Abstract
We have investigated the expression of the three components of the interleukin-2 receptor (IL-2Rα, IL-2Rβ, and IL-2Rγ) on the surface of the various peripheral blood mononuclear cell (PBMC) subsets by flow cytometry analysis. The PBMC were immediately isolated (ficoll) from blood collected on heparin as anticoagulant. The three IL-2R components are absent or only marginally detectable on CD4 T lymphocytes. No expression of the IL-2R chains is found for the B lymphocytes. In most donors, the three chains are not detectable on CD8 T lymphocytes, but for a few of them, IL-2Rβ or IL-2Rγ are clearly expressed. CD56 high (IL-2Rα+) and CD56 low (IL-2Rα−) natural killer (NK) cells express IL-2Rβ, but not IL-2Rγ. IL-2Rγ is expressed by monocytes of all donors although with variable intensity. When blood is collected on other anticoagulants or when cells are isolated 1 day after collection, IL-2Rα, IL-2Rβ, and IL-2Rγ are largely expressed on the surface of most PBMC. This observation provides a possible explanation for divergent data previously reported on IL-2R expression. Finally, we show that IL-2Rγ, which is not detectable on the cell surface of lymphocytes, is nevertheless expressed and stored as an intracellular component. This result is in agreement with the constitutive expression of the IL-2Rγ gene and suggests a specific regulatory mechanism for IL-2Rγ membrane translocation.
INTERLEUKIN-2 (IL-2) was one of the first cytokines to be discovered and characterized.1 IL-2 is a main cytokine in the immune system, as it has pleiotropic effects on lymphocytes including T, B, and natural killer (NK) cells, as well as on some hematopoietic cells,2 and beneficial therapeutic roles of IL-2 have been reported.3-5
The effects of IL-2 are mediated through specific cell surface receptors (IL-2R)6-8 comprising at least three subunits. The first IL-2R component to be identified, IL-2Rα (CD25, Tac antigen),9,10 is a 55-kD protein that binds IL-2 with low affinity (10−8 mol/L). The mode of action of IL-2Rα and the role of IL-2 on IL-2Rα gene expression have been reported.11-13 The second component IL-2Rβ (CD122), a 74-kD protein,14 and the third component IL-2Rγ (CD132), a 64-kD protein,15 belong to the hematopoietin receptor family (for review, see Thèze16). IL-2Rα resembles IL-15Rα.17In humans, two functional receptors can transmit IL-2 signals. The high-affinity receptor (10-11 mol/L) is composed of the three IL-2R subunits. The intermediate receptor (10-9mol/L) is formed by the association of IL-2Rβ and IL-2Rγ. IL-2Rβ not only belongs to IL-2R, but is also a component of IL-15R, and IL-2Rγ is shared by several cytokine receptors: IL-4R, IL-7R, IL-9R, IL-15R (for review, see Thèze18). Signal transduction requires heterodimerization of IL-2Rβ with IL-2Rγ and involves p56lck, Shc, Syk, JAK1, and JAK3 tyrosine kinases and STAT3 and STAT5 transcriptional activators.18-21
Various conflicting data have been reported concerning the expression of the three IL-2R subunits on human peripheral blood mononuclear cells (PBMC) from healthy individuals. For example, some studies reported no detectable expression of IL-2Rα on CD4 T lymphocytes,22,23 whereas others showed positive IL-2Rα expression.24,25 Similar discrepancies have been reported for IL-2Rβ expression on monocytes.25-27 Furthermore, cell-surface expression of IL-2Rγ on T lymphocytes were found positive in some reports,25 but insignificant in others.28 29
We describe here a pattern of IL-2R component expression using blood collected on heparin and freshly isolated PBMC. We show that cells involved in acquired immunity express very low levels of the IL-2R subunits, whereas cells of innate immunity clearly express either IL-2Rβ or IL-2Rγ subunit. We show that this profile is highly sensitive to the conditions of the blood sample, with significant induction of the IL-2R subunits on the cell surface. Our experimental conditions also show that IL-2Rγ is present inside all lymphocyte subsets, but remains undetectable on their surface.
MATERIALS AND METHODS
Antibodies and reagents.
Monoclonal antibodies (MoAbs) anti–IL-2Rα (33B3, IgG2a) and anti–IL-2Rβ (CF1, IgG1) were purchased from Immunotech (Marseille, France). The properties of IgG MoAb anti–IL-2Rγ (3B5) were previously described.28
Mouse MoAbs anti-CD3 (UCHT1, IgG1), anti-CD4 (MT310, IgG1), anti-CD8 (DK25, IgG1), anti-CD14 (Tük4, IgG2a), anti-CD19 (HD37, IgG1), anti-CD20 (B-Ly1, IgG1), anti-CD56 (MOC-1, IgG1), anti-CD71 (Ber-T9, IgG1), anti-Bcl-2 (124, IgG1) and isotype-matched control MoAbs were purchased from DAKO A/S (Glostrup, Denmark). When indicated these antibodies were labeled with R-Phycoerythrin (PE). Anti-CD16 (3G8, IgG1) and anti-CD28 (IOT28, IgG1) were obtained from Immunotech. Fluorescein isothiocyanate (FITC)-conjugated Fab fragment goat antimouse IgG (H+L) was purchased from Jackson Immunoresearch (West Grove, PA). Tri-color (FL3)-conjugated anti-CD14 (Tük4) and isotype-matched control MoAb were obtained from Caltag (Burlingame, CA). All these antibodies were used at saturating concentrations.
Blood collection and PBMC preparation.
Venous blood of healthy donors (Centre de Transfusion Sanguine ≪Jean Julliard», Clamart, France) and of hemochromatosis patients (Site Transfusionnel de l'Hôpital Necker-Enfants Malades, Paris, France) was typically collected on sodique heparin as anticoagulant. At the day of blood sample, PBMC were isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). PBMC were then immediately stained and analyzed with three-color cytometry as described below. When indicated, blood was collected on either CPD (citrate phosphate dextrose) or ACD (acid citrate dextrose), anticoagulants with divalent metal ion chelator activity. In one set of experiments, PBMC were isolated by Ficoll-Hypaque from heparinized blood stored one day at 18°C in a dark room.
Flow cytometric analysis.
Cells were analyzed by three-color flow cytometry. PBMC were first incubated for 30 minutes on ice with MoAbs for IL-2Rα, IL-2Rβ, or IL-2Rγ. Cells were also incubated with MoAbs directed against markers specific for the different PBMC subsets or for activation markers as indicated in the figures. After washing, cells were incubated with FITC-conjugated Fab fragment goat antimouse IgG (H+L) under the same conditions. For FL2 (PE) and FL3 (Tri-color) staining, cells were incubated with CD4-PE and CD14-FL3, CD20-PE and CD14-FL3, CD56-PE and CD14-FL3, or with CD8-PE MoAbs and washed again. Cells were then fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS). A total of two 104 PBMC per sample was acquired for CD4, CD56, and CD8 cells and of five 104 for CD20 cells. Analysis was performed with a FACScan flow cytometer using CellQuest 3.1 software (Becton Dickinson, Mountain View, CA).
Intracellular detection of IL-2Rγ and Bcl-2 was performed as previously described.30 Briefly, cells were fixed in 4% paraformaldehyde in PBS, washed, and permeabilized by a solution of 0.05% (wt/vol) saponin detergent in PBS. Cells were then incubated with anti–IL-2Rγ chain or anti-Bcl2 MoAbs, washed, and stained with FITC-conjugated goat antimouse Fab fragment. A cell-surface staining was then performed as described above to discriminate the different PBMC subsets. For intracellular IL-2Rγ staining, the YT cell line and an Epstein-Barr virus (EBV)-B cell line derived from an X-linked severe combined immune deficiency (IL-2Rγ mRNA−) child (patient P2 in Hacein-Bey et al31) were used as positive and negative controls, respectively.
mRNA detection in PBMC subsets.
PBMC subsets were highly purified by negative selection with magnetic beads (purity > 95%) as previously described.30 Total RNA was then extracted and cDNA was synthesized using an oligo(dT) primer. Polymerase chain reaction (PCR) amplification was performed with already published specific primers.30 PCR products were hybridized with specific γ32-adenosine triphosphate (ATP)-labeled oligonucleotides30 and staining was recorded with the PhosphoImager using the ImagQuant software.
Western blot analysis.
Western blots were performed as previously described.32,33Briefly, lysates were prepared from PBMC subsets purified as described above, by addition of sample buffer (125 mmol/L Tris-HCl pH6.8, 2% sodium dodecyl sulfate (SDS), 10% β-mercaptoethanol, 10% glycerol, 0.01% bromophenol blue). YT and the IL-2Rγ− B-EBV cell lines were used as positive and negative controls, respectively. The protein samples (50 μg) were electrophoresed under reducing conditions on an 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and tranferred onto Immobilon P nitrocellulose membranes (Millipore Corp, Bedford, MA). Filters were blocked for 2 hours at room temperature (RT) with blocking buffer (7% bovine serum albumin (BSA), 0.1% tween 20, 1× PBS) and subsequently incubated for 2 hours at RT in blocking buffer containing the anti–IL-2Rγ rabbit antiserum at a dilution of 1:1,000.34Membranes were washed and incubated for 1.5 hours at RT in blocking buffer containing 1:1,000 peroxidase-labeled goat antirabbit antibody (Biosys, Compiègne, France) and extensively washed. ECL Western blotting detection kit (Amersham International, Burchinghamshire, UK) was used according to the manufacter's instruction and the filters were autoradiographed for a few seconds.
RESULTS
Analysis of IL-2Rα, IL-2Rβ, and IL-2Rγ expression on monocytes and CD4 T lymphocytes.
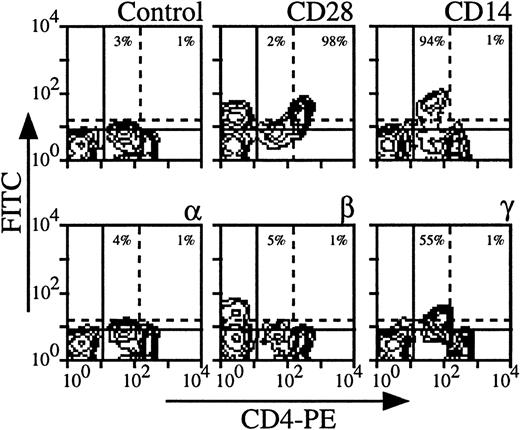
Expression of the three IL-2R subunits on CD4 cells derived from nine healthy individuals was examined; Fig 1shows the results obtained with a representative donor. The intensity of CD4 staining allowed us to distinguish monocytes (CD4 low, CD14+) from CD4 T lymphocytes (CD4 high, CD28+) (Fig 1). It was also verified that monocytes also expressed CD33 and CD11b markers and that CD4 lymphocytes were CD3+ (data not shown). Under these experimental conditions, monocytes clearly expressed IL-2Rγ (55% for the donor presented in Fig 1). The intensity of IL-2Rγ expression on monocytes varies according to the donors (from 15% to 75%), resulting in a mean expression of 38% in the group. IL-2Rα and IL-2Rβ were not detectable or expressed at very low levels depending on the donor (mean expression in the group, 2% for IL-2Rα and 4% for IL-2Rβ). Concerning CD4 T lymphocytes, IL-2Rα, IL-2Rβ, and IL-2Rγ were not detectable or sometimes detectable at very low levels depending on the donor with mean expressions of 4%, 2%, and 4%, respectively. Under our experimental conditions, CD4 T lymphocytes were found to be in a resting stage as measured by size and by the absence of CD71 expression (data not shown and see below).
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by CD4 T lymphocytes and monocytes. PBMC from blood collected on heparin were treated by MoAbs 33B3 (anti–IL-2Rα), CF1 (anti–IL-2Rβ), and 3B5 (anti–IL-2Rγ). Further characterization of the two populations was achieved by treatment with anti-CD14 and anti-CD28 MoAbs. FITC-labeled Fab fragment anti-IgG was used to stain the cells, followed by PE-conjugated anti-CD4 MoAb. Quadrant settings distinguishing positive immunofluorescence from background fluorescence were determined by staining with isotype-matched control MoAbs: solid horizontal line for lymphocytes and dotted horizontal line for monocytes. The vertical dotted line separates CD4 low (monocytes) from CD4 high (lymphocytes) cells. The percentage of positive cells for the different markers is indicated.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by CD4 T lymphocytes and monocytes. PBMC from blood collected on heparin were treated by MoAbs 33B3 (anti–IL-2Rα), CF1 (anti–IL-2Rβ), and 3B5 (anti–IL-2Rγ). Further characterization of the two populations was achieved by treatment with anti-CD14 and anti-CD28 MoAbs. FITC-labeled Fab fragment anti-IgG was used to stain the cells, followed by PE-conjugated anti-CD4 MoAb. Quadrant settings distinguishing positive immunofluorescence from background fluorescence were determined by staining with isotype-matched control MoAbs: solid horizontal line for lymphocytes and dotted horizontal line for monocytes. The vertical dotted line separates CD4 low (monocytes) from CD4 high (lymphocytes) cells. The percentage of positive cells for the different markers is indicated.
B lymphocytes did not express IL-2Rα, IL-2Rβ, and IL-2Rγ at their surface.
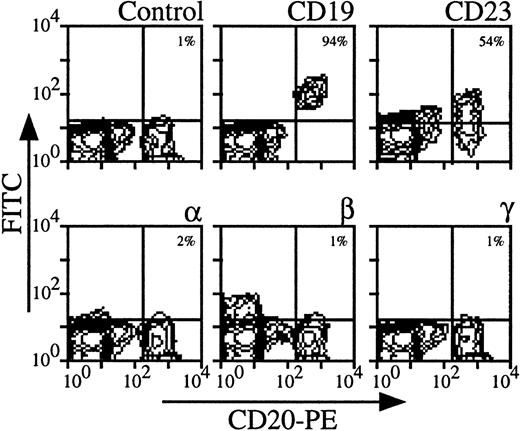
Using three color-flow cytometry with anti-CD20 MoAb on whole PBMC, we could clearly detect B lymphocytes, which were also CD19, CD23+ (see Fig 2 for a representative analysis). For all donors, IL-2Rα, IL-2Rβ, and IL-2Rγ were undetectable (mean expressions < 1%). Similar results were obtained on PBMC enriched in B lymphocytes after depletion of monocytes and CD3 T lymphocytes (data not shown). Comparing the results obtained with CD4 T and B lymphocytes, the mean expression of IL-2R subunits was around 4% for CD4 T lymphocytes and always less than 1% for B lymphocytes. This shows that for a few donors low levels of IL-2R components on CD4 T lymphocytes were observed, whereas these chains were never detectable on B lymphocytes among the different donors.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by B lymphocytes. PBMC from blood collected on heparin were treated by MoAbs 33B3, CF1, and 3B5. Characterization of the population was achieved by treatment with anti-CD19 and anti-CD23 MoAbs. FITC-labeled Fab fragment anti-IgG was used, followed by PE-conjugated anti-CD4 + FL3-conjugated anti-CD14 MoAbs. CD14+ monocytes were excluded for easier analysis. Quadrant setting distinguishing positive immunofluorescence from background fluorescence was determined by staining with isotype-matched control MoAbs. The percentage of positive cells for the different markers is indicated.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by B lymphocytes. PBMC from blood collected on heparin were treated by MoAbs 33B3, CF1, and 3B5. Characterization of the population was achieved by treatment with anti-CD19 and anti-CD23 MoAbs. FITC-labeled Fab fragment anti-IgG was used, followed by PE-conjugated anti-CD4 + FL3-conjugated anti-CD14 MoAbs. CD14+ monocytes were excluded for easier analysis. Quadrant setting distinguishing positive immunofluorescence from background fluorescence was determined by staining with isotype-matched control MoAbs. The percentage of positive cells for the different markers is indicated.
High expression of IL-2Rβ by NK cells.
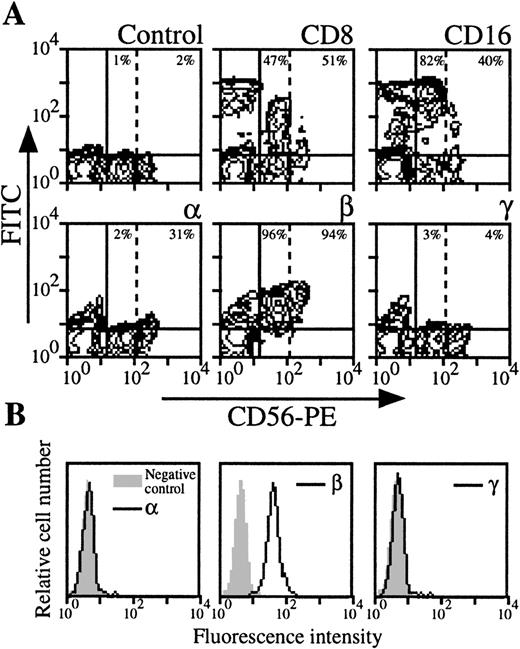
NK cells were analyzed by three color-flow cytometry on PBMC from nine healthy donors by studying the expression of NK-specific CD56 marker. In roughly one third of the donors, NK cells appeared heterogeneous in terms of intensity of the CD56 marker (CD56 low NK population could be distinguished from CD56 high NK population, Fig3A). As previously reported,22these two populations expressed CD8 marker with comparable intensity, but CD16 expression was higher in the CD56 low NK population. IL-2Rα was only present in the CD56 high NK population, whereas IL-2Rβ was present in both. Moreover, IL-2Rγ was not detectable in CD56 low and CD56 high NK cells (Fig 3A). For donors in which CD56 high NK cells could not be identified, IL-2Rα and IL-2Rγ were not detectable, or present at very low levels (mean expression, 1% and 3%, respectively), whereas IL-2Rβ was always strongly expressed (mean expression, 76%). IL-2R expression was also examined in purified NK cells (Fig 3B): this population was also found IL-2Rα−, IL-2Rβ+, and IL-2Rγ−.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by NK cells. (A) PBMC from blood collected on heparin were treated by MoAbs 33B3, CF1, and 3B5. Further characterization of the population was achieved by treatment with anti-CD8 and anti-CD16 MoAbs. FITC-labeled Fab fragment anti-IgG was used, followed by PE-conjugated anti-CD56 MoAb + FL3-conjugated anti-CD14 MoAbs. CD14+ cells were excluded for easier analysis. Quadrant setting distinguishing positive immunofluorescence from background fluorescence was determined by staining with isotype-matched control MoAbs. Vertical dotted line separates CD56 low from CD56 high cells. The percentage of positive cells for the different markers is indicated. (B) NK cells were highly purified from PBMC as previously described.47 The resulting CD56 population was treated by MoAbs 33B3, CF1, and 3B5 followed by FITC-labeled Fab fragment anti-IgG. The percentage of positive cells for the different markers is indicated.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by NK cells. (A) PBMC from blood collected on heparin were treated by MoAbs 33B3, CF1, and 3B5. Further characterization of the population was achieved by treatment with anti-CD8 and anti-CD16 MoAbs. FITC-labeled Fab fragment anti-IgG was used, followed by PE-conjugated anti-CD56 MoAb + FL3-conjugated anti-CD14 MoAbs. CD14+ cells were excluded for easier analysis. Quadrant setting distinguishing positive immunofluorescence from background fluorescence was determined by staining with isotype-matched control MoAbs. Vertical dotted line separates CD56 low from CD56 high cells. The percentage of positive cells for the different markers is indicated. (B) NK cells were highly purified from PBMC as previously described.47 The resulting CD56 population was treated by MoAbs 33B3, CF1, and 3B5 followed by FITC-labeled Fab fragment anti-IgG. The percentage of positive cells for the different markers is indicated.
Fine analysis of the expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by CD8 T lymphocytes.
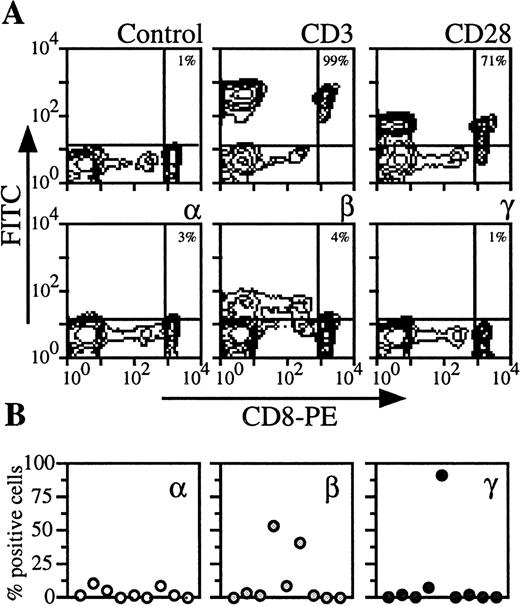
By analyzing PBMC of nine donors with CD8 marker, two populations could be distinguished (Fig 4). CD8 low cells contained NK cells (ie, cells that were CD56+, IL-2Rβ+, CD3−, CD28−). We examined CD8 high cells, which were also CD3 and CD28+ (Fig 4A). On the CD8 T lymphocytes from the majority of the donors, IL-2Rα, IL-2Rβ, and IL-2Rγ could not be detected or were present at very low levels (<5%) (Fig 4A). However, for the remainig healthy donors, IL-2Rβ or IL-2Rγ were strongly expressed (Fig 4B). When these are included in the group of healthy donors, the data are heterogeneous and the mean expression is then more than 10% (Figs 5 and6).
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by CD8 T lymphocytes. (A) PBMC from blood collected on heparin were treated by MoAbs 33B3, CF1, and 3B5. Further characterization of the population was achieved by treatment with anti-CD3 and anti-CD28 MoAbs. FITC-labeled Fab fragment anti-IgG was used, followed by PE-conjugated anti-CD8 MoAb. Quadrant setting distinguishing positive immunofluorescence from background fluorescence was determined by staining with isotype-matched control MoAbs. The percentage of positive cells for the different markers is indicated. (B) Staining and analysis was performed as in (A). The percentage of positive cells for the different markers is shown for a group of nine individuals.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by CD8 T lymphocytes. (A) PBMC from blood collected on heparin were treated by MoAbs 33B3, CF1, and 3B5. Further characterization of the population was achieved by treatment with anti-CD3 and anti-CD28 MoAbs. FITC-labeled Fab fragment anti-IgG was used, followed by PE-conjugated anti-CD8 MoAb. Quadrant setting distinguishing positive immunofluorescence from background fluorescence was determined by staining with isotype-matched control MoAbs. The percentage of positive cells for the different markers is indicated. (B) Staining and analysis was performed as in (A). The percentage of positive cells for the different markers is shown for a group of nine individuals.
Expression of the IL-2R by cells from blood collected with different anticoagulants. (A) Blood of one donor was collected on CPD and PBMC were isolated the same day as the blood sample. Staining and analysis of CD4 T lymphocytes was performed as indicated in Fig 1except that FL3-labeled anti-CD14 MoAb was used. (B) Blood was collected on sodique heparin (n = 9) or on CPD (n = 3) or on ACD (n = 3), and PBMC were isolated the same day as the blood sample (d = 0) or 1 day after (d = 1) in some cases with sodique heparin (n = 4). Staining and analysis was performed as in (A) for CD4 T lymphocytes and as indicated in Fig 4 for CD8 T lymphocytes. Significant differences are indicated as follows, a, P ≤ .0001; b, .0001 < P ≤ .001; c, .001 < P ≤ .01; and d, .01 < P < .05 (nonpaired t-test). (C) Blood was collected on sodique heparin (H) or on CPD (C). CD4 T lymphocytes and monocytes were purified from PBMC isolated at d=0. IL-2Rα and β specific mRNAs were measured as previously described.30HPRT detection is also shown as positive control; N, PCR negative control.
Expression of the IL-2R by cells from blood collected with different anticoagulants. (A) Blood of one donor was collected on CPD and PBMC were isolated the same day as the blood sample. Staining and analysis of CD4 T lymphocytes was performed as indicated in Fig 1except that FL3-labeled anti-CD14 MoAb was used. (B) Blood was collected on sodique heparin (n = 9) or on CPD (n = 3) or on ACD (n = 3), and PBMC were isolated the same day as the blood sample (d = 0) or 1 day after (d = 1) in some cases with sodique heparin (n = 4). Staining and analysis was performed as in (A) for CD4 T lymphocytes and as indicated in Fig 4 for CD8 T lymphocytes. Significant differences are indicated as follows, a, P ≤ .0001; b, .0001 < P ≤ .001; c, .001 < P ≤ .01; and d, .01 < P < .05 (nonpaired t-test). (C) Blood was collected on sodique heparin (H) or on CPD (C). CD4 T lymphocytes and monocytes were purified from PBMC isolated at d=0. IL-2Rα and β specific mRNAs were measured as previously described.30HPRT detection is also shown as positive control; N, PCR negative control.
Expression of IL-2R subunits on the different PBMC subsets in healthy donors and hemochromatosis patients. Blood was collected on sodique heparin and PBMC were isolated the same day as the blood sample, for healthy donors (HD, n = 9) and hemochromatosis patients (HHP, n = 5). Staining and analysis was performed as in Figs1-4. Significant differences are indicated as in Fig 5.
Expression of IL-2R subunits on the different PBMC subsets in healthy donors and hemochromatosis patients. Blood was collected on sodique heparin and PBMC were isolated the same day as the blood sample, for healthy donors (HD, n = 9) and hemochromatosis patients (HHP, n = 5). Staining and analysis was performed as in Figs1-4. Significant differences are indicated as in Fig 5.
Changes in IL-2R expression when blood is collected on Ca2+ chelators.
As shown in Fig 5A, CD4 T lymphocytes from the blood of one donor collected on CPD clearly expressed the three IL-2R chains. The appearance of the three IL-2R subunits on CD4 T lymphocytes was observed when blood from different patients was collected on CPD or ACD (Fig 5B, upper panel). For example, with CPD, the mean expressions of IL-2Rα, IL-2Rβ, and IL-2Rγ were 30%, 32%, and 37%, respectively. When PBMC were isolated 1 day after the blood sample was taken, a similar pattern of expression was found. These results were significantly different from basal levels obtained with heparin and PBMC isolated at day 0. These results have been verified at the mRNA level (Fig 5C). CD4 T lymphocytes purified from blood collected on CPD expressed IL-2Rα and IL-2Rβ mRNAs contrary to cells purified from blood collected on heparin. Results obtained with IL-2Rγ mRNA are not shown because this mRNA is constitutively transcribed.15 30 At the cell surface level, similar results were obtained for CD8 T lymphocytes (Fig 5B, lower panel). On these cells, the IL-2R expression due to the anticoagulant and/or to the time effects was, however, more important compared with CD4 T lymphocytes. Surprisingly, expression of CD71 activation marker was not affected under these conditions (Fig 5B), with the exception of a small increase when PBMC were purified 1 day after collection. Similarly, unchanged expression was observed for CD69 and HLA-DR markers (data not shown).
With monocytes, the use of other anticoagulants and/or PBMC isolation at day 1 triggered the appearance of IL-2Rα and IL-2Rβ and the over-expression of IL-2Rγ (Table1). As for CD4 T lymphocytes, the use of CPD also resulted in IL-2Rα and IL-2Rβ mRNA expression (Fig 5C). For B lymphocytes, a low but significant appearance of the three IL-2R subunits was observed (mean expression of 10% in the various cases, Table 1). On the other hand, the pattern of IL-2R subunit expression on NK cells was not influenced by the experimental conditions (Table 1). As for T lymphocytes, we found no concomitant increase in CD71 expression except for B lymphocytes when PBMC were prepared at day 1 (Table 1). Variations of CD69 in the three subsets presented in Table1, of CD16 on monocytes, and of CD23 on B lymphocytes were insignificant (data not shown).
Expression of CD71 and IL-2R Chains on Monocytes, B Lymphocytes, and NK Cells
| Cell Type . | Anticoagulant/Time . | Percentage of Positive Cells . | |||
|---|---|---|---|---|---|
| CD71 . | IL-2 Receptor . | ||||
| α . | β . | γ . | |||
| Monocytes | Hep Na (d = 0) | 6.6 ± 5.1 | 2.3 ± 3.1 | 4.5 ± 5.2 | 38 ± 23 |
| CPD (d = 0) | 1.5 ± 1.4 | 24 ± 16* | 32 ± 2.8† | 61 ± 19 | |
| ACD (d = 0) | 6.9 ± 1.4 | 57 ± 19† | 74 ± 9.5† | 60 ± 23 | |
| Hep Na (d = 1) | 5.3 ± 5.5 | 6.4 ± 3.6‡ | 16 ± 4.8§ | 71 ± 15‡ | |
| B lymphocytes | Hep Na (d = 0) | 2.1 ± 1.5 | 0.93 ± 1.3 | 0.55 ± 1.1 | 0.81 ± 1.3 |
| CPD (d = 0) | 1.1 ± 1.5 | 4.1 ± 1.4§ | 7.5 ± 5.9§ | 5.2 ± 2.0* | |
| ACD (d = 0) | 3.9 ± 3.4 | 14 ± 4.8† | 24 ± 13† | 10 ± 5.4* | |
| Hep Na (d = 1) | 7.6 ± 1.5† | 5.5 ± 0.69† | 16 ± 12* | 11 ± 9.1§ | |
| NK cells | Hep Na (d = 0) | 1.8 ± 1.2 | 1.5 ± 1.3 | 76 ± 11 | 2.9 ± 1.8 |
| CPD (d = 0) | 0.12 ± 0.21 | 0.61 ± 0.85 | 83 ± 6.6 | 0.71 ± 0.11 | |
| ACD (d = 0) | 0.11 ± 0.12 | 0.33 ± 0.58 | 70 ± 12 | 0.13 ± 0.16 | |
| Hep Na (d = 1) | 2.7 ± 2.4 | 1.6 ± 1.1 | 81 ± 17 | 4.3 ± 2.2 | |
| Cell Type . | Anticoagulant/Time . | Percentage of Positive Cells . | |||
|---|---|---|---|---|---|
| CD71 . | IL-2 Receptor . | ||||
| α . | β . | γ . | |||
| Monocytes | Hep Na (d = 0) | 6.6 ± 5.1 | 2.3 ± 3.1 | 4.5 ± 5.2 | 38 ± 23 |
| CPD (d = 0) | 1.5 ± 1.4 | 24 ± 16* | 32 ± 2.8† | 61 ± 19 | |
| ACD (d = 0) | 6.9 ± 1.4 | 57 ± 19† | 74 ± 9.5† | 60 ± 23 | |
| Hep Na (d = 1) | 5.3 ± 5.5 | 6.4 ± 3.6‡ | 16 ± 4.8§ | 71 ± 15‡ | |
| B lymphocytes | Hep Na (d = 0) | 2.1 ± 1.5 | 0.93 ± 1.3 | 0.55 ± 1.1 | 0.81 ± 1.3 |
| CPD (d = 0) | 1.1 ± 1.5 | 4.1 ± 1.4§ | 7.5 ± 5.9§ | 5.2 ± 2.0* | |
| ACD (d = 0) | 3.9 ± 3.4 | 14 ± 4.8† | 24 ± 13† | 10 ± 5.4* | |
| Hep Na (d = 1) | 7.6 ± 1.5† | 5.5 ± 0.69† | 16 ± 12* | 11 ± 9.1§ | |
| NK cells | Hep Na (d = 0) | 1.8 ± 1.2 | 1.5 ± 1.3 | 76 ± 11 | 2.9 ± 1.8 |
| CPD (d = 0) | 0.12 ± 0.21 | 0.61 ± 0.85 | 83 ± 6.6 | 0.71 ± 0.11 | |
| ACD (d = 0) | 0.11 ± 0.12 | 0.33 ± 0.58 | 70 ± 12 | 0.13 ± 0.16 | |
| Hep Na (d = 1) | 2.7 ± 2.4 | 1.6 ± 1.1 | 81 ± 17 | 4.3 ± 2.2 | |
Staining and analysis of these PBMC subsets have been performed as in Figs 1-3.
.0001 < P ≤ .001.
P ≤ .0001.
.01 < P < .05.
.001 < P ≤ .01.
Heparin did not impede IL-2R subunit detection.
To verify that heparin did not interfere with IL-2R subunit expression or detection, we analyzed IL-2R chain expression in some pathologic situations with blood collected on sodique heparin and PBMC isolated at day 0. Hemochromatosis is due to a defect in iron metabolism and is easily treated by periodic blood-letting. As shown in Fig 6, we found in this pathology that the appearance of the three IL-2R subunits on CD4 T lymphocytes (mean expressions, 40%, 42%, and 22% for IL-2Rα, IL-2Rβ, and IL-2Rγ, respectively), B lymphocytes (13%, 31%, and 26%) and CD8 T lymphocytes (36%, 37%, and 45%). On monocytes, there was appearance of IL-2Rα and IL-2Rβ (26% and 35%) and an increase in IL-2Rγ expression (to 73%). In hemochromatosis, the profile of IL-2R expression on NK cells was not affected.
Intracellular expression of IL-2Rγ in the different lymphocyte populations.
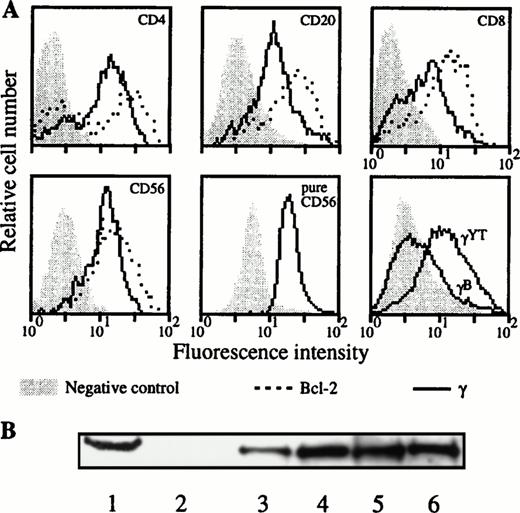
Due to an apparent contradiction between the constitutive IL-2Rγ gene expression and the absence of IL-2Rγ chain cell-surface detection in the lymphocyte subsets when blood was collected on sodique heparin and PBMC was purified at day 0, we analyzed the intracellular expression of this chain on these subsets. Such experiments were performed for three donors. The absence of IL-2Rγ surface expression in the different lymphocyte populations for these donors was verified (data not shown). Figure 7A shows a representative donor. In the four different PBMC populations we could clearly detect the intracellular expression of IL-2Rγ. This result has been confirmed for a purified population of NK cells (Fig 7A, lower middle panel). Although the IL-2Rγ subunit was present as intracellular protein in these four lymphocyte subsets, the IL-2Rα component was not detected and IL-2Rβ chain was not detected in B and T lymphocytes (data not shown). Intracellular expression of IL-2Rγ on the different PBMC subsets has been verified by Western blot (Fig 7B). PBL (which represents all of the PBMC subsets with nondetectable IL-2Rγ expression on cell surface) and purified CD4 T lymphocyte extracts showed the characteristics 64-kD band also found in the YT cell line. Results obtained with the monocyte population, which express IL-2Rγ on their surface, are also shown.
Intracellular expression of IL-2Rγ in the different lymphocyte populations. (A) After cell permeabilization, intracellular staining was performed against IL-2Rγ and Bcl-2 protein as positive control. Two-color flow cytometry was performed on PBMC isolated from blood collected on sodique heparin for the upper and lower left panels. Results were confirmed for NK cells on purified CD56 cells as indicated (lower middle panel). Lower right panel shows a positive (YT) and a negative B-EBV cell line (B) derived from a XSCID child; background controls are identical with these two cell lines. (B) Blood was collected on sodique heparin and PBMC were isolated at d = 0. PBL, CD4 T lymphocytes and monocytes were purified and Western blots performed on their lysates as explained in Materials and Methods. Lane 1, YT cell line (positive control); lane 2, B-EBV cell line from a XSCID patient (negative control); lane 3, PBMC; lane 4, PBL; lane 5, monocytes; and lane 6, CD4 T lymphocytes.
Intracellular expression of IL-2Rγ in the different lymphocyte populations. (A) After cell permeabilization, intracellular staining was performed against IL-2Rγ and Bcl-2 protein as positive control. Two-color flow cytometry was performed on PBMC isolated from blood collected on sodique heparin for the upper and lower left panels. Results were confirmed for NK cells on purified CD56 cells as indicated (lower middle panel). Lower right panel shows a positive (YT) and a negative B-EBV cell line (B) derived from a XSCID child; background controls are identical with these two cell lines. (B) Blood was collected on sodique heparin and PBMC were isolated at d = 0. PBL, CD4 T lymphocytes and monocytes were purified and Western blots performed on their lysates as explained in Materials and Methods. Lane 1, YT cell line (positive control); lane 2, B-EBV cell line from a XSCID patient (negative control); lane 3, PBMC; lane 4, PBL; lane 5, monocytes; and lane 6, CD4 T lymphocytes.
DISCUSSION
Analysis of freshly prepared PBMC from blood collected on heparin allowed the identification of two group of cells: (1) T and B lymphocytes involved in acquired immunity that do not express the three IL-2R components, or express them at very low levels, and (2) monocytes and NK cells classically considered as the cells of innate immunity that strongly express IL-2Rγ or IL-2Rβ, respectively. On CD8 T lymphocytes and monocytes, some variations are observed in IL-2Rβ and IL-2Rγ expressions between healthy donors (Fig 4). The mRNA profile from purified cells, detected by reverse transcriptase (RT)-PCR, is in agreement with these results based on flow cytometry. Indeed, CD4 T and B lymphocytes express only IL-2Rγ mRNA. NK cells express both IL-2Rβ and IL-2Rγ mRNAs, whereas monocytes express only IL-2Rγ mRNA. For CD8 T lymphocytes, IL-2Rγ mRNA is found and not IL-2Rα mRNA whereas, as expected, IL-2Rβ mRNA could be expressed, depending on the donor (Karine Sun, unpublished data). The pattern of expression of IL-2R subunits at the PBMC cell-surface is highly dependent on the preparation conditions of blood and cells, whereas activation markers are apparently less affected by these parameters. The use of freshly prepared PBMC from heparinized blood allows us to clarify the question of IL-2Rγ expression. Indeed, IL-2Rγ is not detectable on the surface of T, B lymphocytes, and NK cells, but is nevertheless clearly present as an intracellular protein. Our conditions also allow monitoring of the appearance of IL-2R subunits in a pathologic situation.
Various conflicting data have been reported about IL-2R subunit expression on the different PBMC subsets. On CD4 T lymphocytes, the data are divergent for the expression of the three IL-2R chains.22-25,28,29,36,37 Contradictory results have also been reported for IL-2Rβ and IL-2Rγ expression on CD8 T lymphocytes.22,24,25,28,29,36,37 For the other PBMC subsets, the differences are mainly focused on the expression of one IL-2R component: IL-2Rα for B lymphocytes,25,36 IL-2Rβ for monocytes,25-27,36,38 and IL-2Rγ for NK cells.25,28 39 In most of these reports, the method of blood collection and PBMC preparation are not always well specified and in some reports, results obtained from only one donor are presented.
Our results on the role of the used anticoagulant and of the day of PBMC isolation may provide an explanation for some of the discrepancies in the published data. Ca2+ chelators are typically used as anticoagulants. As CPD and ACD were Ca2+ chelators, it seems paradoxical that use of these products results in an increase of IL-2R subunit expression, whereas Ca2+ is necessary for cell activation. However, Vanham et al40 have also shown a strong expression of IL-2Rα and IL-2Rβ on both CD4 and CD8 T lymphocytes with blood collected on EDTA, another Ca2+chelator. Moreover, blood conservation temperature41 and blood age42 have some effect on the detection of different markers. These factors are important, as some centers deliver blood after serological testing, ie, 1 day after blood collection. As IL-2R expression is very sensitive, we can also hypothesize that commonly used methods for monocyte purification could enhance the expression of IL-2Rβ, which is often detected.26 27 Furthermore as shown here, IL-2R chain expression displays some variations among donors on some PBMC subsets, CD8 T lymphocytes mainly, and this factor may have been neglected in previous studies.
Under our experimental conditions, we observe a broad change in the IL-2R expression profile in hemochromatosis patients, compared with healthy donors (Fig 6). Thus blood drawing on heparin do not impede the detection of the three IL-2R subunits on various PBMC populations. Moreover, the increase of IL-2R expression seen in this pathology, particularly on CD8 T lymphocytes, would have been undetectable with ACD or CPD. The same situation would have probably occurred during studies of the activation of purified CD4 T lymphocytes: in a resting stage, the three IL-2R subunits are not detectable on the cell-surface, and only appear after anti-CD3 stimulation.30 Our observations have two consequences. First, blood collected during the treatment of hemochromatosis is a common source of PBMC for many laboratories in cellular immunology. The expression of the IL-2R on these cells may have had some influences on previous results, which could not be taken into account. Second, the use of this protocol allows detection of IL-2R expression variations between the resting and activated stages and between normal and pathologic situations. This procedure has also been useful in the analysis of IL-2R expression defects in other pathologies, such as hepatitis (Denis David, unpublished data).
In this study we have shown that whereas IL-2R chain expression is very sensitive to the conditions of blood collection and of PBMC preparation, expression of the activation markers CD69, CD71, and HLA-DR are only weakly affected by these conditions. Therefore, specific induction of IL-2R chains may prime cells to become responsive to IL-2 in the absence of activation marker expression. In this view, one could speculate that cell IL-2 sensitivity might also depend on the conditions of blood collection and PBMC purification. Comparison with previously published data may be difficult because the anticoagulant used and the time of PBMC purification are not always indicated, and measurement of activation markers may not be relevant. As IL-2Rβ and IL-2Rγ belong to the large hematopoietin receptor family,16 other receptor components of this family may also display very sensitive patterns of expression.
When blood is collected on heparin and PBMC isolated at day 0, CD4 T lymphocytes, B lymphocytes, NK cells, and CD8 T lymphocytes do not express IL-2Rγ on their surface, or express at very low levels, depending on the donors (Figs 1-4). However, we show that even in these conditions, IL-2Rγ is nevertheless largely present as intracellular protein in these different PBMC subsets (Fig 7A). This result confirms and extends our previous data showing that purified resting CD4 T lymphocytes also express IL-2Rγ only inside the cell.30 The intracellular expressions we show here are consistent with the fact that IL-2Rγ gene promoter has the characteristics of a constitutively active promoter43 and that IL-2Rγ mRNA is constitutively expressed in PBL15 and in monocytes.44 For human monocytes, IL-2Rγ has been shown to migrate as several Western blot bands, in addition to the classical 64-kD band,15 which correspond to different levels of N-linked glycosylation.33 We can thus hypothesize that this protein maturation process might also exist in the other PBMC subsets where we have detected IL-2Rγ as an intracellular protein. In the different lymphocyte subsets, IL-2Rγ may be unable to translocate to the cell-surface. In this view, a “helper” protein would be necessary for this process. In some conditions, IL-2Rβ or another protein might play this role. Some examples of proteins stored inside the cell, which require the presence of such a “helper” protein, have been described.45 46
The present study provides a description of the pattern of IL-2R subunit expression by T and B lymphocytes, NK cells, and monocyte populations. These results should provide some insight into the immune mechanisms controlling IL-2 responsiveness to the various cellular components of the immune system and should be helpful in designing new therapeutic strategies based on the use of IL-2 or cytokines sharing IL-2Rβ and IL-2Rγ with IL-2R.
ACKNOWLEDGMENT
We are indebted to Drs R. Robb, K. Sugamura, W.J. Leonard, M. Noguchi, and Y. Jacques for kindly providing the antibodies used in these experiments. We are grateful to Dr R. Weil and S. Herblot for their valuable advice and to Drs G. de Saint Basile and A. Fischer for their generous gift of the IL-2Rγ− B-EBV cell line. We thank B. Bénard, M. Minet, and P. Trumbic for their expert technical assistance. We also thank Dr F. Saul for kindly reviewing this manuscript.
Supported by grants from ANRS (Agence Nationale de Recherche sur le SIDA, Paris, France) and National Institutes of Health Grant No. CA41619. D.D. is a fellow of the SIDACTION (Fondation pour la Recherche Médicale, Paris France). L.B. and C.D. are supported by the ANRS and by the AFM (Agence Française contre les Myopathies, Paris, France), respectively.
L.B. and J.-L. M. contributed equally to this work.
Address reprint requests to Jacques Thèze, MD, PhD, Unité d'Immunogénétique Cellulaire, Département d'Immunologie, Institut Pasteur, 25 & 28 rue du Dr Roux, 75724 Paris cedex 15, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal