Abstract
Activation of natural killer (NK) cells in the presence of interleukin-12 (IL-12) augments the capacity of these effector cells to recognize B7-1– and B7-2–expressing target cells. These effector cells also efficiently lyse autologous B7-positive progenitor or organ-derived dendritic cells, suggesting a physiologic regulatory pathway between IL-12, NK cells, and B7-expressing antigen-presenting cells. Although IL-12–activated NK cells secreted higher levels of interferon-γ, this cytokine did not play a role in synergistic effects of IL-12 and B7 on NK activation. The B7-counterreceptor was found to be selectively upregulated on IL-2/IL-12 as compared with IL-2–activated NK cells. CD28 is functionally involved in the recognition of B7 on target cells since IL-2/IL-12–activated NK cells derived from CD28 knockout mice were strongly reduced in their capacity to lyse syngeneic B7-positive tumor cells as well as antigen-presenting cells. However, recognition of B7 on allogeneic targets did not require the expression of CD28 on the IL-2/IL-12–activated NK cells. Hence, IL-12 triggers the expression of both CD28-dependent and CD28-independent mechanisms that allow NK cells to eliminate B7-positive target cells including autologous dendritic cells.
NATURAL KILLER (NK) cells constitute phenotypically and functionally a diverse population of large granular lymphocytes in peripheral blood and spleen. They are spontaneously cytotoxic against a variety of target cells, including certain tumor cell lines and virus-infected cells as well as allogeneic bone marrow (BM) and lymphoid cells. Although this activity was initially considered to be nonspecific and non-major histocompatability complex (MHC) restricted, NK cells are capable to discriminate specifically between their targets.1 Although NK cell specificity involves both activating and inhibitory receptors, in general a target inhibition type of activation mechanism determines this specificity.2-5 Indeed, we and others have shown that NK target engagement could be inversely correlated to the expression of specific MHC class I molecules on the targets.6-12 These observations have been strengthened by the identification of the MHC class I–specific Ly49 C-type lectin family of inhibitory receptors on mouse NK13-16 and KIRs (killer cell inhibitory receptors) on human NK cells.17-19 Furthermore, recent evidence suggests that the NK-activating stimuli, delivered by target cells, could be overruled by class I–mediated inhibition.20
In contrast to the inhibitory pathway of NK regulation, less is known about the triggering and activation events. In fact, the target inhibition model could not account for the observations that some virus-infected, transformed, and even autologous cells were NK-sensitive notwithstanding a normal expression of class I molecules.21-23 Hence, appropriate activation signals may overrule the class I–mediated inhibition and this notion was strengthened by the observation that expression of B7-1 costimulatory molecules on NK-resistant tumor cell variants rendered these target cells NK-sensitive in vivo and in vitro.24-27
NK cell stimulatory factor (NKSF) or interleukin-12 (IL-12) is a heterodimeric cytokine produced by macrophages, B cells, and other accessory cell types, which exerts immunomodulatory effects on T and NK cells.28 In particular, IL-12 has been shown to (1) synergize with low doses of IL-2 in generation of LAK activity,29 (2) enhance the cytotoxic activity of NK cells,30,31 (3) induce the secretion of IFN-γ by either T or NK cells,28 (4) mediate direct mitogenic effects on activated T and NK cells,32 (5) induce the secretion of tumor necrosis factor (TNF)-α and -β by purified NK cells,30 and (6) augment the expression of IL-2Rα and TNFR30,31 as well as cell adhesion molecules on human T and NK cells32 and to negatively regulate the proliferation of IL-2–stimulated NK cells.31 Interestingly, Kubin et al33 showed that IL-12 synergized with B7 costimulation to increase the proliferation of and cytokine release by T cells in vitro. These observations were confirmed by demonstrating that the antitumor effect of B7 transfection into a tumor-cell vaccine could be further amplified by administration of IL-12 at the vaccine site34or by a combination of B7-1– and IL-12–transfected tumor cells.35
In these different investigations all the reported effects were ascribed solely to the interaction between CD28 on T cells and B7-1 on the target cells.34,35 In view of the involvement of both B7 and IL-12 in NK cell activation, it was of interest to investigate whether IL-12 could also play a role in the interaction between B7 (on target cells) and NK cells. To this end the activity of LAK cells, generated in the presence of IL-2 with or without IL-12, toward diverse targets expressing B7-1 and/or B7-2 was compared. Furthermore, these two different NK effector populations were used to delineate the NK receptors that may be involved in the target (B7)/NK interaction. Finally, the differential activated NK cells were tested for their capacity to recognize B7 on syngeneic normal cells such as dendritic cells (DCs) aiming at further clarifying the role of NK cells in the elimination of autologous antigen presenting cells.23 27
MATERIALS AND METHODS
Mice.
Specific pathogen-free female AKR (Thy 1.1, H-2k), C57BL/6 (Thy 1.2, H-2b), B10.BR (Thy 1.2, H-2k), and CB-17 SCID/SCID (H-2d) mice were obtained from Harlan CPB (Zeist, The Netherlands). CD28−/−knockout mice were kindly provided by C. Thompson (Gwen Knapp Center, University of Chicago, Chicago, IL) to M.M. and approved to be used in the described experiments.36
Tumor cell lines.
The highly malignant BW-Li (H-2K+, H-2D++) is a spontaneous metastasizing variant derived from the original, nonmetastatic BW5147 T-cell lymphoma (AKR origin; Salk Institute, La Jolla, CA). The BW-LiDhigh(H-2K−, H-2D+++) variant was obtained from BW-Li by successive fluorescence-activated cell sorter (FACS) sortings for high H-2D and low H-2K expression. Transfection of BW-Li and BW-LiDhigh with pcDNAneo1::MoB7-124 or pcDNA3::MoB7-2 (obtained by cloning mouse B7-2 cDNA in the pcDNA3 expression vector (Invitrogen BV, Leek, The Netherlands) viaEcoRI restriction) gave rise to BW-Li(B7-1)/BW-LiDhigh(B7-1) and BW-Li(B7-2)/BW-LiDhigh(B7-2), respectively.6,24YAC-1 is a Moloney murine leukemia virus–induced line of A/Sn origin. RMA-S is an antigen-processing defective mutant of RMA (C57BL/6 origin) and was kindly provided by L. Eisenbach (ILRAD, Israel). RMA-S(B7-1) was derived from RMA-S after transfection and was kindly provided by M. Bellone (Instituto Scientifico H. San Raffaele, Milan, Italy).37 All tumor cell lines were cultured in RPMI supplemented with 10% fetal calf serum, antibiotics, and L-glutamine (GIBCO, Grand Island, NY).
Generation of A-LAK cells.
Mouse spleen cells are isolated by flushing mouse spleens with erythrocyte lysis buffer (8.29 g NH4Cl, 1.0 g KHCO3, and 0.0372 g EDTA/L, pH 7.4) by means of a needle and syringe. 2 × 108 spleen cells are loaded on a 0.4-g nylon wool column (Wako Chemicals, Osaka, Japan), pre-equilibrated with Dulbecco's modified Eagle's medium (DMEM) containing 5% heat-inactivated fetal calf serum (FCS). After 45 minutes' incubation at 37°C, the nonadherent cell fraction (mainly T cells) is collected and counted. The cells are brought to a concentration of 2 × 106/mL in RPMI medium, supplemented with 0.3 mg/mL L-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 10% FCS, 5 × 10−5 mol/L mercaptoethanol (=CM), and 1,000 U/mL IL-2 (Eurocetus, Amsterdam, The Netherlands), and incubated at 37°C in 5% CO2 and 100% humidity during 5 days. For the generation of IL-2/IL-12 LAK, recombinant mouse IL-12 (generously provided by Genetics Institute, Inc, Cambridge, MA) was added to the IL-2 LAK cultures at 100 U/mL, 48 hours after initiation of the cultures for another 72 hours. The total LAK cell recovery varied from 125% to 150% for IL-2 LAKs and from 60% to 85% for IL-2/IL-12 LAKs as compared with the initial cell number at the start of the LAK culture in all experiments performed. The adherent cell fraction, comprising between 20% to 40% of the total LAK cell population, was harvested with 0.01% EDTA in phosphate-buffered saline (PBS) and was used as effector cell population (A-LAK) in [111In]-release cytotoxicity assays. The viability of the IL-2 A-LAK population was always greater than 84% for the IL-2 A-LAK and greater than 87% for the IL-2/IL-12 A-LAKs in all experiments performed.
Dendritic cell preparations.
DCs were purified from spleens according to a procedure described by Crowley et al.38 Briefly, spleens were digested with collagenase (CLS III; Worthington, Freehold, NJ) and separated into low- and high-density fractions on bovine serum albumin gradient (Bovuminar Cohn Fraction V powder; Armour Pharmaceutical Co, Tarrytown, NJ). Low-density cells were cultured during 2 hours and nonadherent cells were removed by vigorous pipetting. The same procedure was repeated with a shorter incubation (1 hour) in serum-free media. After overnight culture, nonadherent cells were collected and contained at least 90% of DC as assessed by morphology and specific staining using N418 monoclonal antibody (MoAb).39 DCs were generated from BM progenitors according to a procedure modified from a protocol of Inaba et al.40 Briefly, BM was flushed from tibias and femurs and depleted of lymphocytes, granulocytes, and Ia+cells using a cocktail of MoAbs and sheep anti-rat IgG DYNABEADS M-450 (Dynal, Oslo, Norway). The MoAbs were anti-CD8, anti-CD4, GR-1 anti-granulocyte, anti-B220/CD45R, and anti-I-Ab/I-Eb (Pharmingen, San Diego, CA). Cells were plated in 24-well culture plates (2.5 × 105/mL, 1 mL/well) in DMEM supplemented with 10% heat-inactivated FCS and 200 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and cultured for 10 days. The cultures were usually fed every 2 days by gently swirling of plates, aspirating 75% of medium, and adding fresh medium with GM-CSF. Cultured cells were readily identified as DCs on the basis of their distinct morphology and expression of antigens (anti-I-Ab/I-Eb, N418, anti-B7-1, and anti-B7-2).24
[111In]-release cytotoxicity assay.
The target cells were labeled with [111In] by incubating 106 cells in 100 μL (RPMI + 5% FCS) with 0.1 mCi [111In]Cl (Amersham, Gent, Belgium) during 10 minutes.24 After extensive washing, 104 target cells were incubated with twofold dilutions of effector cells in a total volume of 200 μL CM in 96-well round-bottom plates. In blocking experiments, labeled targets were incubated with 0.5 mg/mL anti-B7-1 or anti-B7-2 F(ab)2 fragments during 30 minutes at room temperature and immediately included in the [111In]-release assay. In parallel, F(ab)2fragments of isotype controls (hamster IgG for anti-B7-1 and rat IgG2a, κ for anti-B7-2) were generated in the lab and added to the targets as described above. All experiments were performed in triplicates and at least twice repeated. After 4 hours at 37°C, the plates are centrifuged 3 minutes at 600 rpm, 100 μL of supernatants is collected and radiation is counted in a γ-counter. The percentage of specific lysis was calculated as ([Experimental Release − Spontaneous Release]/[Maximal Release − Spontaneous Release]) × 100, where the spontaneous release was determined from labeled target cells incubated without effector cells and maximal lysis from target cells incubated 1 hour before harvesting in 2% sodium dodecyl sulfate (SDS).
FACS staining and analysis of A-LAK cells.
Cell samples comprising 106 adherent LAK cells were incubated with the appropriate dilutions of antibodies, as suggested by the distributors, at 4°C for periods of 30 minutes. Mouse anti-NK1.1-PE and anti-Ly49A-FITC (both with corresponding fluorochrome labeled mouse IgG2a,κ [anti-TNP] isotype controls), rat anti-CD8a-FITC, anti-CD4-PE (RM4-5) and anti-CD8a-PE (with fluorochrome rat IgG2a,κ isotype control), rat anti-mouse Pan-NK cell marker DX-5-FITC (with rat IgM,κ-FITC isotype control) and hamster anti-CD28-PE (with syrian hamster [anti-Vγ3 TCR-PE (536)] IgG isotype control) and anti-CTLA-4-PE (with armenian hamster IgG (anti-TNP-PE) isotype control) were purchased from Pharmingen. Rat anti-mouse IL-2R was purchased from the ATCC (cat no. CRL1698). To prevent FcR-mediated binding of staining antibodies to the LAK cells, the Fc-receptors were blocked with 2.4G2 derived F(ab)2antibodies, before the addition of the indicated antibodies. The isotype controls were included during FACS staining and did not give a significant aspecific background staining. The proper isotype controls were used as a reference to delineate the negative populations of the specifically stained LAKs. The stained cells were subjected to FACS analysis with the Becton Dickinson (Sunnyvale, CA) FACStar coupled to an Apple Macintosh FACStation. The results were analyzed with the CellQuest program.
Cytokine assays.
The production of IL-2 and IFN-γ was quantified by subjecting culture supernatants to an IL-2– or IFN-γ–specific sandwich enzyme-linked immunosorbent assay applying purified rat anti-mouse and biotinylated rat anti-mouse IL-2 and IFN-γ (Pharmingen) and comparing the results with a dilution series of recombinant mouse IL-2 and IFN-γ.
The production of IFN-γ in IL-2/IL-12 LAK cultures was blocked by adding 3 μg/mL of neutralizing anti-mouse IFN-γ (HB170 [ATCC], generously provided by A. Darji [GBF, Braunschweig, Germany]), at the same time that IL-12 was added to the LAK cultures. Up to the time of harvesting, no IFN-γ could be detected in the culture supernatant of the anti-IFN-γ–treated LAK cultures, while the untreated IL-2/IL-12 LAKs produced significant amounts of IFN-γ, as measured by sandwich ELISA (see above). In parallel, IL-2/IL-12 LAK cultures were also treated with isotype control antibodies, purchased from Pharmingen. Inclusion of the isotype control antibodies did not influence the cytotoxic activity, nor the production of IFN-γ, by the LAK cells.
Statistical analysis.
The statistical relevance of differences in specific release was evaluated with a two-way ANOVA test, applying GraphPad Prism software (GraphPad Software Inc, San Diego, CA). Differences are considered as not significant for P ≥ .05, significant for P < .05, and very significant for P ≤ .01.
RESULTS
Presence of IL-12 during LAK cell generation augments B7-1 and B7-2 triggered NK-mediated cytotoxicity toward B7 target cell lines.
To investigate the cytolytic activity of IL-12–activated NK cells toward BW5147 T-lymphoma variants and thereof derived B7 transfectants, IL-12–activated LAK cells were generated by culturing nylon wool purified spleen cells in the presence of 1,000 U/mL IL-2 or IL-12 during 5 days. Because addition of IL-12 at the initiation of the LAK culture was found to abrogate completely LAK cell generation (data not shown), conditions were optimized to obtain IL-12–activated LAK cells. IL-12–activated LAK cells were generated by preactivating nylon wool purified spleen cells during 2 days with IL-2 and supplementing 100 U/mL of IL-12 for 3 additional days. The cytotoxic activity of the adherent fraction of IL-2–activated LAK (IL-2 A-LAK) cells as compared with the adherent fraction of IL-2/IL-12 LAK (IL-2/IL-12 A-LAK) cells was tested on two different MHC class I–positive BW variants and thereof derived B7-1 and B7-2 transfectants. In accordance with earlier observations,24 expression of B7-1 on BW cells strongly enhanced (P < .0001) the sensitivity of the target cells to IL-2 A-LAK lysis (Fig 1). Interestingly, including IL-12 during LAK generation further potentiated (P < .0001) the cytotoxic activity of LAK cells toward B7-1-expressing BW cells (Fig 1). Furthermore, even if the expression of B7-2 on BW-LiDhigh cells was not as potent as B7-1 to confer sensitivity to IL-2 A-LAK–mediated lysis, activation of LAK cells with IL-12 increased the potency of these effector cells to lyse efficiently BW(B7-2) transfectants (P < .0001) (Fig 1). To show that T cells were not implicated in the B7-induced cytolytic capacity of IL-2 or IL-2/IL-12 A-LAK cells, LAK populations were generated from nylon wool purified severe combined immunodeficiency disease (SCID) spleens. As shown in Fig2, SCID A-LAKs exerted a similar activity toward BW(B7) as normal A-LAKs because they also lysed preferentially B7-1–expressing targets (P < .0001) and this activity could be further potentiated by incubating the LAK cells with IL-12 during 3 days (P = .0025) (Fig 2). Hence, T cells were not involved in the lysis of BW(B7) transfectants.
A-LAK cytotoxicity toward BW variants transfected with B7-1 or B7-2. AKR-derived LAK cells were generated in the presence of IL-2 alone or in combination with IL-12 and the cytolytic activity of the respective adherent fractions was tested against BW T-lymphoma variants and the NK-sensitive tumor YAC-1. The results are represented as the mean cytolysis of the respective targets as percent specific release (±SD). (A) Sensitivity of BW-LiDhigh (×), BW-LiDhigh (B7-1) (+), BW-LiDhigh (B7-2) (▴), and YAC-1 (*) to IL-2 versus IL-2/IL-12 A-LAK effector cells. (B) Sensitivity of BW-Li (▪), BW-Li(B7-1) (⧫), BW-Li(B7-2) (•), and YAC-1 (*) to IL-2 versus IL-2/IL-12 A-LAK effector cells. Spontaneous release in all assays was less than 15%. Both experiments were performed in triplicate and repeated at least five times, giving similar results from which one is shown. Standard deviations less than ±3% are not shown for the sake of clarity.
A-LAK cytotoxicity toward BW variants transfected with B7-1 or B7-2. AKR-derived LAK cells were generated in the presence of IL-2 alone or in combination with IL-12 and the cytolytic activity of the respective adherent fractions was tested against BW T-lymphoma variants and the NK-sensitive tumor YAC-1. The results are represented as the mean cytolysis of the respective targets as percent specific release (±SD). (A) Sensitivity of BW-LiDhigh (×), BW-LiDhigh (B7-1) (+), BW-LiDhigh (B7-2) (▴), and YAC-1 (*) to IL-2 versus IL-2/IL-12 A-LAK effector cells. (B) Sensitivity of BW-Li (▪), BW-Li(B7-1) (⧫), BW-Li(B7-2) (•), and YAC-1 (*) to IL-2 versus IL-2/IL-12 A-LAK effector cells. Spontaneous release in all assays was less than 15%. Both experiments were performed in triplicate and repeated at least five times, giving similar results from which one is shown. Standard deviations less than ±3% are not shown for the sake of clarity.
Lytic activity of SCID-spleen-derived A-LAK cells. The activity of IL-2-activated (filled symbols) versus IL-2/IL-12-activated (open symbols) A-LAK cells was measured in an in vitro cytotoxicity assay. The specific lysis of BW-Li (squares) and BW-Li(B7-1) (diamonds) target cells was compared and here represented as the mean percent specific [111In]-release of triplicates (±SD). Spontaneous release was less than 5%. One of two similar experiments is shown. Standard deviations less than ±3% are not shown for the sake of clarity.
Lytic activity of SCID-spleen-derived A-LAK cells. The activity of IL-2-activated (filled symbols) versus IL-2/IL-12-activated (open symbols) A-LAK cells was measured in an in vitro cytotoxicity assay. The specific lysis of BW-Li (squares) and BW-Li(B7-1) (diamonds) target cells was compared and here represented as the mean percent specific [111In]-release of triplicates (±SD). Spontaneous release was less than 5%. One of two similar experiments is shown. Standard deviations less than ±3% are not shown for the sake of clarity.
Because IL-12 was found to induce the cytolytic activity of NK cells toward NK-sensitive (P ≤ .01 for YAC-1) but not to NK-resistant targets (P = .08 for BW-LiDhigh) (Fig1), it was of importance to determine whether the increased activity of IL-2/IL-12 A-LAKs against BW(B7) targets involved recognition of B7. To avoid Fc receptor-mediated antibody-dependent cellular cytotoxicity activities of the A-LAK cells, target cells were treated with the F(ab)2 fragments of anti-B7-1 or anti-B7-2 antibodies. As shown in Table 1, blocking B7-1 abrogated almost completely the B7-1-induced lytic activity of IL-2 A-LAKs at the different E/T ratios. The increased cytolytic activity of IL-2/IL-12 A-LAKs toward B7-1 and B7-2 transfected, as compared with the nontransfected BW variants, was reversed with at least 80% at the highest E/T ratios and strongly reduced at the lowest E/T ratios (Table1). This specific effect shows that most of the enhanced cytolysis of B7 transfectants by IL-2/IL-12 A-LAKs is due to the recognition of B7-1 or B7-2 costimulatory molecules by NK cells.
Effect of Anti-B7-1 or Anti-B7-2 F(ab)2Treatment on the Lysis of BW Targets by IL-2 A-LAK and IL-2/IL-12 A-LAK Effector Cells
| Target Cells . | . | BW-Li(B7-1) . | BW-Li(B7-2) . | ||||
|---|---|---|---|---|---|---|---|
| Treatment . | . | None . | AntiB7-1* . | Control* . | None . | AntiB7-2* . | Control* . |
| Effector cells . | E:T . | % Increase† . | % Inhibition‡ . | % Increase† . | % Inhibition‡ . | ||
| IL-2 LAK | 5 | 0 | — | — | 0 | — | — |
| 10 | 266 | 74 | 0 | 207 | 32 | 0 | |
| 20 | 179 | 80 | 1 | 134 | 65 | 16 | |
| IL-2/IL-12 LAK | 5 | 177 | 100 | 0 | 206 | 75 | 0 |
| 10 | 309 | 68 | 0 | 259 | 52 | 7 | |
| 20 | 116 | 80 | 0 | 181 | 100 | 25 | |
| Target Cells . | . | BW-Li(B7-1) . | BW-Li(B7-2) . | ||||
|---|---|---|---|---|---|---|---|
| Treatment . | . | None . | AntiB7-1* . | Control* . | None . | AntiB7-2* . | Control* . |
| Effector cells . | E:T . | % Increase† . | % Inhibition‡ . | % Increase† . | % Inhibition‡ . | ||
| IL-2 LAK | 5 | 0 | — | — | 0 | — | — |
| 10 | 266 | 74 | 0 | 207 | 32 | 0 | |
| 20 | 179 | 80 | 1 | 134 | 65 | 16 | |
| IL-2/IL-12 LAK | 5 | 177 | 100 | 0 | 206 | 75 | 0 |
| 10 | 309 | 68 | 0 | 259 | 52 | 7 | |
| 20 | 116 | 80 | 0 | 181 | 100 | 25 | |
*Targets were treated during 30 minutes with anti-B7-1, anti-B7-2, and isotype control F(ab)2 fragments of antibodies before inclusion in 111In-release assay (see Materials and Methods).
Percent increase in the specific lysis of BW-Li transfected with B7-1 or B7-2 as compared with nontransfected cells.
Percent inhibition of the B7-1/B7-2–induced increase in LAK-mediated lysis of BW-Li variants due to B7-1, B7-2, or isotype control F(ab)2 antibody treatment.
IFN-γ is not required for the increased lytic activity of IL-2/IL-12 A-LAK cells on B7 target cell lines. Comparing IL-2 LAK with IL-2/IL-12 LAK cultures, we observed that addition of IL-12 during LAK generation induces strongly the production of IFN-γ (data not shown). Locally produced IFN-γ may in turn increase the expression of IL-2R on IL-2/IL-12 LAK cells and may consequently result in a higher consumption of IL-2 in these cultures. In fact, IL-2/IL-12 A-LAKs were found to express higher levels of IL-2R and no IL-2 could be detected in supernatants of IL-2/IL-12 LAK cell cultures (data not shown). According to different reports, the effect of IL-12 on NK activation has been ascribed to IFN-γ.41 42 Therefore, the possible contribution of IFN-γ on the increased cytotoxic activity of IL-2/IL-12 A-LAKs toward BW(B7-1) and BW(B7-2) was investigated. To this end, neutralizing amounts of anti–IFN-γ antibodies were added to the LAK cultures simultaneous with IL-12. Blocking IFN-γ in the LAK cultures, as verified in enzyme-linked immunosorbent assay (ELISA) (data not shown), did not significantly influence BW(B7-1) (P = .5) or BW(B7-2) (P = .05) directed lysis (Fig3). These results refute a direct involvement of IFN-γ on the induced cytotoxic activity of IL-2/IL-12 A-LAKs.
Blocking of IFN-γ during generation of IL-2/IL-12 LAK cells does not reverse the IL-12-promoting effect on LAK cell lysis of B7-1– or B7-2–expressing tumor cells. The cytolytic activity of AKR-derived IL-2 A-LAKs is compared with the cytolytic activity of untreated or anti–IFN-γ MoAb-treated IL-2/IL-12 A-LAKs on BW-Li, BW-Li(B7-1), and BW-Li(B7-2) targets. The results are represented as the percentage specific [111In]-release (±SE) at different E/T ratios. Standard deviations less than ±3% are not shown for the sake of clarity. Spontaneous release was less than 4%. The experiment was repeated twice. (▧), IL-2 A-LAK; (▧), IL-2 + IL-12 A-LAK; (▩), IL-2 + IL-12 A-LAK + α-IFN-γ.
Blocking of IFN-γ during generation of IL-2/IL-12 LAK cells does not reverse the IL-12-promoting effect on LAK cell lysis of B7-1– or B7-2–expressing tumor cells. The cytolytic activity of AKR-derived IL-2 A-LAKs is compared with the cytolytic activity of untreated or anti–IFN-γ MoAb-treated IL-2/IL-12 A-LAKs on BW-Li, BW-Li(B7-1), and BW-Li(B7-2) targets. The results are represented as the percentage specific [111In]-release (±SE) at different E/T ratios. Standard deviations less than ±3% are not shown for the sake of clarity. Spontaneous release was less than 4%. The experiment was repeated twice. (▧), IL-2 A-LAK; (▧), IL-2 + IL-12 A-LAK; (▩), IL-2 + IL-12 A-LAK + α-IFN-γ.
Presence of IL-12 during LAK cell generation alters the morphotype and the membrane phenotype of A-LAK cells.
The morphotype of IL-2/IL-12 A-LAK cells was strikingly different from IL-2 A-LAK cells because these populations contained a larger proportion of bigger/granulous cells (Fig4A and B), which showed an upregulation of the side scatter signal as evaluated by FACS scatter signal analysis (Fig 4C through E). These cells have the tendency to stick stronger to plastic than IL-2 A-LAK because longer incubation times and higher EDTA concentrations were required to detach efficiently the adherent IL-2/IL-12 LAK cell fractions from the culture plates.
Presence of IL-12 during LAK cell production generates an adherent subpopulation with a specific morphotype. In contrast to IL-2 LAKs (A), IL-12 induces the formation of granulous, large cells (B). Adherent AKR-derived LAK cells were stained with Crystal violet and photographed (original magnification × 100) in culture plate. This morphological shift can also be visualized by FACS scatter analysis of AKR-derived IL-2 A-LAK cells (C) as compared with IL-2/IL-12 A-LAK cells (E). The population with highest cellular density is situated in region 1 (R1) for IL-2 A-LAK and in region 2 (R2) for IL-2/IL-12 A-LAK. The overlay histogram (D) compares the side scatter signals of both LAK populations.
Presence of IL-12 during LAK cell production generates an adherent subpopulation with a specific morphotype. In contrast to IL-2 LAKs (A), IL-12 induces the formation of granulous, large cells (B). Adherent AKR-derived LAK cells were stained with Crystal violet and photographed (original magnification × 100) in culture plate. This morphological shift can also be visualized by FACS scatter analysis of AKR-derived IL-2 A-LAK cells (C) as compared with IL-2/IL-12 A-LAK cells (E). The population with highest cellular density is situated in region 1 (R1) for IL-2 A-LAK and in region 2 (R2) for IL-2/IL-12 A-LAK. The overlay histogram (D) compares the side scatter signals of both LAK populations.
Because BW(B7) transfectants were lysed more efficiently by IL-2 and IL-2/IL-12 A-LAK cells, the expression of B7 counterreceptors on the effector cell populations was analyzed. In accordance with Nandi et al,43 A-LAK cells express higher levels of CD28. Before the addition of IL-2 to the nylon wool-purified spleen cells, CD28 was expressed solely on a CD4/CD8-positive population (Fig5A). Yet after 5 days' culture in the presence of IL-2, a CD4−/CD8−population positive for CD28 expression appeared (Fig 5B). Adding IL-12 to the LAK cultures on day 3 induced drastically the expression of CD28 on the CD4−/CD8− population (Fig5C). This population of CD4−/CD8−/CD28+ cells corresponded to the subpopulation of IL-2/IL-12 A-LAK cells with high granulosity. This result was confirmed by analyzing the expression of CD28 on A-LAK cells derived from SCID mice and as shown in Fig 5D through F, the profile of CD28 expression was comparable with that of the CD4−/CD8− subpopulation of A-LAK cultures generated from immunocompetent spleen cells. The effect of IL-12 on the expression of CD28 and other T and NK cell antigens on IL-2 A-LAK cells are compiled in Fig 6A through C. Although the expression of CD28 was strongly induced by addition of IL-12, the expression of CTLA-4, the second counterreceptor for B7-1 and B7-2, was not found to be elevated during LAK cell generation either with IL-2 or with IL-2 and IL-12 (data not shown). To evaluate the expression of the commonly known NK cell markers such as Ly49 and NK1.1 (not expressed in the H-2k haplotype, syngeneic with BW tumor cells), IL-2 and IL-2/IL-12 LAK were generated from spleens of allogeneic C57BL/6 (H-2b haplotype) and congenic B10.BR (H-2k haplotype) mice strains. The expression of Ly49A and NK1.1 was strongly upregulated during IL-2 LAK culture (result not shown), yet adding IL-12 during LAK cell generation did not further induce the expression of these NK cell–specific membrane antigens (Fig 6B and C). The expression of the NK cell marker DX5 also refutes any positive role of IL-12 in the enrichment of NK cells in the LAK preparations (Fig 6A and C).
IL-12 induces the expression of CD28 on a subpopulation of adherent LAK cells. The expression of CD28 is represented in function of CD4/CD8 expression for AKR-derived nylon wool purified spleen (A), IL-2 A-LAK (B), and IL-2/IL-12 A-LAK (C) cells as compared with SCID-derived nylon wool purified spleen (D), IL-2 A-LAK (E), and IL-2/IL-12 A-LAK (F) cells. The A-LAK cells were pretreated with 2.4G2 antibodies to block Fc receptors and subsequently incubated with phycoerythrin (PE)-labeled CD28 and fluorescein isothiocyanate (FITC)–labeled CD8 antibodies. The dot plots indicate the percentage of positive cells calculated via quadrant statistics using the suitable isotype controls.
IL-12 induces the expression of CD28 on a subpopulation of adherent LAK cells. The expression of CD28 is represented in function of CD4/CD8 expression for AKR-derived nylon wool purified spleen (A), IL-2 A-LAK (B), and IL-2/IL-12 A-LAK (C) cells as compared with SCID-derived nylon wool purified spleen (D), IL-2 A-LAK (E), and IL-2/IL-12 A-LAK (F) cells. The A-LAK cells were pretreated with 2.4G2 antibodies to block Fc receptors and subsequently incubated with phycoerythrin (PE)-labeled CD28 and fluorescein isothiocyanate (FITC)–labeled CD8 antibodies. The dot plots indicate the percentage of positive cells calculated via quadrant statistics using the suitable isotype controls.
NK and T-cell markers on H-2k– and H-2b–derived IL-2 and IL-2/IL-12 A-LAK populations. Flow cytometric analysis of (A) AKR (H-2k), (B) C57BL/6 (H-2b), and (C) B10.BR (H-2k)–derived IL-2 versus IL-2/IL-12 A-LAK preparations, stained with FITC (Y axis) and PE (X axis)–labeled MoAbs as described in Materials and Methods. The cells were preincubated with 2.4G2 for 30 minutes before staining. The results are represented in dot plots indicating the percentage of positive cells. Positive populations were calculated via quadrant statistics applying the proper isotype controls to outline the negative populations (top panels represent FITC-labeled rat IgM and PE-labeled syrian hamster Ig, other isotype controls are not shown).
NK and T-cell markers on H-2k– and H-2b–derived IL-2 and IL-2/IL-12 A-LAK populations. Flow cytometric analysis of (A) AKR (H-2k), (B) C57BL/6 (H-2b), and (C) B10.BR (H-2k)–derived IL-2 versus IL-2/IL-12 A-LAK preparations, stained with FITC (Y axis) and PE (X axis)–labeled MoAbs as described in Materials and Methods. The cells were preincubated with 2.4G2 for 30 minutes before staining. The results are represented in dot plots indicating the percentage of positive cells. Positive populations were calculated via quadrant statistics applying the proper isotype controls to outline the negative populations (top panels represent FITC-labeled rat IgM and PE-labeled syrian hamster Ig, other isotype controls are not shown).
CD28-dependent and -independent recognition of B7 target cell lines by A-LAK cells.
Because IL-2/IL-12 A-LAKs lysed more efficiently B7 target cells and expressed more strongly CD28, it was of interest to test whether CD28-B7 interactions are functionally involved in the increased cytolysis. To this end IL-2 and IL-2/IL-12 LAK cultures were generated from spleens of CD28−/− knockout mice (H-2b haplotype). FACS analysis confirmed that CD28 was not expressed on spleen cells derived from these mice and that CD28 was not induced during culture of nylon wool purified fractions in the presence of IL-2 or IL-2 and IL-12 (data not shown). Testing the CD28−/− A-LAK activity toward NK-sensitive YAC-1 targets, a reduced cytolytic activity that reached between 75% and 50% of normal A-LAK activity, was systematically observed (P < .0001) (Fig 7). Comparing the sensitivity of BW(B7-1) target cells to either syngeneic (AKR, H-2k), congenic (B10.BR, H-2k), or allogeneic (C57BL/6, H-2b) effectors, with CD28−/− (H-2b) IL-2/IL-12 A-LAK cell effectors, absence of CD28 did not influence strongly the B7-1–mediated effect (P < .0001 for all effectors). Indeed, taking into account that CD28−/− IL-2/IL-12 A-LAK cells showed an overall reduced cytolytic activity (as tested on YAC-1 cells), these effector cells still recognized more efficiently B7-1 target cells (P < .0001). These results indicate that CD28 is not solely responsible for the B7-1–mediated recognition and lysis of B7-1 target cells by IL-2/IL-12 A-LAK cells. However, it should be emphasized that the target cells and effector cells were allogeneic in this experimental set-up. Therefore, B7-1 positive, syngeneic tumor cells (RMA-S) were also tested and as shown in Fig 7, the lysis of RMA-S(B7-1) cells was completely reduced to the background lysis (RMA-S target cells) when CD28−/−IL-2/IL-12 A-LAK cells were used (P > .1). Hence, depending on the target cell population, B7 recognition by IL-2/IL-12 A-LAK cells appears to be CD28-dependent or -independent.
Involvement of CD28 expression in the A-LAK–mediated lysis of B7-1 expressing tumor cells. (A) The lytic activity of AKR (H-2k haplotype), (B) B10.BR (H-2k haplotype), (C, E) C57BL/6 (H-2b haplotype), and (D, F) CD28−/− knockout mice (H-2bhaplotype)–derived IL-2/IL-12 A-LAK cells was tested against (A through D) parental and B7-1 transfected BW-Li (H-2khaplotype) and (E through F) RMA-S (H-2b haplotype) tumor cell variants in an [111In]-release assay. The specific lysis of YAC-1 was used as a reference for the lytic capability of the IL-2/IL-12 A-LAK. Spontaneous release was ≤10% for all cell lines. One representative of three experiments is shown, indicating the mean percentage specific release of targets in triplicate (±SD). Standard deviations less than ±3% are not shown for the sake of clarity. (▪), BW-Li; (⧫), BW-Li(B7-1); (*), YAC-1; (▴), RMA-S; (×), RMA-S(B7-1).
Involvement of CD28 expression in the A-LAK–mediated lysis of B7-1 expressing tumor cells. (A) The lytic activity of AKR (H-2k haplotype), (B) B10.BR (H-2k haplotype), (C, E) C57BL/6 (H-2b haplotype), and (D, F) CD28−/− knockout mice (H-2bhaplotype)–derived IL-2/IL-12 A-LAK cells was tested against (A through D) parental and B7-1 transfected BW-Li (H-2khaplotype) and (E through F) RMA-S (H-2b haplotype) tumor cell variants in an [111In]-release assay. The specific lysis of YAC-1 was used as a reference for the lytic capability of the IL-2/IL-12 A-LAK. Spontaneous release was ≤10% for all cell lines. One representative of three experiments is shown, indicating the mean percentage specific release of targets in triplicate (±SD). Standard deviations less than ±3% are not shown for the sake of clarity. (▪), BW-Li; (⧫), BW-Li(B7-1); (*), YAC-1; (▴), RMA-S; (×), RMA-S(B7-1).
Activation with IL-12 endows LAK cells with the capacity to lyse syngeneic DCs via a CD28-dependent recognition.
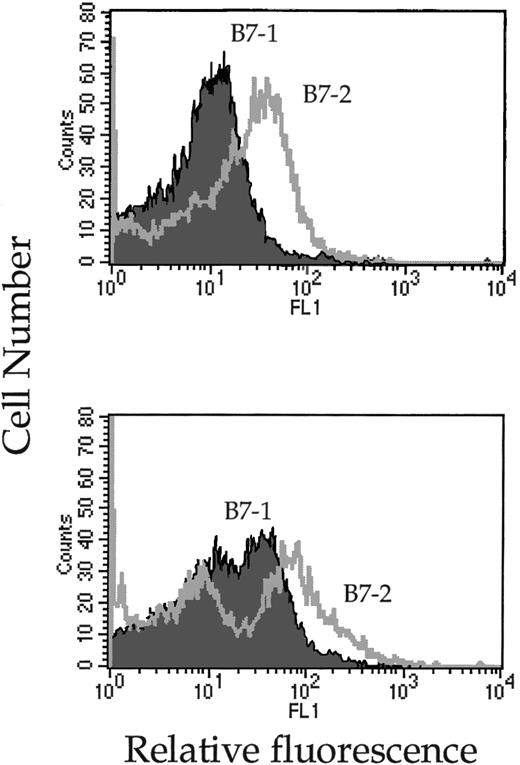
So far the B7 target cell lines used in this study were transformed cells. To test whether high expression of B7-molecules could trigger the lytic machinery of NK cells toward lysis of nontransformed syngeneic B7+ cells, mature DCs were isolated in vitro and confronted with IL-2 and IL-2/IL-12 A-LAKs. Mature DCs, generated from BM (progenitor DCs) or spleen (spleen DCs), were purified and analyzed for expression of B7-1 and B7-2. Both dendritic cell preparations expressed high levels of B7-1 and B7-2 (Fig8) and their susceptibility to A-LAK lysis was tested. Although a high expression of B7-1 and B7-2 was recorded on the DCs, IL-2–activated A-LAK cells did not significantly lyse autologous DCs at similar E/T ratios used for the lysis of B7-expressing tumor cells (Table 2). Yet when the LAKs were activated in the presence of IL-12, spleen-derived (P < .0001) as well as progenitor (P < .0001) autologous DCs became susceptible to NK-mediated destruction (Table 2). Furthermore, the lysis of DCs by IL-2/IL-12 A-LAKs was completely abrogated when the LAKs were generated from CD28−/− mice (P > .5 for spleen DCs,P > .05 for progenitor DCs). Hence, here also recognition and lysis of B7-positive targets by IL-2/IL-12 A-LAKs is CD28-dependent.
Mature splenic (top) and progenitor (bottom) DCs express high levels of B7-1 and B7-2 on their surface. In vitro–activated DCs were, upon harvesting, treated with 2.4G2 antibodies to block Fc receptors and subsequently incubated with FITC-labeled B7-1 or B7-2 antibodies. The cells were analyzed by FACS and their fluorescence signals are represented in overlay histograms.
Mature splenic (top) and progenitor (bottom) DCs express high levels of B7-1 and B7-2 on their surface. In vitro–activated DCs were, upon harvesting, treated with 2.4G2 antibodies to block Fc receptors and subsequently incubated with FITC-labeled B7-1 or B7-2 antibodies. The cells were analyzed by FACS and their fluorescence signals are represented in overlay histograms.
IL-12 Induces the Capability of A-LAK Cells to Kill Autologous DCs When CD28 Is Expressed on the Effector Cell Population
| E:T | % Specific Lysis* | ||||||||
| Experiment 1 | Experiment 2 | ||||||||
| C57BL/6 | CD28−/− | C57BL/6 | CD28−/− | ||||||
| IL-2 | IL-2/12 | IL-2 | IL-2/12 | IL-2 | IL-2/12 | IL-2 | IL-2/12 | ||
| Spleen DCs | 20:1 | 10 (±2) | 20 (±1) | 0 (±3) | 3 (±0) | 5 (±2) | 25 (±3) | 1 (±5) | 0 (±2) |
| 10:1 | 3 (±2) | 15 (±1) | 2 (±0) | 0 (±3) | 1 (±2) | 16 (±4) | 0 (±5) | 0 (±2) | |
| 5:1 | 4 (±6) | 14 (±1) | 2 (±2) | 0 (±2) | 0 (±1) | 13 (±2) | 0 (±5) | 0 (±2) | |
| 2.5:1 | 1 (±2) | 12 (±2) | 1 (±2) | 0 (±1) | 1 (±7) | 12 (±1) | 0 (±4) | 0 (±2) | |
| Progenitor DCs | 20:1 | ND | ND | ND | ND | 4 (±1) | 23 (±1) | 1 (±0) | 6 (±2) |
| 10:1 | ND | ND | ND | ND | 1 (±1) | 18 (±3) | 0 (±1) | 5 (±2) | |
| 5:1 | ND | ND | ND | ND | 2 (±1) | 10 (±1) | 0 (±1) | 1 (±2) | |
| 2.5:1 | ND | ND | ND | ND | 2 (±1) | 9 (±1) | 0 (±1) | 2 (±1) | |
| YAC-1† | 20:1 | 43 (±3) | 44 (±2) | 19 (±1) | 25 (±2) | 70 (±1) | 72 (±2) | 40 (±3) | 49 (±1) |
| 10:1 | 29 (±1) | 36 (±2) | 14 (±1) | 18 (±1) | 58 (±1) | 61 (±1) | 29 (±1) | 39 (±2) | |
| 5:1 | 22 (±1) | 28 (±1) | 8 (±1) | 17 (±3) | 46 (±0) | 52 (±3) | 19 (±1) | 30 (±1) | |
| 2.5:1 | 13 (±0) | 19 (±2) | 5 (±0) | 8 (±1) | 34 (±1) | 43 (±1) | 12 (±0) | 21 (±0) | |
| E:T | % Specific Lysis* | ||||||||
| Experiment 1 | Experiment 2 | ||||||||
| C57BL/6 | CD28−/− | C57BL/6 | CD28−/− | ||||||
| IL-2 | IL-2/12 | IL-2 | IL-2/12 | IL-2 | IL-2/12 | IL-2 | IL-2/12 | ||
| Spleen DCs | 20:1 | 10 (±2) | 20 (±1) | 0 (±3) | 3 (±0) | 5 (±2) | 25 (±3) | 1 (±5) | 0 (±2) |
| 10:1 | 3 (±2) | 15 (±1) | 2 (±0) | 0 (±3) | 1 (±2) | 16 (±4) | 0 (±5) | 0 (±2) | |
| 5:1 | 4 (±6) | 14 (±1) | 2 (±2) | 0 (±2) | 0 (±1) | 13 (±2) | 0 (±5) | 0 (±2) | |
| 2.5:1 | 1 (±2) | 12 (±2) | 1 (±2) | 0 (±1) | 1 (±7) | 12 (±1) | 0 (±4) | 0 (±2) | |
| Progenitor DCs | 20:1 | ND | ND | ND | ND | 4 (±1) | 23 (±1) | 1 (±0) | 6 (±2) |
| 10:1 | ND | ND | ND | ND | 1 (±1) | 18 (±3) | 0 (±1) | 5 (±2) | |
| 5:1 | ND | ND | ND | ND | 2 (±1) | 10 (±1) | 0 (±1) | 1 (±2) | |
| 2.5:1 | ND | ND | ND | ND | 2 (±1) | 9 (±1) | 0 (±1) | 2 (±1) | |
| YAC-1† | 20:1 | 43 (±3) | 44 (±2) | 19 (±1) | 25 (±2) | 70 (±1) | 72 (±2) | 40 (±3) | 49 (±1) |
| 10:1 | 29 (±1) | 36 (±2) | 14 (±1) | 18 (±1) | 58 (±1) | 61 (±1) | 29 (±1) | 39 (±2) | |
| 5:1 | 22 (±1) | 28 (±1) | 8 (±1) | 17 (±3) | 46 (±0) | 52 (±3) | 19 (±1) | 30 (±1) | |
| 2.5:1 | 13 (±0) | 19 (±2) | 5 (±0) | 8 (±1) | 34 (±1) | 43 (±1) | 12 (±0) | 21 (±0) | |
*The percent specific lysis (±SD) was determined in standard 4-hour 111In-release cytotoxicity experiments. Spontaneous release was < 3% for YAC-1, <12% for progenitor DCs, and <35% for spleen DCs. Both experiments were performed in triplicates and repeated three times.
The specific lysis of YAC-1 cells was used as a reference for the overall lytic capability of C57BL/6 versus CD28−/−–derived IL-2 and IL-2/IL-12 A-LAK cultures.
DISCUSSION
We herein confirm that NK cells, though being subjected to MHC class I–mediated inhibitory signals, can still be effectively activated to destroy tumor targets when appropriate activation signals are provided. Indeed, we and others have recently reported that expression of B7-1 on tumor cells can activate NK cell–mediated lysis. This was shown in the mouse: (1) in vitro via cytotoxicity assays with IL-2–activated LAK cell fractions and polyI:polyC-activated NK cells,24,25,27and (2) in vivo where immune effectors rejecting B7-1 expressing tumors were shown to reside primarily in the nonadaptive NK arm of immune response.24-26 Similar observations were made for a human NK leukemia cell line, YT2C2, because these cells could lyse B7-1-expressing targets in vitro.44 In the present study we have extended these observations by showing that activation of NK cells with IL-12 synergizes with the B7-induced effect. Furthermore, evidence is provided that IL-12 induces, besides the B7-1–, also the B7-2–mediated NK responses. Consequently, NK cells can recognize the B7-2 antigen for engagement and subsequent cytolysis of diverse targets, provided appropriate activation signal(s) (ie, IL-12) are supplemented. Collectively, these observations stress the importance of specific activation signals in NK cell function and indicate that NK cells can cope with inhibitory and activating stimuli in such a way that strong activating signals can overrule the MHC class I inhibition.
Although the effect of IL-12 on NK cell activity has been extensively investigated, several questions concerning the mechanism of enhanced cytolysis remain unsolved. In accordance with other studies,45 IL-12 was found to enhance the lytic activity of LAK cells toward NK-sensitive BW variants, but not toward NK-resistant variants (data not shown). IL-12 was proposed to augment the NK cell-mediated cytotoxicity at a postbinding level of the lytic interaction by increasing the maximal level of granule exocytosis.46 We observed that the granulosity of IL-2/IL-12 A-LAK cells is increased as compared with IL-2 A-LAKs. IL-12, in the presence of IL-2, can also upregulate NK cell activation markers and surface adhesion molecules, including CD69, CD71, CD56, LFA-1, ICAM-1 and CD2 and it has been speculated that the subsequent enhanced conjugate formation is sufficient to trigger a cytolytic response.32,46 Yet other investigators failed to confirm this significant upregulation in adhesion molecules or related enhancement in E/T conjugate formation.47 We found that the expression of certain surface molecules, among which CD28 and the IL-2R, was induced on IL-2/IL-12 A-LAK cells, yet other membrane markers such as CD4, CD8, or NK1.1 were not modulated. Furthermore, blocking of B7-2 or B7-1 was sufficient to abrogate the B7-specific induced lytic activity of IL-2 and IL-2/IL-12 A-LAK cells, indicating that the enhanced lysis of B7-1/B7-2 expressing targets is not simply due to an overall stimulating effect of IL-12 on the cytolytic machinery of NK cells, but rather that engagement of B7 appears to be the limiting step.
The present report is not the first one to show that IL-12 can synergize with B7-1–mediated effects. In vitro it has been reported that IL-12 synergizes with B7/CD28 interactions by inducing (1) efficient proliferation and cytokine production (mostly IFN-γ, but also TNF-α and GM-CSF) by human T cells48 and (2) IL-2Rα expression by mouse Th1 clones.49 In vivo, B7-1 and IL-12 were reported to cooperate in the induction of effective antitumor immunity and therapy. This cooperative effect was ascribed to the presence of CD4+ and CD8+ T cells and/or involved IFN-γ.34,35,50 Coughlin et al34 observed an effect of anti-asialoGM1 antibody treatment on the IL-12/B7 cooperative antitumor activity. These experiments enforce the possible relevance of our observations in immune responses and cancer therapy.
In general, these different studies proposed that the IL-12–induced lysis of B7-expressing targets involved both T cells and IFN-γ production. As far as IFN-γ is concerned, antibody neutralization experiments have shown that IFN-γ was essential for the in vivo antitumor efficacy of IL-12 alone.41,42 Also, the IFN regulatory factor-1 (IRF-1) seemed to play a crucial role in the induction of NK cell–mediated cytotoxic and effector functions in vivo.51 In fact, IFN-γ was shown to be implicated in inducing NK proliferation, converting a noncytolytic NK precursor cell into a functional NK cell, increasing the recycling of already existing NK cells, enhancing target-binding capacity of NKs, and increasing the lytic efficiency of conjugated effector cells.52 Although we observed a strong elevation of IFN-γ production during IL-2/IL-12 LAK cell generation, neutralization of this cytokine during LAK cell generation did not significantly reduce the cytolysis of B7-positive targets, indicating that IFN-γ was not required for the B7-mediated induction of LAK lysis. Similarly, IFN-γ was not found to be involved in the cytolytic activity of short time cultures of NK + IL-12.53 Finally, because IL-2/IL-12 A-LAK cells generated from SCID spleens or normal mice manifested similar features, T cells were clearly not required for the particular activities of these effector cells.
Because tumor cells do normally not express B7-molecules on their surface, the physiologic relevance of our observations was explored by testing the cytotoxic activity of IL-2 versus IL-12/IL-2 A-LAK cells against normal cells that express abundantly B7-1 and B7-2. DCs, following purification and maturation in vitro, were found to be relative resistant to IL-2 A-LAK cells, while significant lysis was observed with IL-2/IL-12 A-LAK cells. These results differ to some extent from a recent report showing that BM-derived cells, in contrast to resting peritoneal DCs and macrophages, were efficiently lysed by autologous IL-2 LAK cells.27 Different reasons may account for this opposed result, such as (1) differences in the preparation of LAK cells, (2) the effector/target (E/T) ratio used that was rather low in our study yet more physiologically relevant, and (3) the activation status of the target cells used. The last aspect may be important since during activation/maturation of DCs, an evolution in surface marker expression occurs, in particular the expression of B7-1 and B7-2 on immature DCs is extremely low compared with fully activated DCs.38-40Collectively, both studies provide sufficient evidence to postulate a role for NK cells in controlling immune responses via interacting with antigen-presenting cells. In fact, a negative regulatory effect of NKs on antigen-presenting cells (APC) was previously suggested in studies, showing that NK cells can lyse activated monocytes54 and inhibit the generation of CD8+ cytotoxic T lymphocytes (CTLs) by suppressing or eliminating DCs.55 Besides negative regulation of NK cells on APC, a positive regulation may exist as well. Indeed, it was recently reported that IFN-α/β can induce the trafficking of NK cells from the BM to the marginal zone of the spleen where the macrophages, DCs, and monocytes reside. Subsequently, the NK cells would promote the maturation of these cells via cytokine production, when the NK cells get properly activated. Also, it was shown here that IL-12 was implicated in the activation but that full activation may require both exposure to soluble IL-12 and delivery of a costimulatory signal through NK-APC cell contact.56 Our results suggest that these events will be deleterious for APC that are already fully matured or hyperactivated, closing the regulatory loop between APC and NK interactions. Similar regulatory interactions have recently also been established between NK cells and CTLs.57
The role of CD28 expression on NK cells in the lysis of B7-positive target cells is rather controversial. Because B7 expressing targets were more efficiently lysed by IL-2/IL-12 A-LAK cells, which express higher levels of CD28, the importance of CD28 in this phenomenon was addressed using effector cell populations from CD28−/− knockout mice. We could conclude that CD28 plays an important role in the NK-mediated lysis of B7-expressing targets, since CD28−/− IL-2/IL-12 A-LAKs were no longer able to lyse B7-1/B7-2–positive DCs as well as B7-1–expressing syngeneic tumor cells. However, the B7/CD28 interaction seemed not to be primordial in the lysis of allogeneic B7-1/B7-2–expressing tumor cells. These observations may indicate that CD28 is not always involved in the recognition of B7 targets, implying that NK cells may express a third B7-counterreceptor, capable to transduce costimulatory signals and whose expression is controlled by appropriate activation/differentiation signals (such as IL-12). In fact, using other NK cell populations, Chambers et al27failed to show any effect of CD28 in the lysibility of B7-expressing targets. In contrast, in the human system it was demonstrated that CD28 was functionally responsible for the destruction of B7-expressing targets because anti-CD28 treatment of the FcR-negative YT2C2 NK line blocked the B7 mediated lysis of B7 expressing targets.44Alternative costimulatory pathways and new receptors were also substantiated in other systems. Indeed, it was reported that CD28-deficient mice exhibit normal cytotoxic T-lymphocyte activity36 and, furthermore, that CTLA-4 does not fully substitute for all functions of CD28.58 Furthermore, blocking of B7-1 and B7-2 does not interfere with the activation of CD8+ cells by allogeneic MHC class I molecules expressed on Epstein-Barr virus (EBV)-transformed B cells.59 Finally, it was reported that anti-B7 or anti-CD28 antibodies do not always completely block the lytic activity of the human NK-like line YT on B7-positive EBV-transformed cells.60
Collectively our experimental evidence suggests that IL-12 activation of NK cells increases the capacity of these effector cells to lyse B7-expressing target cells via CD28-dependent and CD28-independent mechanisms.
ACKNOWLEDGMENT
We thank L. Brijs, M. Gobert, E. Vercauteren, E. Omasta, and F. Thielemans for an excellent technical assistance and both Hoffman-LaRoche, Inc and Genetics Institute, Inc (Cambridge, MA) for supplying the recombinant IL-12 used for this work.
Supported by grants for the Sportvereniging tegen de Kanker (A.B.G.), the GOA (P.D.B., K.T.), the Governmental Vlaams Actieprogramma Biotechnologie (P.D.B.), and the Vereniging voor Kankerbestrijding (P.D.B.).
Address reprint requests to Anja B. Geldhof, PhD, Laboratory of Cellular Immunology, VIB-VUB/IMOL II, Paardenstraat 65, B-1640 Sint-Genesius Rode, Belgium.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 2. Lytic activity of SCID-spleen-derived A-LAK cells. The activity of IL-2-activated (filled symbols) versus IL-2/IL-12-activated (open symbols) A-LAK cells was measured in an in vitro cytotoxicity assay. The specific lysis of BW-Li (squares) and BW-Li(B7-1) (diamonds) target cells was compared and here represented as the mean percent specific [111In]-release of triplicates (±SD). Spontaneous release was less than 5%. One of two similar experiments is shown. Standard deviations less than ±3% are not shown for the sake of clarity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.196/3/m_blod4012802.jpeg?Expires=1767809992&Signature=GMO7V6ig~UBDpK1N8hmrsyzVPJm-BQq7H2g7VmVlMFy5N-LODUXpnmnuGpZu4jjjK~l3M2pOKkFJ2eFOMt9POZlwA~6zii~WTRmOWiZmyL8y6MnxGYBhuzHf7lzq1N-z4gtcx9Y6Mhjkkv6inbiOOuj2sgJ7Desw9hRvbXLzdoF2-HHvcqnJfa2F2Gz9YMp7OB9cLDBRfbCa7G653DzFawMxXkAco24QMhU9k3DXWZqKgMjx1Cq0opS3d0bwAB66j2ayNnK1TnLbB-pAgMUaJSqusO-5IPqXCPIbO5VmW4rRJS2bzoHjUsW5NDTHEOprpV2cq7WGBxy33y4ups8h-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Blocking of IFN-γ during generation of IL-2/IL-12 LAK cells does not reverse the IL-12-promoting effect on LAK cell lysis of B7-1– or B7-2–expressing tumor cells. The cytolytic activity of AKR-derived IL-2 A-LAKs is compared with the cytolytic activity of untreated or anti–IFN-γ MoAb-treated IL-2/IL-12 A-LAKs on BW-Li, BW-Li(B7-1), and BW-Li(B7-2) targets. The results are represented as the percentage specific [111In]-release (±SE) at different E/T ratios. Standard deviations less than ±3% are not shown for the sake of clarity. Spontaneous release was less than 4%. The experiment was repeated twice. (▧), IL-2 A-LAK; (▧), IL-2 + IL-12 A-LAK; (▩), IL-2 + IL-12 A-LAK + α-IFN-γ.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.196/3/m_blod4012803.jpeg?Expires=1767809992&Signature=YGa54B7HOGZBxR22n9c5Q~Tj63WRikAOhl~Ujo6uJUGhWt0FSqNV~z6I4~Hv0dJuAOsVUTdR71FCSQ-L3BMP5bdSD4wGGWgxkWe7TySZR-QNgafGT~UV7RQu7YyY9zFBlRhaHvY097NNLv4oQGdIDXUslmwpoaWu3msrk76V64iD2YyQtY3Czl7cLfNlqDBHv3wtEb4u7ZT9t4iEpTpMpVjDDss17U27z305RmXYdA4-qe4VIQNfhIatQcdVhneaB2MRylVu39ga05ogOC5BYqlm3AZGfrXMLW48A3W0nuH3~J4f~N~y0ipeyVgukWIXeWghw8ueKnJD-KV90zxUIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Fig. 7. Involvement of CD28 expression in the A-LAK–mediated lysis of B7-1 expressing tumor cells. (A) The lytic activity of AKR (H-2k haplotype), (B) B10.BR (H-2k haplotype), (C, E) C57BL/6 (H-2b haplotype), and (D, F) CD28−/− knockout mice (H-2bhaplotype)–derived IL-2/IL-12 A-LAK cells was tested against (A through D) parental and B7-1 transfected BW-Li (H-2khaplotype) and (E through F) RMA-S (H-2b haplotype) tumor cell variants in an [111In]-release assay. The specific lysis of YAC-1 was used as a reference for the lytic capability of the IL-2/IL-12 A-LAK. Spontaneous release was ≤10% for all cell lines. One representative of three experiments is shown, indicating the mean percentage specific release of targets in triplicate (±SD). Standard deviations less than ±3% are not shown for the sake of clarity. (▪), BW-Li; (⧫), BW-Li(B7-1); (*), YAC-1; (▴), RMA-S; (×), RMA-S(B7-1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.196/3/m_blod4012807.jpeg?Expires=1767809992&Signature=K3oVliN7oDurLZSee0ZujFOT-cqW1tQDY~1lekLxo~FtBDxwvY8Vp7MyxsHYAvm8U3tr12xjdTUrlAF8Jdx547oN5U6QhQXB3YBJHGo8X1JiGlchUcpXltN5pXEs8UKoY46kJdEtPvqWyGWTxnUK-aiAmYYGi5nwaaqgTJfSYTLFviMxq9WIhJsNZTlYPSA6iEMEqO3XIli-W8h7Jzm15K3JcOucT7U5-Wngpkbh-BIMMw4q1GSuyk0P0VoGT-q20Qo6y6NROJX1YqLOL2Bq8B1A1io1XBNpBsfDJGg59xaOasXWPXaFR~URxhu7rHkNO-8AyzoeJFLUQXW4T~Z5DQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal