Abstract
Allogeneic cord blood is now being widely used as a source of stem cells for hematologic reconstitution after myeloablative therapy, with reported significantly lower levels of graft-versus-host disease (GVHD) compared with the use of allogeneic bone marrow (BM). This study was undertaken to investigate biologic aspects of natural killer (NK) cell activity, as recognized effector cells of the GVHD and graft-versus-leukemia (GVL) response, from cord blood and conventional BM. NK-cell activity levels of freshly isolated cells from cord blood and BM against K562 targets were comparable. Lymphokine activated killer (LAK) cells from both hematopoietic cell sources were compared for their ability to kill target cells by necrotic or apoptotic mechanisms using specific target cell lines. Cord blood cells had significantly higher necrosis-mediated cytotoxic activity against Daudi target cells compared with BM-derived cells. Cord blood LAK cells had relatively high levels of apoptotic-mediated cytotoxicity against YAC-1 target cells, whereas BM-derived LAK cells were unable to induce apoptosis in these cells. Interleukin-2 (IL-2) induced significant granzyme B activity in cord cells in contrast to BM cells, in which very little activity was measured. Western blotting confirmed these findings, with IL-2 inducing granzyme B protein expression in cord cells but not detectable levels in BM cells. BM cells had significantly lower cell surface expression of IL-2R and prolonged culture in IL-2 was only partially able to restore their deficient apoptotic cytotoxic activity. Thus, major differences exist between cord blood-derived and BM-derived mononuclear cells with respect to their NK-cell–associated cytotoxic behavior. This could have important implications for stem cell transplantation phenomena, because it suggests that cord blood may have increased potential for a GVL effect.
NATURAL KILLER (NK) cells play a number of recognized roles in bone marrow transplantation (BMT).1They have been identified as an effector cell in graft-versus-host disease (GVHD)2,3 and also as an effector cell in the beneficial graft-versus-leukemia (GVL)4,5 effect. Cord blood is now being used as an alternative source of stem cells to BM for hematologic reconstitution.6 Results thus far show a significantly reduced incidence of GVHD in cord blood transplant patients even when unrelated donors are used.7,8 However, the follow-up period is still too short for any impact on GVL to be known. Cord blood cells have been reported to have reduced NK-cell activity relative to adult peripheral blood.9,10 This may contribute to the increased morbidity and mortality associated with neonatal infections.11 Cord cells can be induced by various stimuli, in particular interleukin-2 (IL-2), to develop high lymphokine activated killer (LAK) activity.12 13
LAK cells have been shown to kill target cells by at least two mechanisms, necrosis and apoptosis.14 Perforin, which is found in the cyotoxic granules of NK cells, is accredited as the protein responsible for necrosis, which primarily involves target cell membrane damage.15 Apoptosis of target cells may be induced by a number of mechanisms, including fas/fas-ligand interactions16 and tumor necrosis factor-α (TNF-α) secretion,17 or via granzymes.18,19 Granzymes are a family of serine proteases found in the cytotoxic granules of activated cytotoxic lymphocytes.20 Granzyme B has been reported to be essential for the rapid induction of apoptosis in susceptible target cells,21,22 although the exact mechanism by which this happens remains to be elucidated. It is now accepted that apoptosis represents the physiologic form of cell death in vivo.23 Therefore, it seems reasonable to expect it to also represent the primary mechanism involved in cell-mediated cytotoxicity, and there is a growing body of evidence in the literature to support this hypothesis.19 24 However, relatively little is known about this pathway at regulatory or effector mechanism levels.
Data in the literature describing cytotoxic activities of BM NK cells are varied with respect to potential and kinetics of induction. Freshly isolated BM is generally reported to have low killing activity.25,26 Agah et al27 have reported rapidly induced, potent killing in BM by IL-2, whereas others have reported slower induction with much donor variation.25 26Similar to cord blood, nothing is known about the potential of BM LAK cells to induce apoptosis in target cells. This study was undertaken to compare aspects of cord blood and BM cytotoxic activities using model systems to measure both necrotic and apoptotic death of various tumor target cell lines.
MATERIALS AND METHODS
Effector cells.
Approximately 10 mL of BM was obtained from the posterior ileac crests of anesthetized healthy adolescent and adult patients undergoing elective orthopedic surgery. Cord blood samples were obtained from full-term, normal delivery, healthy infants. Samples were collected into 0.01 mol/L EDTA in phosphate-buffered saline (PBS), pH 7.4. Mononuclear cells were isolated by lymphoprep (Nycomed, Oslo, Norway) density-gradient centrifugation.28Cells cultured in vitro were grown in RPMI-1640 medium supplemented with 10% fetal calf serum (GIBCO, Paisley, UK), 20 mmol/Ll-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 20 mmol/L HEPES, referred to as complete medium (CM). IL-2 (Cetus Corp, Emeryville, CA) was used at 500 U/mL and was added at the start of culture.
Phenotyping.
Mononuclear cells isolated by density-gradient centrifugation28 were stained with monoclonal antibodies and examined for cell surface expression of NK cell antigens by flow cytometry. Directly conjugated anti-CD56 phycoerythrin (PE) (Becton Dickinson, Oxford, UK), anti-CD16 fluorescein isothiocyanate (FITC) (DAKO, High Wycombe, Bucks, UK), and anti-CD8 PE (DAKO) antibodies were used.
NK cell purification.
CD56+ cells were purified using the Mini MACS system (Miltenyi Biotec, Bergisch-Gladback, Germany). Briefly, isolated mononuclear cells were incubated with anti-CD56 magnetic beads for 15 minutes at 4°C. Cells were washed, resuspended in 0.5 mL wash buffer (PBS, 2 mmol/L EDTA, 0.5% bovine serum albumin, pH 7.2), and passed through a magnetized column. Negative cells were eluted by washing. The column was then removed from the magnet and CD56+ cells were eluted using 1 mL wash buffer.
51Cr release assay.
A standard 4-hour 51Cr release assay was used to measure necrotic death of target cells.29 Briefly, 106target cells were labeled with 50 μCi Na251CrO4 for 1 hour at 37°C. Cells were washed twice with PBS and resuspended in CM, and 1 × 104 cells were aliquoted per well in 100 μL volumes. Effector cells in an equal volume were added, in quadruplicate, to give the desired effector:target (E:T) cell ratios. The plate was centrifuged at 150g for 5 minutes to initiate cell contact and incubated at 37°C for 4 hours. The plate was then centrifuged at 350g for 10 minutes, supernatants were decanted, and samples were counted using a γ-counter (Wallac-LKB, Milton-Keynes, UK). CM alone or HCl (1 mol/L) was added to labeled target cells for calculation of spontaneous (spons) or maximum (max) release, respectively. The percentage of kill (% kill) was calculated using the following equation: % Kill = (Experimental − Spons)/(Max − Spons) × 100%.
125I-UdR release assay.
This assay was used to measure apoptosis of target cells induced by LAK effectors.30 Target cells (5 × 105) were labeled with 10 μCi 125I-deoxyuridine (125I-UdR) in 200 μL for 2 hours at 37°C. Cells were washed twice with PBS, resuspended in CM at 106 cells/mL, and aliquoted in 0.5 mL volumes into 1.5-mL eppendorf tubes in triplicate. Effector cells in CM were added to give the desired E:T cell ratios. Tubes were centrifuged at 800 rpm in a minifuge for 5 minutes to inititate cell contact. After 4 hours, the tubes were centrifuged at 5,000 rpm in a minifuge for 5 minutes. Supernatants were collected into tubes and cell pellets were resuspended in 1 mL lysis buffer (5 mmol/L Tris/HCl, pH 8.0; 0.1 mol/L EDTA, pH 8.0; 0.5% Triton X-100). After a brief vortex, tubes were centrifuged at full speed in a minifuge for 20 minutes to separate fragmented from bulk DNA. Supernatants were combined with those collected previously and counted on a γ-counter for 1 minute each. Spons count was obtained from tubes in which CM alone was added to the radiolabeled target cells and max release was calculated by adding the radioactivity remaining in the spons pellets to the supernant counts: % Kill = (Experimental − Spons)/(Max − Spons) × 100%.
DNA extraction, electrophoresis, and autoradiography.
YAC-1 cells (5 × 105) were labeled with 10 μCi125I-UdR for 2 hours at 37°C. Cells were washed and incubated with 5 × 106 cord blood LAK effectors to give a final E:T of 10:1. Cells were centrifuged at 500 rpm for 5 minutes in a minifuge to inititate cell contact. After 4 hours, pelleted cells were subjected to protein and RNAse digests and two phenol-chloroform-amyl alcohol DNA extractions.31 Purified DNA was diluted in TE buffer (10 mmol/L Tris/HCl, 1 mmol/L EDTA, pH 7.4) and centrifuged at full speed in the minifuge to separate fragmented from intact DNA. Supernatants were precipitated in 2 vol of 100% ethanol overnight. Precipitated DNA was pelleted by centrifugation and, after removal of the ethanol, air-dried for 30 minutes. Each DNA sample was resuspended in 50 μL TE buffer with 5 μL loading buffer and electrophoresed through a 100 mL, 1.5% agarose minigel at 35 V for 4 hours, using TAE as running buffer (0.04 mol/L Tris-acetate, 0.001 mol/L EDTA, pH 8.0).32 One microgram of λHindIII molecular weight markers was used and ethidium bromide (3 μL of 10 mg/mL stock) was added directly to the gel to visualize DNA. The resulting gel was dried using a Hoefer Drygel Jr (San Francisco, CA) onto a sheet of Whatmann No.1 filter paper (Maidstone, UK). This was exposed to x-ray film overnight and an autoradiograph was developed using standard techniques.33
Asp-ase assay for granzyme B enzyme activity.
Granzyme B has a rare enzyme substrate specificity for aspartic acid.18 Effector cell lysates were prepared by resuspending cultured cells at 5 × 106/mL or freshly isolated cells at 1 × 107/mL in 1 mL lysis buffer (0.1 mol/L HEPES, 0.05 mol/L CaCl2, 0.5% NP40, pH 7.5). Cells were freeze-thawed twice and centrifuged in a minifuge at 10,000 rpm for 15 minutes. The resulting supernatants were diluted (0.1 mol/L HEPES, 0.05 mol/L CaCl2, pH 7.5) and aliquoted into a 96-well flat-bottomed plate in 50 μL volumes. The substrate used was n-boc-ala-ala-asp-pNA (Bachem, Bubendorf, Switzerland). Fifty microliters of 2 mmol/L substrate was added per well. Control samples consisted of substrate and diluent alone. Substrate conversion was calculated by measuring the change in optical density (OD) at 405 nm after 3 days of incubation at 37°C.
Western blotting.34
Cells were extracted at 5 × 107/mL into lysis buffer (10 mmol/L Tris/HCl, pH 8.0, 100 mmol/L NaCl, 1% NP40, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 μg/mL aprotinin, 0.001 mol/L EDTA, 50 μmol/L leupeptin, 1 μg/mL pepstatin, and 1 μg/mL antipain). Thirty-microliter cell extracts were diluted with nonreducing loading buffer (62.5 mmol/L Tris, 3% wt/vol sodium dodecyl sulfate [SDS], 10% wt/vol glycerol, and 0.01% wt/vol bromophenol blue) and electrophoresed on a 15% nonreducing SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel. Samples were electroblotted onto nitrocellulose paper and probed with an anti-granzyme B monoclonal antibody (Pharmacell, France). 3-Amino-9-ethyl carbazole (AEC) was used to visualize band staining.
IL-2R detection.
IL-2R expression was measured using a fluorokine receptor assay (R&D Systems, Abingdon, UK), as per the manufacturer's instructions. The primary reagent consisted of a human IL-2 biotin conjugate. This reagent did not discriminate between the various IL-2R subunits. An avidin-fluorescein conjugate was added to detect bound primary reagent and flow cytometry was used to quantify cells staining positive for the reagent.
Statistics.
The Mann Whitney statistical test was used to compare cord blood and BM data.
RESULTS
NK cell activity of freshly isolated cord and BM cells against K562 target cells.
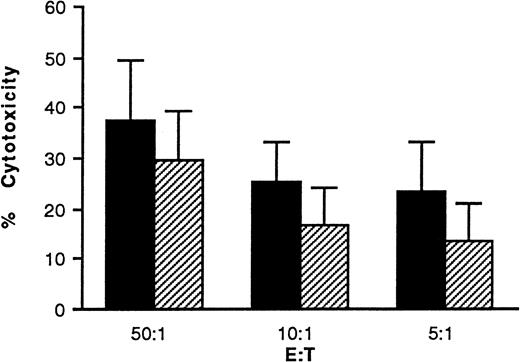
The NK activity of freshly isolated cells was measured against K562 target cells using a 51Cr release assay and the results are shown in Fig 1. Cord cells had higher cytotoxicity at all E:T cell ratios tested, but the differences were not statistically significant.
NK activity of freshly isolated cord blood and BM mononuclear cells. (▪) Cord blood (n = 9) and (□) BM (n = 7) mononuclear cells were isolated by lymphoprep gradient centrifugation. Cells were washed and set up in a 51Cr release assay at the E:T cell ratios indicated. Bars show the % kill + 1 standard deviation (SD). Data were compared using the Mann Whitney statistical test.
NK activity of freshly isolated cord blood and BM mononuclear cells. (▪) Cord blood (n = 9) and (□) BM (n = 7) mononuclear cells were isolated by lymphoprep gradient centrifugation. Cells were washed and set up in a 51Cr release assay at the E:T cell ratios indicated. Bars show the % kill + 1 standard deviation (SD). Data were compared using the Mann Whitney statistical test.
Cord blood and BM contain similar percentages of NK cells.
A total of 12.2% ± 10.0% and 13.6% ± 10.6% of cord blood lymphocytes (n = 6) and 10.6% ± 5.1% and 8.1% ± 4.7% of BM lymphocytes (n = 6) expressed CD56 and CD16 antigens, respectively. CD8 was expressed on 16.0% ± 3.8% (n = 5) of cord blood lymphocytes and on 22.0% ± 11.9% (n = 5) BM lymphocytes.
Cord LAK cells can kill target cells by apoptosis.
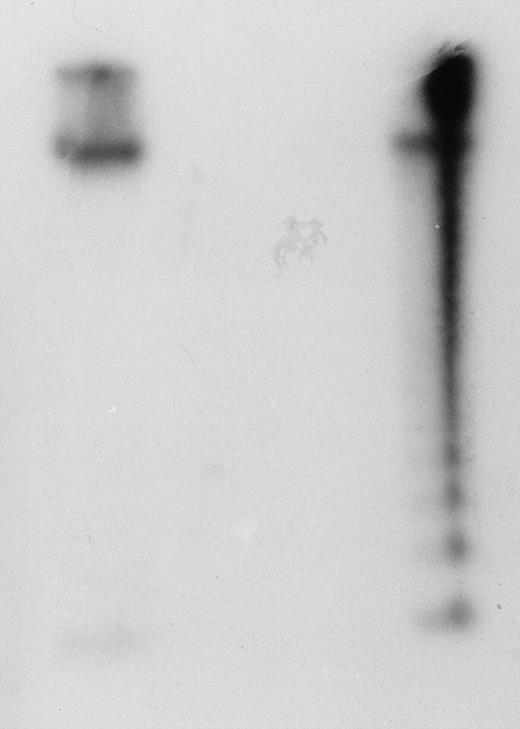
Cord LAK cells have been shown to kill by necrotic means,10but their potential to kill by inducing apoptotic death of target cells has not been demonstrated. Cord LAK cells cultured in IL-2 (500 U/mL) for 3 days were incubated with 125I-UdR–labeled YAC-1 cells for a period of 4 hours. After this time, cell mixture DNA was extracted, electrophoresed, and autoradiographed. The resulting autoradiograph is shown in Fig 2. It clearly shows that DNA extracted from target cells alone remains intact, whereas target cell DNA from the cell mixture has a characteristic DNA ladder pattern indicative of apoptosis. Thus, cord LAK cells are capable of killing their target cells by apoptosis.
Cord blood LAK cells can kill YAC-1 target cells by apoptosis. Cord blood mononuclear cells were cultured in IL-2 (500 U/mL) at 37°C. After 3 days, the cells were washed and 5 × 106 cells were incubated with 5 × 105 YAC-1 target cells that had been prelabeled with 125I-UdR. After 4 hours, DNA was extracted from the cell mixture, electrophoresed, and autoradiographed. The left-hand lane shows DNA extracted from YAC-1 cells in isolation. The right-hand lane shows YAC-1 DNA extracted from the cell mixture preparation. The DNA ladder is indicative of apoptosis. This figure is representative of three experiments.
Cord blood LAK cells can kill YAC-1 target cells by apoptosis. Cord blood mononuclear cells were cultured in IL-2 (500 U/mL) at 37°C. After 3 days, the cells were washed and 5 × 106 cells were incubated with 5 × 105 YAC-1 target cells that had been prelabeled with 125I-UdR. After 4 hours, DNA was extracted from the cell mixture, electrophoresed, and autoradiographed. The left-hand lane shows DNA extracted from YAC-1 cells in isolation. The right-hand lane shows YAC-1 DNA extracted from the cell mixture preparation. The DNA ladder is indicative of apoptosis. This figure is representative of three experiments.
LAK activity of cord blood and BM mononuclear cells against Daudi and YAC-1 target cells.
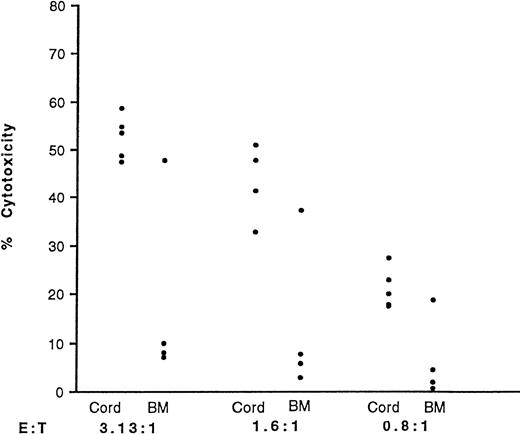
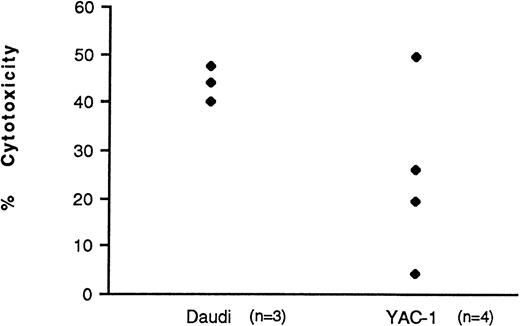
Cord blood and BM mononuclear cells were cultured in IL-2 for 3 days and their LAK activity against both Daudi and YAC-1 target cell lines was quantified. LAK cells kill the Daudi cell line by necrotic means, as measured by the 51Cr release assay. In contrast, they kill YAC-1 cells by inducing them to undergo apoptosis. DNA fragmentation, which is indicative of apoptosis, was measured using an125I-UdR release assay. Table 1shows the data obtained. Cord LAK cells had high cytotoxicity against both Daudi and YAC-1 target cells. In contrast, BM had significantly lower killing of Daudi cells (P < .001) and a virtual absence of any killing of YAC-1 target cells. This absence of killing of YAC-1 cells by unfractionated BM-derived NK cells was confirmed using purified NK-cell preparations. Three of four purified BM-derived NK-cell preparations displayed minimal apoptotic killing of YAC-1 targets compared with purified cord blood-derived NK cells at similar E:T cell ratios (Fig 3; P < .05 at E:T of 3.13:1).
LAK Activity of Cord Blood and BM Cells Against Daudi and YAC-1 Target Cells
| . | Cord . | BM . | P Value . |
|---|---|---|---|
| Necrosis (Daudi) | 86.0 ± 11.6 (n = 8) | 45.7 ± 25.0 (n = 11) | <.001 |
| Apoptosis (YAC-1) | 66.1 ± 9.7 (n = 5) | 2.2 ± 2.7 (n = 7) | <.001 |
| . | Cord . | BM . | P Value . |
|---|---|---|---|
| Necrosis (Daudi) | 86.0 ± 11.6 (n = 8) | 45.7 ± 25.0 (n = 11) | <.001 |
| Apoptosis (YAC-1) | 66.1 ± 9.7 (n = 5) | 2.2 ± 2.7 (n = 7) | <.001 |
Cord blood and BM mononuclear cells were isolated and cultured in IL-2 (500 U/mL). After 3 days, the cells were washed and their cytotoxicity against Daudi and YAC-1 cells was measured using the51Cr and 125I-UdR release assays, respectively. Figures show the % kill ± 1 SD. A constant E:T of 25:1 was used. The average spons/max ratios were 14.8% for the Daudi cell experiments and 10.0% for the YAC-1 cell experiments. The Mann Whitney statistical test was used to compare data.
LAK activity of purified cord blood and BM NK cells against YAC-1 target cells. NK cells were purified and cultured in IL-2 (500 U/mL). CD56+ cells comprised 86.7% ± 7.0% and 82.8% ± 9.0% of purified populations in cord blood (n = 5) and BM (n = 4), respectively. After 3 days, the cells were washed and their cytotoxicity against YAC-1 cells measured using the125I-UdR release assay and at three E:T ratios. The points represent individual samples. The average spons/max ratio was 8.0%.
LAK activity of purified cord blood and BM NK cells against YAC-1 target cells. NK cells were purified and cultured in IL-2 (500 U/mL). CD56+ cells comprised 86.7% ± 7.0% and 82.8% ± 9.0% of purified populations in cord blood (n = 5) and BM (n = 4), respectively. After 3 days, the cells were washed and their cytotoxicity against YAC-1 cells measured using the125I-UdR release assay and at three E:T ratios. The points represent individual samples. The average spons/max ratio was 8.0%.
Measurement of granzyme B enzyme activity.
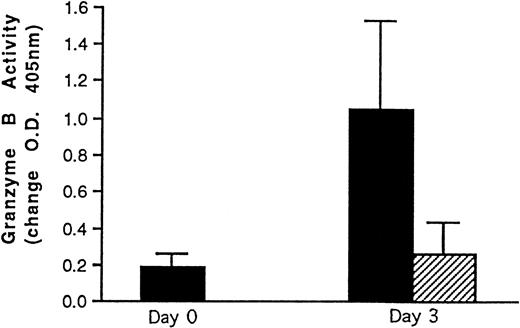
Granzyme B activity levels were measured in cord blood and BM LAK cell extracts. The results obtained are shown in Fig4. Freshly isolated cord cells had low granzyme B enzyme activity. Incubation with IL-2 induced granzyme B activity in cord LAK cells. This was significantly higher than that of BM LAK cell extracts, supporting the cytotoxicity data obtained.
Granzyme B enzyme activity data. (▪) Cord blood (n = 8) and (□) BM (n = 4) mononuclear cell preparations were incubated in IL-2 (500 U/mL). After 3 days, the cells were washed and lysed and the resulting cell extracts were incubated with a substrate for granzyme B. Freshly isolated cord blood mononuclear cell extracts (n = 8) were also analyzed. Enzyme activity was followed colorimetrically at 405 nm and is expressed as a change in OD + 1 SD with time. Readings were taken after 3 days of incubation at 37°C. The Mann Whitney statistical test was used to compare data. *P< .02.
Granzyme B enzyme activity data. (▪) Cord blood (n = 8) and (□) BM (n = 4) mononuclear cell preparations were incubated in IL-2 (500 U/mL). After 3 days, the cells were washed and lysed and the resulting cell extracts were incubated with a substrate for granzyme B. Freshly isolated cord blood mononuclear cell extracts (n = 8) were also analyzed. Enzyme activity was followed colorimetrically at 405 nm and is expressed as a change in OD + 1 SD with time. Readings were taken after 3 days of incubation at 37°C. The Mann Whitney statistical test was used to compare data. *P< .02.
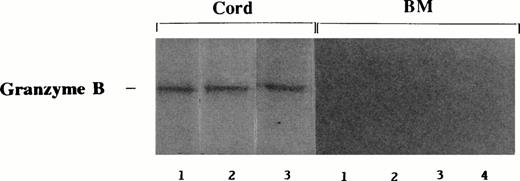
Granzyme B expression in cord blood and BM LAK cells.
Cord blood and BM LAK cells were examined for granzyme B protein expression by Western blotting. Figure 5shows that all cord blood samples that had been cultured in IL-2 had strong granzyme B bands present. In contrast, none of the BM samples tested had detectable levels of the protein.
Expression of granzyme B in IL-2–stimulated cord blood and BM cells. Cord blood (n = 3) and BM (n = 4) mononuclear cells were cultured in IL-2 (500 U/mL) for 3 days. Samples were extracted, electrophoresed on 15% nonreducing SDS-PAGE gel, and immunoblotted using anti-granzyme B monoclonal antibody. AEC was used to visualize immunostaining.
Expression of granzyme B in IL-2–stimulated cord blood and BM cells. Cord blood (n = 3) and BM (n = 4) mononuclear cells were cultured in IL-2 (500 U/mL) for 3 days. Samples were extracted, electrophoresed on 15% nonreducing SDS-PAGE gel, and immunoblotted using anti-granzyme B monoclonal antibody. AEC was used to visualize immunostaining.
IL-12 does not induce BM cells to kill YAC-1 target cells by apoptosis.
In an effort to induce apoptotic killing activity, BM mononuclear cells were cocultured with IL-2 and IL-12. IL-12 has been reported to induce NK activity and to synergize with IL-2 in the induction of LAK activity.35 36 BM cells were cultured for 3 days at 500 U/mL IL-2 and 1 ng/mL IL-12. The % kill for the cocultured BM cells at E:T 25:1 was 1.3% ± 2.0%, compared with 4.0% ± 4.4% for cells cultured in IL-2 alone (n = 3).
BM cells have variable kinetics of induction of LAK activity.
In further experiments to try to induce BM cells to kill YAC-1 targets, BM cells were incubated for longer time periods in IL-2. LAK activity against Daudi target cells reached a consistent level of killing after 7 days of incubation in IL-2 (Fig 6). However, the data for the YAC-1 target cells were variable: one sample had adequate killing, two samples developed low killing activity, and one sample failed to induce any apoptosis in YAC-1 cells.
BM cytotoxicity after prolonged exposure to IL-2. BM mononuclear cells were isolated and set up in culture with IL-2 (500 U/mL) for 7 days. After this time, the cells were washed and their cytotoxicity against Daudi and YAC-1 cells measured in cytotoxicity assays. Individual results are shown. A standard E:T of 25:1 was used. The average spons/max ratios were 11.1% and 7.0% for Daudi and YAC-1 cells, respectively.
BM cytotoxicity after prolonged exposure to IL-2. BM mononuclear cells were isolated and set up in culture with IL-2 (500 U/mL) for 7 days. After this time, the cells were washed and their cytotoxicity against Daudi and YAC-1 cells measured in cytotoxicity assays. Individual results are shown. A standard E:T of 25:1 was used. The average spons/max ratios were 11.1% and 7.0% for Daudi and YAC-1 cells, respectively.
A reduced number of BM cells express the IL-2R.
Cord blood and BM mononuclear cells were phenotyped for their expression of IL-2R. A total of 18.0% ± 9.8% of cord blood gated lymphocytes (n = 7) expressed IL-2R. On double label analysis, 13.4% ± 5.2% CD56+ cord cells and 17.6% ± 4.7% CD16+ cord cells (n = 4) coexpressed IL-2R. Only 2.5% ± 2.6% of lymphocyte gated BM cells (n = 4) expressed IL-2R (P < .01).
DISCUSSION
NK and LAK cells can induce both necrotic and apoptotic death of target cells.14 Apoptosis of target cells can be induced by a number of mechanisms, including fas/fas-ligand interactions,16 triggering by TNF-α17(secreted by effector cell), or triggering by granzyme proteins.18,19 Perforin, the key molecule involved in necrosis, exerts its effect upon insertion into the target cell membrane, where it polymerizes to form a pore, causing cell death by osmotic lysis.15 Research on cytotoxicity mechanisms to date has focused on necrosis. However, the distinction between the two processes may not be as clear-cut as was first assumed. Recent literature suggests that perforin may represent part of the apoptosis mechanism of killing,19,24 where it may function to facilitate transfer of cytotoxic granule contents into a target cell where they can trigger apoptosis. Whereas there is no doubt that perforin can cause cell lysis,15 it is possible that this may be restricted to particular situations, eg, in target cells, where necrosis may be the only option available, due to inactivation or disruption of the indigenous apoptosis biochemical pathway. This situation occurs in many cell lines. The K562 cell line, which has long served as the target cell line for measurement of NK activity,29 has been reported to be relatively resistant to induction of apoptosis due to the presence of the bcr-abl complex.37 Therefore, one can conclude that, in vitro, or indeed in vivo, these cells will invariably die by necrosis, as detected in a 51Cr release assay. In this study, the YAC-1 cell line was chosen for the apoptosis assay, because DNA fragmentation is readily measured and the cells are recognized by LAK effectors. Daudi cells, which do not undergo measurable DNA fragmentation (data not shown), were used as targets to measure necrosis.
The NK-cell–mediated killing potential of freshly isolated cord blood and BM cells was examined using the K562 target cell line, and, although cord cells had higher killing at all E:T cell ratios tested, the differences seen were not significant. Data in the literature regarding cytotoxicity of BM cells are somewhat variable, with low,25 no,26 or variable38 killing figures reported. Reported kinetics of LAK induction also vary, ranging from 139 to 626 days. In this study, BM LAK cells (3-day cultures) had variable killing against Daudi cells, as indicated by the high standard deviation figure. Cord LAK activity, generally reported to be higher than that of adult peripheral blood cells,12 13 was also significantly higher than in BM cells.
Cord blood LAK cells were shown qualitatively to induce apoptosis in target cells by agarose electrophoresis. This positive finding was paralleled in the 125I-UdR release assay, which is used to quantify DNA fragmentation as an indicator of apoptosis. In contrast, BM-derived cells had a complete inability to induce apoptosis of YAC-1 target cells, a finding confirmed using purified NK-cell preparations. The significance of one purified NK-cell BM sample mediating apoptotic killing activity is not immediately apparent and may reflect peripheral blood NK-cell contamination of the BM aspirate. The contrasting finding of high cord LAK apoptotic activity versus absent BM LAK activity was confirmed at the effector molecule level. It is unlikely that cytotoxic T cells contributed to this difference because the percentages of CD8 cells in cord blood and BM were similar. BM cells were also relatively resistant to efforts to stimulate their apoptotic mechanism: culture in IL-12 did not, and prolonged culture in IL-2 was only partially successful in overcoming this nonresponsiveness. The basis for the absence of apoptotic cytotoxicity in BM cells remains unclear. BM NK cells may represent a functionally immature cell type, compared with cord cells, with acquisition of apoptotic cytotoxic potential reflecting a more mature effector phenotype. Alternatively, BM NK cells might be fully mature cells that have been potently downregulated, at a functional level, by the immunosuppressive environment of the BM. High concentrations of TGF-β in BM, previously reported by this group40 and others,41 may play a key role in the observed IL-2 nonresponsiveness. IL-2 only partially restored cytotoxicity of adult42 and cord mononuclear cells that had been preincubated in TGF-β (data not shown). It seems likely that a combination of altered effector cell maturation status and environmental influences contribute to the cytotoxicity characteristics of BM NK cells that distinguish them from their cord blood counterparts.
The high concentration of TGF-β in BM may also contribute to the observation that relatively few BM cells expressed any IL-2R, compared with almost 20% of cord blood lymphocytes. Low CD25 (IL-2Rα chain) has previously been reported on lymphocyte-gated BM cells.43 TGF-β reduces IL-2Rα expression and inhibits protein phosphorylation induced by IL-2.42 It is known that culture of lymphocytes in IL-2 results in increased IL-2R expression.44 This fact, combined with observations from the extended IL-2 incubation experiments performed here, suggests that longer time incubations may possibly restore the apoptotic pathway of killing in BM cells.
It is known that NK cells play a role in the regulation of hematopoiesis,45,46 and it is possible that they may do this by a number of mechanisms, including direct cytotoxic activity47,48 and/or cytokine secretion.49,50 If apoptosis is the physiologic form of cell death, the resistance of BM NK cells to IL-2 induction of cytotoxicity, as reported here, suggests that cytotoxicity is not a key effector mechanism. Other recent reports support this finding51 and even suggest that graft rejection mediated by NK cells is not at the level of cytotoxicity but rather by NK-cell regulation of hematopoiesis.52-54 Given the cytokine secretion profile of NK cells that include IL-3,55,56TNF-α,57 interferon-γ,58TGF-β,59 and granulocyte-macrophage colony-stimulating factor,55 56 it seems probable that they will function as regulatory cells via the cytokines they produce.
Despite not being able to kill YAC-1 cells by inducing them to undergo apoptosis, IL-2–stimulated BM cells were still capable of killing Daudi target cells. This indicates a tighter regulation of molecules, eg, granzyme B, involved in apoptosis versus necrosis. This, in turn, suggests that apoptosis is the more important mechanism in vivo, thereby further supporting earlier presented arguments.
It is known that BM NK cells mediate a GVL effect in BMT patients.4,5 The potential GVL effect of cord blood is as yet unknown, but the data presented here suggest that cord blood may have an increased potential for GVL compared with BM. This is indicated by the consistently higher LAK activity of cord blood cells. Freshly isolated cells from both cord blood and BM had comparable killing, and it may therefore be necessary to activate cells with cytokines for their potential to be realized. In human studies, IL-2 infusion into patients suffering from metastatic melanoma resulted in increased expression of both perforin and granzyme B genes by circulating cells.60 Therefore, in vivo activation of cytotoxic cells could potentially be used in a transplant situation to boost GVL. In a murine transplantation model system, it has been found that in vitro activation of BM cells and subsequent IL-2 infusion were both required for an optimal GVL effect.39 However, the potential beneficial effects of the high responsiveness of cord cells to IL-2, in mediating increased GVL, are as yet unknown and deserve further investigation.
ACKNOWLEDGMENT
The authors thank the staff of the Coombe Women's Hospital, Dublin 8, and the orthopedic and theatre staff of Our Lady's Hospital for Sick Children, Dublin 12, and St. Vincent's Hospital, Dublin 4, for their cooperation in supplying cord blood and BM samples, respectively.
Address reprint requests to Denis J. Reen, PhD, The Children's Research Centre, Our Lady's Hospital for Sick Children, Crumlin, Dublin 12, Ireland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal