyte count.
CEREBROVASCULAR accident (CVA) is a catastrophic complication of sickle cell disease (SCD) and a leading cause of death in both children1 and adults.2The reported risk of first CVA in the first 20 years of life is 0.761 per 100 patient-years.3 A cohort study in Jamaica estimated the prevalence of CVA to be 7.8% among 310 patients of all genotypes aged 9 to 17 years who were observed since birth.4
Among patients with the common genotypes of SCD, CVA is most frequent in those with sickle cell anemia (SS).5 The rate of CVA for patients with other genotypes (SC, S-β+ thalassemia, and S-β0 thalassemia) has not been reported. The influence of age, clinical events, and hematologic and genetic factors on the risk of CVA needs to be clarified so that accurate counseling can be provided to patients, their families, and couples at risk for producing children with these genotypes.
To address these and other questions related to CVA, the Cooperative Study of Sickle Cell Disease (CSSCD) prospectively collected data on CVA in a large cohort of patients.6 7 These data comprise the largest series of CVAs in a group of SCD patients. The younger patients in this cohort were observed since birth and provide the most accurate rates of CVA in children with SCD living in the United States. In this report, the prevalence and incidence of CVA and the effects of age, genotype, and other risk factors are described.
MATERIALS AND METHODS
The CSSCD, a longitudinal clinical study, observed in its first phase 4,082 patients from 23 clinical centers across the United States between October 1978 and September 1988. The study was approved by the Institutional Review Boards of the participating centers. The study design, recruitment process, and characteristics of patients enrolled are described in detail elsewhere.6-8 In addition to newborns, all patients who had visited a participating clinic for any medical reason between 1975 and 1978 were eligible subjects. Enrollment was closed in May 1981, except for newborns who were enrolled throughout the study period. Patients were observed for an average of 5.2 ± 2.0 years. A hemoglobin (Hb) diagnosis was not confirmed for 139 of the 4,082 patients because they were on transfusion therapy; 31 of the 139 and 122 other patients had had at least one CVA before study entry. An additional 174 patients were observed for routine visits but not clinical events. Estimates of the incidence of CVA were based on the remaining 3,647 patients. The genotype distribution of the patient sample at entry is shown in Table 1.
Patient Sample at Entry
| Hb Type . | N . | % . | Mean Age ± SD (yr) . |
|---|---|---|---|
| SS | 2,675 | 67.8 | 14.1 ± 12.2 |
| SC | 873 | 22.1 | 13.5 ± 13.9 |
| S-β+ thalassemia | 203 | 5.2 | 16.2 ± 13.2 |
| S-β0 thalassemia | 192 | 4.9 | 15.6 ± 11.6 |
| Total | 3,943 | 100 | 14.2 ± 12.7 |
| Unknown Hb type | 139 | — | 10.9 ± 13.3 |
| Grand total | 4,082 | — | 14.1 ± 12.7 |
| Hb Type . | N . | % . | Mean Age ± SD (yr) . |
|---|---|---|---|
| SS | 2,675 | 67.8 | 14.1 ± 12.2 |
| SC | 873 | 22.1 | 13.5 ± 13.9 |
| S-β+ thalassemia | 203 | 5.2 | 16.2 ± 13.2 |
| S-β0 thalassemia | 192 | 4.9 | 15.6 ± 11.6 |
| Total | 3,943 | 100 | 14.2 ± 12.7 |
| Unknown Hb type | 139 | — | 10.9 ± 13.3 |
| Grand total | 4,082 | — | 14.1 ± 12.7 |
Laboratory diagnosis.
The Centers for Disease Control (CDC) determined the Hb phenotype by cellulose acetate and citrate agar electrophoresis methods9and the presence of β-thalassemia by quantification of Hb A2 levels using column chromatography.10 The percentage of fetal Hb (Hb F) was measured using the method of alkali denaturation11 by the CDC for patients entered at 2 years of age and older and by local centers for younger patients. Steady-state complete blood counts were performed at local centers from samples taken during routine clinic visits. α-Globin gene mapping to determine the presence of a thalassemia was performed on samples from 2,002 of the 2,675 SS patients (75%) by one of us (S.E.) using the blot hybridization method.12 13
Definition of clinical syndromes.
CVA was defined as an acute neurologic syndrome secondary to occlusion of an artery or hemorrhage with resultant ischemia and neurologic symptoms and signs. In this study, CVA included transient ischemic attack (TIA), completed infarctive stroke (neurologic deficits lasting more than 24 hours), and hemorrhagic stroke. TIA was defined as neurologic signs with vascular distribution that resolve within 24 hours (or 48 hours if basilar system is involved). Strokes were classified by the investigator at the center as hemorrhagic or infarctive based on the available clinical and imaging studies. Ninety-five percent of the patients classified as having infarctive stroke underwent computer tomography (CT) scan, brain scan, and/or magnetic resonance imaging (MRI; 1 patient) at the time of the event. Ninety-three percent of the patients classified as having hemorrhagic stroke underwent CT scan, brain scan, arteriogram, and/or autopsy after the event. MRI information was not collected during the first 8 years of the CSSCD (ie, before December 1986). Studies were performed uniformly across all ages.
The CSSCD definitions of acute anemic episode, acute chest syndrome, meningitis, and painful event are presented elsewhere.14Seizure included major or minor motor seizures or psychomotor seizures that were not secondary to central nervous system infection, tumor, or stroke. Priapism was defined as a painful erection of the penis lasting more than 1 hour. Only events severe enough to bring the patient to seek medical care were recorded.
Statistical analyses.
Crude and age-specific prevalence rates were calculated as the number of patients with at least one CVA before study entry divided by the total number of patients in the relevant subgroup. Crude incidence rates of the first CVA on study were calculated as the number of first CVAs occurring during the specified time interval divided by the number of person-years of observation in the relevant subgroup. The direct method of standardization was used for all age-adjusted rates, with the age distribution of the entire study sample used as the standard. Incidence rates were compared using a test of binomial proportions.15 The SAS macro SMOOTH (Paul Allison, University of Pennsylvania) was used to obtain kernel-smoothed hazard estimates for CVA as a function of age.16 Event-free survival curves were estimated using the Kaplan-Meier method, with adjustment of the risk sets to account for differing entry ages,17 and age at CVA used as the time measure. The proportional hazards model score function test was used to compare survival curves.
Cox proportional hazards regression with risk set adjustment was used to determine the risk factors for an initial CVA. Separate models were fit for hemorrhagic and completed infarctive CVA. For all patients who did not experience a CVA, observation time was truncated at the earliest of the following: the end of the study, the time of loss to follow-up, or the date of death from any cause. Potential covariates were gender, systolic blood pressure, Hb F level, mean Hb level, mean leukocyte count, mean platelet count, α-thalassemia; history of meningitis; presentation for seizure, surgery, priapism, acute anemia, acute chest syndrome, and transfusion within 2 weeks before CVA; and rates of painful episodes and acute chest syndrome. Mean values were calculated using all values collected during regular clinic visits after 1 year of age and before the CVA. Blood pressure was not averaged; rather, the values were collected over all annual visits after 2 years of age were incorporated into the model. TIA was examined also as a risk factor for completed stroke, because, in practice, many physicians may not consider TIA as a CVA event. Of the 2,436 SS patients who had no history of CVA before study entry, 36 were excluded because they were not observed past 1 year of age, leaving 2,400 in the analysis. A stepwise procedure was used to arrive at a final multivariate model. α-Thalassemia was examined individually but not included in the stepwise regression due to unknown α-globin gene number for 25% of SS patients.
Descriptive statistics are presented as percentages and means ± 1 standard deviation. All P values are two-sided, and a Pvalue of .05 is considered significant.
RESULTS
Prevalence and incidence of CVA.
At entry into the study 153 of 4,082 patients had a history of CVA resulting in an overall crude prevalence rate of 3.75%. The highest age-adjusted prevalence estimate, 4.01%, was in patients with SS, followed in decreasing order by the rates of 2.43% for S-β0 thalassemia, 1.29% for S-β+thalassemia, and 0.84% for SC. One hundred thirty-nine patients had unknown genotype due to chronic transfusion. Assuming that those patients are SS results in an age-adjusted prevalence of 4.96% in that group. The age-specific prevalence rates for each genotype, separating those with unconfirmed genotype (“unknown”), are shown in Table2. There was no difference in prevalence between male and female patients.
Prevalence of CVA at Study Entry
| Age at Entry (yr) . | SS . | SC . | S-β+ . | S-β0 . | Unknown* . | Total . |
|---|---|---|---|---|---|---|
| N | 2,675 | 873 | 203 | 192 | 139 | 4,082 |
| <2 | 0.00 | 0.00 | 0.00 | 4.76 (1) | 0.00 | 0.11 (1) |
| 2-5 | 2.29 (8) | 0.00 | 0.00 | 0.00 | 30.0 (3) | 2.32 (11) |
| 6-9 | 4.93 (14) | 1.02 (1) | 0.00 | 0.00 | 64.7 (11) | 5.79 (26) |
| 10-19 | 5.52 (39) | 1.06 (2) | 1.64 (1) | 0.00 | 48.2 (13) | 5.27 (55) |
| 20-29 | 5.33 (26) | 1.60 (2) | 2.56 (1) | 0.00 | 10.0 (2) | 4.35 (31) |
| 30-39 | 7.22 (14) | 1.32 (1) | 0.00 | 7.69 (1) | 33.3 (2) | 5.84 (18) |
| 40-49 | 8.57 (6) | 0.00 | 16.67 (1) | 33.3 (1) | 0.00 | 7.62 (8) |
| ≥50 | 7.14 (2) | 5.26 (1) | 0.00 | 0.00 | 0.00 | 5.17 (3) |
| Overall | 4.07 (109) | 0.80 (7) | 1.48 (3) | 1.56 (3) | 22.3 (31) | 3.75 (153) |
| Age-adjusted | 4.01 | 0.84 | 1.29 | 2.43 | 27.2 |
| Age at Entry (yr) . | SS . | SC . | S-β+ . | S-β0 . | Unknown* . | Total . |
|---|---|---|---|---|---|---|
| N | 2,675 | 873 | 203 | 192 | 139 | 4,082 |
| <2 | 0.00 | 0.00 | 0.00 | 4.76 (1) | 0.00 | 0.11 (1) |
| 2-5 | 2.29 (8) | 0.00 | 0.00 | 0.00 | 30.0 (3) | 2.32 (11) |
| 6-9 | 4.93 (14) | 1.02 (1) | 0.00 | 0.00 | 64.7 (11) | 5.79 (26) |
| 10-19 | 5.52 (39) | 1.06 (2) | 1.64 (1) | 0.00 | 48.2 (13) | 5.27 (55) |
| 20-29 | 5.33 (26) | 1.60 (2) | 2.56 (1) | 0.00 | 10.0 (2) | 4.35 (31) |
| 30-39 | 7.22 (14) | 1.32 (1) | 0.00 | 7.69 (1) | 33.3 (2) | 5.84 (18) |
| 40-49 | 8.57 (6) | 0.00 | 16.67 (1) | 33.3 (1) | 0.00 | 7.62 (8) |
| ≥50 | 7.14 (2) | 5.26 (1) | 0.00 | 0.00 | 0.00 | 5.17 (3) |
| Overall | 4.07 (109) | 0.80 (7) | 1.48 (3) | 1.56 (3) | 22.3 (31) | 3.75 (153) |
| Age-adjusted | 4.01 | 0.84 | 1.29 | 2.43 | 27.2 |
The percentage of patients with CVA history, by age group and hemoglobin genotype, is shown. Numbers of patients with CVA before entry are in parentheses.
Patients whose Hb genotype was not confirmed because of transfusion therapy. When these patients are combined with the SS group, the modified prevalence estimate is 4.98% (age-adjusted rate, 4.96%) for the SS patients.
During the study, 87 of the 3,647 patients with no history of CVA at entry had a CVA, yielding an overall incidence rate of 0.46 per 100 patient-years. Age-specific incidence rates for each genotype are shown in Table 3. The highest incidence of first CVA was in the SS group, with an age-adjusted rate of 0.61 per 100 patient-years. We compared the incidence rates in broader age groups, isolating those less than 1 year of age, none of whom had a CVA. Incidence rates of CVA in SS patients less than 1 year of age, 1 to 9 years of age, 10 to 19 years of age, and 20+ years of age were 0.00, 0.84, 0.41, and 0.59, respectively. When compared with each other, only the difference in incidence between the 1 to 9 years of age and 10 to 19 years of age groups was statistically significant (P = .026). The 2 youngest of the 7 SC patients who had first CVA on study were 5 years of age; they both had TIA. There was no significant difference in incidence of first CVA between male and female patients at any age.
Incidence of First CVA on Study
| Age (yr) . | SS . | SC . | S-β+ . | S-β0 . | Total . |
|---|---|---|---|---|---|
| N | 2,436 | 839 | 188 | 184 | 3,647 |
| <2 | 0.13 (1) | 0.00 | 0.00 | 0.00 | 0.08 (1) |
| 2-5 | 1.02 (20) | 0.27 (2) | 0.00 | 0.00 | 0.75 (22) |
| 6-9 | 0.79 (15) | 0.00 | 0.00 | 0.00 | 0.55 (15) |
| 10-19 | 0.41 (15) | 0.09 (1) | 0.00 | 0.00 | 0.30 (16) |
| 20-29 | 0.52 (14) | 0.16 (1) | 0.46 (1) | 0.43 (1) | 0.45 (17) |
| 30-39 | 0.59 (8) | 0.00 | 0.00 | 0.00 | 0.39 (8) |
| 40-49 | 0.74 (3) | 1.01 (2) | 0.00 | 0.00 | 0.76 (5) |
| ≥50 | 1.28 (2) | 0.76 (1) | 0.00 | 0.00 | 0.91 (3) |
| Overall | 0.61 (78) | 0.17 (7) | 0.11 (1) | 0.10 (1) | 0.46 (87) |
| Age-adjusted | 0.61 | 0.15 | 0.09 | 0.08 |
| Age (yr) . | SS . | SC . | S-β+ . | S-β0 . | Total . |
|---|---|---|---|---|---|
| N | 2,436 | 839 | 188 | 184 | 3,647 |
| <2 | 0.13 (1) | 0.00 | 0.00 | 0.00 | 0.08 (1) |
| 2-5 | 1.02 (20) | 0.27 (2) | 0.00 | 0.00 | 0.75 (22) |
| 6-9 | 0.79 (15) | 0.00 | 0.00 | 0.00 | 0.55 (15) |
| 10-19 | 0.41 (15) | 0.09 (1) | 0.00 | 0.00 | 0.30 (16) |
| 20-29 | 0.52 (14) | 0.16 (1) | 0.46 (1) | 0.43 (1) | 0.45 (17) |
| 30-39 | 0.59 (8) | 0.00 | 0.00 | 0.00 | 0.39 (8) |
| 40-49 | 0.74 (3) | 1.01 (2) | 0.00 | 0.00 | 0.76 (5) |
| ≥50 | 1.28 (2) | 0.76 (1) | 0.00 | 0.00 | 0.91 (3) |
| Overall | 0.61 (78) | 0.17 (7) | 0.11 (1) | 0.10 (1) | 0.46 (87) |
| Age-adjusted | 0.61 | 0.15 | 0.09 | 0.08 |
The number of CVAs per 100 patient-years, by age group at occurrence and hemoglobin genotype, is shown. The number in parentheses represents the number of events.
Age at first CVA.
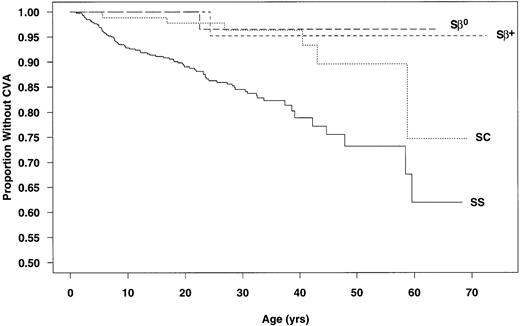
Data from the 3,647 patients used to calculate incidence rates were used to determine CVA-free survival curves. The estimated age at first CVA was significantly different for SS and SC patients (P < .001; Fig 1). The chances of having a first CVA by 20 years of age, 30 years of age, and 45 years of age were estimated at 11%, 15%, and 24%, respectively, for SS patients and 2%, 4%, and 10%, respectively, for those with SC.
Age at first CVA by genotype. (—) SS; (···) SC; (---) S-β+ thalassemia; (– –) S-β0thalassemia.
Age at first CVA by genotype. (—) SS; (···) SC; (---) S-β+ thalassemia; (– –) S-β0thalassemia.
Age and type of CVA in SS patients.
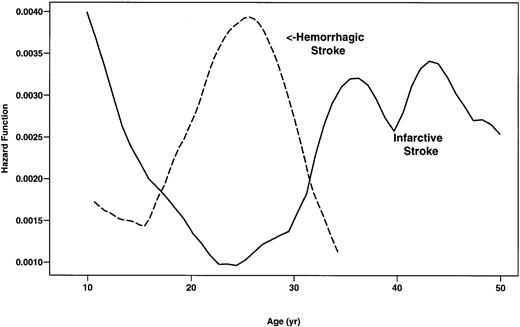
During the study, there were 78 first CVAs in SS patients. The type of CVA was not available for 2 patients. Forty-one (53.9%) of these CVAs were infarctive, 26 (34.2%) were hemorrhagic, 8 (10.5%) were TIA, and 1 (1.3%) had both infarctive and hemorrhagic features (Table4). Although the incidence of infarctive CVA was highest in SS patients younger than 20 years of age (0.44 per 100 patient-years), adults more than 30 years of age were also found to be at risk (Fig 2). Conversely, the rate of hemorrhagic stroke was highest in patients 20 to 29 years of age (0.44 per 100 patient-years) and was low in children and older patients (Fig2). Eight patients younger than 10 years of age experienced hemorrhagic stroke (0.17 per 100 patient-years).
Incidence of First CVA in SS Patients
| Age (yr) . | TIA . | Type of CVA* . | ||
|---|---|---|---|---|
| Infarctive . | Hemorrhagic . | Unidentified . | ||
| <2 | 0.00 (0) | 0.13 (1) | 0.00 (0) | 0.00 (0) |
| 2-5 | 0.15 (3) | 0.70 (14) | 0.15 (3) | 0.00 (0) |
| 6-9 | 0.00 (0) | 0.51 (10) | 0.25 (5) | 0.00 (0) |
| 10-19 | 0.05 (2) | 0.24 (9)* | 0.14 (5)* | 0.00 (0) |
| 20-29 | 0.04 (1) | 0.04 (1) | 0.44 (12) | 0.00 (0) |
| 30-39 | 0.15 (2) | 0.37 (5) | 0.07 (1) | 0.00 (0) |
| 40-49 | 0.00 (0) | 0.24 (1) | 0.24 (1) | 0.24 (1) |
| ≥50 | 0.00 (0) | 0.62 (1) | 0.00 (0) | 0.62 (1) |
| Overall | 0.06 (8) | 0.32 (42) | 0.21 (27) | 0.02 (2) |
| Age (yr) . | TIA . | Type of CVA* . | ||
|---|---|---|---|---|
| Infarctive . | Hemorrhagic . | Unidentified . | ||
| <2 | 0.00 (0) | 0.13 (1) | 0.00 (0) | 0.00 (0) |
| 2-5 | 0.15 (3) | 0.70 (14) | 0.15 (3) | 0.00 (0) |
| 6-9 | 0.00 (0) | 0.51 (10) | 0.25 (5) | 0.00 (0) |
| 10-19 | 0.05 (2) | 0.24 (9)* | 0.14 (5)* | 0.00 (0) |
| 20-29 | 0.04 (1) | 0.04 (1) | 0.44 (12) | 0.00 (0) |
| 30-39 | 0.15 (2) | 0.37 (5) | 0.07 (1) | 0.00 (0) |
| 40-49 | 0.00 (0) | 0.24 (1) | 0.24 (1) | 0.24 (1) |
| ≥50 | 0.00 (0) | 0.62 (1) | 0.00 (0) | 0.62 (1) |
| Overall | 0.06 (8) | 0.32 (42) | 0.21 (27) | 0.02 (2) |
The number of CVAs per 100 patient-years, by type of CVA and age group, is shown. The number in parentheses represents the number of events.
One CVA in the 10 to 19 years of age group had both infarctive and hemorrhagic features.
Smoothed hazard rates of infarctive and hemorrhagic stroke in SS patients by age. (—) Infarctive stroke; (---) hemorrhagic stroke.
Smoothed hazard rates of infarctive and hemorrhagic stroke in SS patients by age. (—) Infarctive stroke; (---) hemorrhagic stroke.
Recurrence of CVA.
Among 72 SS patients who survived the first day of their initial CVA, there were 10 recurrences (14%): 6.4 events per 100 patient-years in patients with initial CVA occurring at less than 20 years of age and 1.6 events per 100 patient-years in patients with initial CVA occurring at ≥20 years of age. Two events followed TIA with a mean time to recurrence of 3.0 months, 2 events followed hemorrhagic stroke with a mean time to recurrence of 7.2 months, and 6 events followed infarctive stroke with a mean time to recurrence of 22.2 months. The 2 TIAs were both followed by infarctive stroke, the 2 hemorrhagic strokes were both followed by a second hemorrhagic stroke, and the 6 infarctive strokes were followed by 1 TIA, 4 infarctive strokes, and 1 with mixed infarctive/hemorrhagic stroke features. One 43-year-old SC patient with infarctive stroke had a recurrence of unknown type 6 months later. Four of the 10 SS patients with recurrent CVA received a blood transfusion within 1 month before the recurrence. However, the CSSCD did not mandate a transfusion protocol for study participants; therefore, no definitive statement can be made regarding the association between transfusion practices and CVA recurrence.
CVA-related mortality.
All deaths occurring less than 14 days after a CVA were considered to be CVA related. A total of 104 patients (87 first and 17 recurrent CVAs) had 133 episodes of CVA on study. Eleven (10.6%) of the 104 patients died, 9 after hemorrhagic stroke and 2 after strokes of unidentified type. Seven of the 11 deaths resulted from the first CVA; of these, 6 had a hemorrhagic stroke and 1 had stroke of unidentified type. All 11 patients were in the SS group (11.7% of SS CVA patients) and ranged in age from 12 to 58 years. Six died on the day of the CVA, whereas the others died within 1 week after the CVA. The mortality rate for hemorrhagic stroke was 24% overall and 26% for SS patients. No deaths occurred within 14 days after 62 infarctive strokes.
α-Thalassemia and CVA.
Of 2,436 SS patients with no history of CVA at study entry, 1,833 had α-globin gene mapping data: 573 (31%) had α-thalassemia (48 with 2 and 525 with 3 α-globin genes) and 1,260 (69%) had 4 (n = 1,242) or 5 (n = 18) α-globin genes. The incidence of CVA of all types for patients with α-thalassemia (0.32 per 100 patient-years) was lower than that for patients without α-thalassemia (0.74 per 100 patient-years), yielding a relative risk of 0.44 (95% confidence interval, 0.23 to 0.85; P = .011). There were no CVA observed in the 48 patients with 2 α-globin genes. The incidence of infarctive stroke was 0.21 per 100 patient-years in those with α-thalassemia and 0.81 per 100 patient-years in those without α-thalassemia (P= .079). The incidence of hemorrhagic stroke was 0.06 per 100 patient-years versus 0.23 per 100 patient-years in those with and without α-thalassemia (P = .076).
Risk factors for CVA.
Risk factors were evaluated separately for first infarctive and hemorrhagic strokes because the two types of strokes may have different pathophysiologies. For these analyses, TIA was considered as a risk factor for having a subsequent completed infarctive or hemorrhagic stroke. No patients experienced seizures, surgical procedures, or acute anemic events within 2 weeks before CVA.
Completed infarctive stroke.
Univariate analyses of the risk of completed infarctive stroke (age at first CVA) identified eight risk factors. Prior TIA, history of meningitis (any type), history of bacterial meningitis, systolic blood pressure, steady-state leukocyte count, acute chest syndrome within 2 weeks before stroke, and rate of acute chest syndrome were positively related to infarctive stroke, whereas steady-state Hb concentration was negatively related; ie, patients with lower steady-state Hb are at greater risk of infarctive stroke (P < .05). The rate of severe painful episodes (P = .671), Hb F level (P = .106), blood transfusion within 2 weeks before stroke (P = .077), and platelet count (P = .097) were not significantly related to occurrence of first completed infarctive stroke.
The final multivariate model for risk of completed infarctive stroke included five variables: prior TIA, steady-state Hb concentration, acute chest syndrome within 2 weeks before CVA, rate of acute chest syndrome, and systolic blood pressure (Table5A). The most significant predictor of completed infarctive stroke was prior TIA; however, the majority of patients had stroke without prior TIA. In this study, 2 of 42 completed infarctive strokes were preceded by TIA, compared with 6 TIA in 2,394 patients who did not experience any stroke. Similarly, 4 of the 42 completed infarctive strokes were preceded within 2 weeks by acute chest syndrome. An additional 3 patients had acute chest syndrome on the same day as the completed infarctive stroke, but these patients were excluded from the analysis because timing of the acute chest syndrome event relative to the onset of infarctive stroke was not documented.
Results of Analyses of Risk of First Stroke in SS Patients
| Predictor . | Relative Risk . | 95% CI . | PValue . |
|---|---|---|---|
| A. Infarctive stroke (final multivariate model: five predictors significant at P < .05) | |||
| Prior TIA | 56.0 | 12.0, 285 | <.001 |
| Steady-state Hb | 1.85 per 1 g/dL* decrease | 1.32, 2.59 | <.001 |
| Acute chest syndrome within 2 weeks prior | 7.03 | 1.85, 26.7 | .001 |
| Acute chest syndrome rate | 2.39 per event/yr | 1.27, 4.48 | .005 |
| Systolic blood pressure | 1.31 per 10 mm Hg increase | 1.03, 1.67 | .033 |
| B. Hemorrhagic stroke (final multivariate model: two predictors significant atP < .05) | |||
| Steady-state Hb | 1.61 per 1 g/dL* decrease | 1.11, 2.35 | .013 |
| Steady-state leukocyte count | 1.94 per 5 × 109/L increase | 1.73, 2.18 | .026 |
| Predictor . | Relative Risk . | 95% CI . | PValue . |
|---|---|---|---|
| A. Infarctive stroke (final multivariate model: five predictors significant at P < .05) | |||
| Prior TIA | 56.0 | 12.0, 285 | <.001 |
| Steady-state Hb | 1.85 per 1 g/dL* decrease | 1.32, 2.59 | <.001 |
| Acute chest syndrome within 2 weeks prior | 7.03 | 1.85, 26.7 | .001 |
| Acute chest syndrome rate | 2.39 per event/yr | 1.27, 4.48 | .005 |
| Systolic blood pressure | 1.31 per 10 mm Hg increase | 1.03, 1.67 | .033 |
| B. Hemorrhagic stroke (final multivariate model: two predictors significant atP < .05) | |||
| Steady-state Hb | 1.61 per 1 g/dL* decrease | 1.11, 2.35 | .013 |
| Steady-state leukocyte count | 1.94 per 5 × 109/L increase | 1.73, 2.18 | .026 |
Abbreviation: CI, confidence interval.
1 g/dL = 0.6206 mmol/L.
Hemorrhagic stroke.
Univariate analyses of the risk of hemorrhagic stroke (age at first CVA) identified three significant risk factors: steady-state leukocyte count and rate of acute chest syndrome were positively related to risk of hemorrhagic stroke, whereas steady-state Hb concentration was negatively related (P < .05). The presence of α-thalassemia provided a marginally significant protection against hemorrhagic stroke in SS patients (P = .054). Unlike infarctive stroke, history of meningitis and systolic blood pressure were not significant univariate predictors; none of the 28 patients with first hemorrhagic stroke on study had had meningitis.
The final multivariate model for risk of hemorrhagic stroke included two significant variables: low steady-state Hb concentration and high leukocyte count (Table 5B).
DISCUSSION
This report represents larger numbers of SCD patients with CVA than any previous study. As expected, the highest incidence rate was found in SS patients. Although SS patients are at the greatest risk of stroke, clinicians and others counseling about SCD should note that strokes occurred also in patients with other genotypes. Children less than 2 years of age had the lowest CVA incidence, suggesting that there may be a protective mechanism operative in early life or that, in SCD, the pathology responsible for CVA develops over time. However, we found the incidence of CVA to be higher in the 1 to 9 years of age group than in the 10 to 19 years of age group. This finding suggests that a subset of patients may have additional risk factors for early stroke.
The type of stroke may be due to different pathophysiologic mechanisms or to progressive cerebrovascular damage. The notion that infarctive strokes occur more commonly in children whereas hemorrhagic strokes occur more frequently in adults is supported partly by this study. Infarctive stroke was more common in SS patients less than 20 years of age than in those older, but patients more than 30 years of age were also at risk. Interestingly, the period of lowest risk for infarctive stroke (20 to 29 years of age) was the period of highest risk for hemorrhagic stroke. However, it should be noted that children less than 10 years of age experienced a higher rate of hemorrhagic stroke (0.17 per 100-patient-years) than reported previously.
Some of our data may be useful in identifying patients at high risk for completed CVA. History of TIA was a strong risk factor for completed infarctive stroke. This fact should alert clinicians to regard TIA as a sign of cerebrovascular disease, use definitive diagnostic studies, and initiate aggressive management to prevent occurrence of completed stroke. However, infarctive strokes are often not preceded by TIA, and in young children mild TIA is likely to go unnoticed.
The temporal association of episodes of acute chest syndrome and CVA found in this study has not been reported previously. It is possible that in patients with damaged cerebral vessels, CVA may be precipitated by hypoxia associated with pulmonary disease. Alternatively, these strokes may be related to the fat embolization syndrome.18We did not find an association between first CVA and priapism19 or transfusion therapy occurring within 2 weeks before the CVA.
Anemia in SCD is a reflection of overall severity of SCD. Patients with the milder genotypes are less anemic than those with more severe genotypes. However, within each genotype, an association between the severity of anemia and major complications of SCD is not always apparent. In SS patients, a high Hb level is related to increased rates of severe pain20 and acute chest syndrome.21Severe anemia may pose an added risk for CVA. It has been suggested that the increased cerebral blood flow and flow velocity associated with chronic anemia22 cause flow disturbances that may lead to cerebrovascular damage.23
A high leukocyte count appears to be a risk factor for many severe complications of SCD: rates of severe pain,20 acute chest syndrome,21 and mortality.2 Association of increased white blood cell count with CVA has been reported4; our data show such a correlation only for hemorrhagic CVA. The contribution of leukocytes, if any, and the various vaso-active and cytoadhesive molecules produced by leukocytes to vasoocclusion has not been defined.
The lack of a protective effect of increased Hb F levels on CVA was surprising, given reports of its inhibiting effect on CVA risk in other series.3,4 Hb F has been inversely correlated with rates of other major vasoocclusive manifestations of sickle cell disease such as severe pain and acute chest syndrome. It remains to be seen whether the ameliorating effect of hydroxyurea therapy on rates of severe pain and acute chest syndrome will be shown with CVA also.24
The effect of α-thalassemia on the incidence of CVA is controversial; whereas some studies have found that it reduces the risk of CVA,14,25,26 others have not.4,27 This study provides some evidence to support earlier reports that α-thalassemia protects SCD patients from CVA.14,25 26 We observed a significant association when all types of CVA were combined, but the effect was marginal (.05 < P < .10) when infarctive and hemorrhagic strokes were considered separately, in part due to the smaller number of events in each subgroup. Nevertheless, it should be noted that the effect of α-thalassemia appears to be similar for infarctive and hemorrhagic strokes. Based on additional multivariate analysis, we conclude that the protective effect of α-thalassemia is largely due to the improvement in Hb concentration. We did not find α-thalassemia to be a significant predictor of CVA after adjusting for Hb.
SCD patients who suffer CVA have a high risk of recurrence that is reduced but not abolished by chronic transfusion therapy.28-32 We were unable to assess the impact of chronic transfusion on CVA recurrence because the study did not mandate any transfusion regimen for patients with CVA.
We observed no mortality after 62 infarctive strokes, but there was a 24% mortality rate after 38 hemorrhagic strokes within 2 weeks after the event. Mortality related to hemorrhagic stroke was rapid, with 6 of the 11 CVA-related deaths occurring on the day of the event.
For the last 2 decades, management of CVA in SCD has been directed mainly towards prevention of recurrence. There is now strong interest in preventing the occurrence of the first CVA. The CSSCD and others are involved in prospective studies using magnetic resonance technology and extensive neuropsychological testing to look for evidence of intracranial pathology that may be predictive of CVA. Cerebral infarcts33,34 and cerebrovascular disease35have been demonstrated in SS patients with no history of CVA. Transcranial Doppler ultrasonography has been shown to be able to select patients at high risk of stroke,36 and a national collaborative study in which patients with abnormal flow velocities are randomized to transfusion or observation has shown recently the risk of first CVA can be reduced significantly with chronic transfusion therapy. Patients with the risk factors described in this study should be particularly evaluated for evidence of cerebrovascular disease. Caretakers of young children with SCD should be educated about signs of TIA and advised to report them. Furthermore, patients with clinical and hematologic risk factors who are found to have cerebrovascular disease should be considered seriously for intervention studies and therapy.
ACKNOWLEDGMENT
The authors thank Sergio Piomelli, Orah Platt, Samuel Charache, William Mentzer, Rebecca Stellato, and Dale Usner for their thoughtful review and helpful comments regarding this manuscript.
APPENDIX
The following were senior investigators in the CSSCD: Clinical Centers: R. Johnson, Alta Bates Hospital (Berkeley, CA); L. McMahon, Boston City Hospital (Boston, MA); O. Platt, Children's Hospital (Boston, MA); F. Gill and K. Ohene-Frempong, Children's Hospital (Philadelphia, PA); G. Bray, J. Kelleher, and S. Leikin, Children's National Medical Center (Washington, DC); E. Vichinsky and B. Lubin, Children's Hospital (Oakland, CA); A. Bank and S. Piomelli, Columbia Presbyterian Hospital (New York, NY); W. Rosse, J. Falletta, and T. Kinney, Duke University (Durham, NC); L. Lessin, George Washington University (Washington, DC); J. Smith and Y. Khakoo, Harlem Hospital (New York, NY); R. Scott, O. Castro, and C. Reindorf, Howard University (Washington, DC); H. Dosik, S. Diamond, and R. Bellevue, Interfaith Medical Center (Brooklyn, NY); W. Wang and J. Wilimas, LeBonheur Children's Hospital (Memphis, TN); P. Milner, Medical College of Georgia (Augusta, GA); A. Brown, S. Miller, R. Rieder, and P. Gillette, State University of New York Health Science Center at Brooklyn (Brooklyn, NY); W. Lande, S. Embury, and W. Mentzer, San Francisco General Hospital (San Francisco, CA); D. Wethers and R. Grover, St Luke's-Roosevelt Medical Center (New York, NY); M. Koshy and N. Talishy, University of Illinois (Chicago, IL); C. Pegelow, P. Klug, and J. Temple, University of Miami (Miami, FL); M. Steinberg, University of Mississippi (Jackson, MS); A. Kraus, University of Tennessee (Memphis, TN); H. Zarkowsky, Washington University (St Louis, MO); C. Dampier, Wyler Children's Hospital (Chicago, IL); H. Pearson and A.K. Ritchey, Yale University (New Haven, CT); Statistical Coordinating Centers: P. Levy, D. Gallagher, A. Koranda, Z. Flournoy-Gill, and E. Jones, University of Illinois School of Public Health (Chicago, IL; 1979-89); S. McKinlay, O. Platt, D. Gallagher, D. Brambilla, and L. Sleeper, New England Research Institutes (Watertown, MA; 1989-1997); M. Espeland, Bowman-Gray School of Medicine (Winston-Salem, NC); Program Administration: M. Gaston, C. Reid, and J. Verter, National Heart, Lung, and Blood Institute (Bethesda, MD).
Supported by the Division of Blood Diseases and Resources of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Address reprint requests to Kwaku Ohene-Frempong, MD, Division of Hematology, Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal