Abstract
B cells can differentiate into the antibody-secreting cells, plasma cells, whereas the crucial signals that positively control the entry into the pathway to plasma cells have been unclear. Triggering via CD27 by CD27 ligand (CD70) on purified peripheral blood B cells yielded an increase in the number of plasma cells in the presence of interleukin-10 (IL-10). Differentiation into plasma cells by a combination of IL-10 and CD70 transfectants occurred in CD27+ B cells but not in CD27− B cells. Moreover, addition of IL-2 to the IL-10 and CD70-transfect activation system greatly induced differentiation into plasma cells. In the presence of only IL-2, IL-4, or IL-6, CD70 transfectants did not promote differentiation into plasma cells. On the other hand, CD40 signaling increased the expansion of a B-cell pool from peripheral blood B cells primarily activated by IL-2, IL-10, and anti-CD40 monoclonal antibody (MoAb). Finally, CD27 signaling also rescued B cells from IL-10–mediated apoptosis. These data demonstrate that CD27 ligand (CD70) is a key molecule to prevent the IL-10–mediated promotion of apoptosis and to direct the differentiation of CD27+ memory B cells toward plasma cells in cooperation with IL-10.
TO PRODUCE ANTIBODIES, the differentiation of B cells into the specific antibody-secreting cells, plasma cells, is required. Triggering via B-cell Ig receptors by antigens, cytokines such as interleukin-2 (IL-2), IL-6, and IL-10, and direct cell-to-cell contact between T and B cells play an important role in the differentiation into plasma cells. It has been shown that activated B cells express IL-6 receptors and IL-6 induces the differentiation to plasma cells.1,2 Continuous high levels of serum or topical IL-6 in several diseases3 may promote the differentiation to plasma cells,4 and multiple myeloma cells proliferate in response to IL-6.5,6 However, IL-6 alone did not promote the differentiation to plasma cells.7Recently, IL-10 was characterized as a cytokine produced by Th2 clones to inhibit cytokine synthesis by Th1 clones. Ig production was enhanced by IL-10, and IL-10 also functions to increase plasma cells.8 9
In regard to the T-cell help by direct T/B-cell interaction, CD40/CD40 ligand (CD154) interaction is important for Ig production. Recent reports demonstrated that the CD40-mediated signal induced B-cell proliferation and differentiation into memory B cells but suppressed their capacity to differentiate along the plasma cell pathway,10,11 indicating that the CD40 signaling pathway is instrumental for clonal expansion of the memory B-cell pool but does not operate in the late phase of the response. What kinds of molecules do regulate the differentiation into plasma cells? CD27, characterized in detail by van Lier et al,12,13 is a type I glycoprotein expressed on T and B cells and B-cell malignancies.14Recently, we demonstrated that the interaction between CD27, which belongs to the nerve growth factor receptor/tumor necrosis factor receptor (NGFR/TNFR) family,15 and CD27 ligand (CD70), which belongs to the TNF family16 and is expressed not only on activated B cells but also on T cells, especially activated CD4+ CD45RO T cells,17 enhanced Ig production by B cells,18 19 suggesting that the CD27/CD70 interaction is involved in the differentiation of B cells into plasma cells.
In this study, we examined the effect of CD27/CD70 interaction on plasma cell generation induced by IL-10 to further characterize the role of CD27-mediated signaling in B-cell differentiation.
MATERIALS AND METHODS
Antibodies and reagents.
The following monoclonal antibodies (MoAbs) were used in this study. Anti-CD27 (8H5, IgG1) was used, which does not block the ligation of CD27/CD70 as described elsewhere.20 Phycoerythrin (PE)-conjugated anti-CD20 MoAb was purchased from DAKO Japan (Tokyo, Japan). Purified anti-CD70 MoAb (HNE51, IgG1), anti-CD40 MoAb (MAB89, IgG1), anti-CD154 MoAb (TRAP-1, IgG1), and fluorescein isothiocyanate (FITC)-conjugated anti-CD38 MoAb (T16, IgG1) were obtained from Immunotech (Westbrook, ME). Staphylococcus aureus Cowan strain (SAC) and propidium iodide were purchased from Sigma (St Louis, MO), human IL-4, IL-6, and IL-10 from Genzyme (Cambridge, MA), and IL-2 from Takeda Seiyaku Co (Osaka, Japan).
Cell preparation.
Adult human peripheral blood mononuclear cells (PBMNCs) were isolated from healthy volunteers by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density-gradient centrifugation. PBMNCs were separated with 5% sheep erythrocytes into erythrocyte rosette-positive (E+) and negative (E−) populations.21 After depleting the monocytes by silica (IBL, Fujioka, Japan) or adherence to the plastic surface, E− cells were further purified into B cells by positive selection with anti-CD19 MoAb–coated immunomagnetic beads (Dynal, Oslo, Norway), and anti-CD19 MoAb was then removed with Detach a bead (Dynal); 97% of cells in the B-cell population thus obtained were reactive with anti-CD20 MoAbs. Adult CD20+CD27− and CD20+CD27+ B cells were isolated from the monocyte-depleted E− cells by sorting using a FACStar Plus (Becton Dickinson, Mountain View, CA) under sterile conditions. Both populations thus obtained were greater than 98% pure. Bone marrow specimens were obtained from multiple myeloma patients whose bone marrow was occupied by greater than 60% myeloma cells. MNCs were separated by Ficoll-Hypaque density-gradient centrifugation.
Preparation of CD70 transfectants.
Total RNA was isolated by the single-step method22 from the CD70+ human B-cell line, Daudi cells. Reverse transcription was conducted using 1 μg total RNA, and polymerase chain reaction was performed with CD70 primers (5′-TCAGAATTCTGACAGGTTGAAGCAAGTAGA-3′ and 5′-CGATCTAGAAACCCTAATCAGCAGCAGTGG-3′). The amplified DNA was digested with EcoRI and XbaI and cloned into the mammalian expression vector pcDLSRα296.23 The resulting plasmid and the SRα expression vector with the neomycin-resistant gene were used to cotransfect into the CD70− murine pre-B-cell line 300-1924 by electroporation using a Gene Pulsar (Bio-Rad, Hercules, CA). The transfectants were selected by growth in culture medium containing the neomycin analog G418 (GIBCO-BRL, Gaithersburg, MD) at 1 mg/mL.
Flow cytometric analysis and detection of B cells undergoing apoptosis.
Purified B cells after activation and MNCs obtained from multiple myeloma patients were stained with a combination of CD38-FITC, CD27-PE, and CD20-PE. Two-color analysis of cell-surface molecules was performed by a FACScan (Becton Dickinson). Dead cells were removed by staining with propidium iodide.
Following incubation of B cells with IL-10 (100 ng/mL) in the presence or absence of CD70 transfectants or anti-CD40 MoAb (1 μg/mL) for 3 days, the B cells were incubated with 70% ethanol overnight at −20°C. After washing with phosphate-buffered saline (PBS), the cells were suspended with 1 mL PBS containing 20 μg/mL propidium iodide (Sigma) and 200 μg/mL RNase (Sigma). The red fluorescence was measured on a FACScan. Cellular debris was excluded from analysis, and the DNA content of intact cells was registered on a logarithmic scale. The viable-cell count was performed by staining with trypan blue (GIBCO) after 3-day culture of B cells under various conditions.
DNA fragmentation was assessed by agarose gel electrophoresis. Briefly, 2 × 106 B cells were incubated for 3 days in the presence of IL-10 (100 ng/mL) with or without transfectants. Following cell lysis, intact DNA and cellular debris were pelleted by centrifugation. After phenol extraction of the supernatant and isopropanol precipitation, the pellets were resuspended in TE buffer containing 0.1 mg/mL RNase. Then, the fragmented DNA was electrophoresed with 1% agarose gel.
Fixation of cells.
The CD70/300-19 and mock/300-19 transfectants were incubated with 1% paraformaldehyde in PBS for 5 minutes. After washing with PBS three times, the cells were cultured in RPMI 1640 + 10% fetal calf serum for 30 minutes and then used for the analysis.
Ig assay by enzyme-linked immunosorbent assay.
Purified peripheral blood B cells were cultured with IL-10 (50 to 100 ng/mL) or IL-2 (50 U/mL) plus IL-10 (50 to 100 ng/mL) in the presence of various concentrations of the fixed CD70/300-19 or mock/300-19 at a final cell density of 1.25 × 105/mL in a volume of 200 μL/well for 8 days at 37°C in a humidified atmosphere with 5% CO2. The cultured supernatants were harvested and added to goat anti-human Ig (Southern Biotechnology, Birmingham, AL)–coated 96-well flat enzyme-linked immunosorbent assay (ELISA) plates with control human IgG, IgM, and IgA (Sigma Chemical) overnight. After discarding the supernatants and washing with 0.05% Tween in PBS, the bound human Ig was detected with alkaline phosphatase–labeled goat anti-human IgG, IgM, or IgA (Sigma) at a dilution of 1/1,500, and color detection was performed. In this ELISA system, no cross-reaction between IgG, IgM, and IgA occurred. Calibration was performed with PBS at standard zero levels.
RESULTS
Morphologic and flow-cytometric analyses of plasma cells induced by CD70 transfectants.
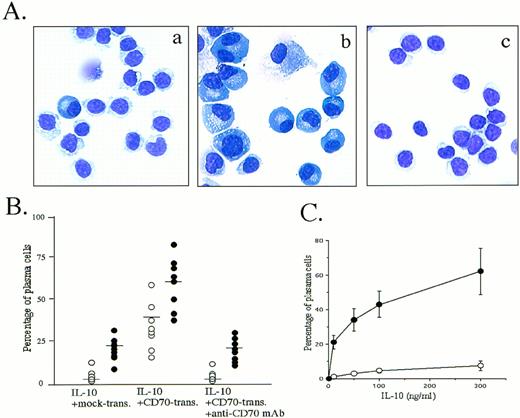
The observation that peripheral blood B cells produced large amounts of Ig by the triggering CD27 in the presence of SAC plus IL-218,19,25 or IL-10 (Table 1) (and Nagumo H, Agematsu K, submitted) prompted us to ascertain whether CD27/CD70 interaction was involved in the promotion of plasma cell generation. Accordingly, we studied the effect of CD27 signaling on peripheral blood B-cell differentiation by morphologic and flow-cytometric analyses in various B-cell activation systems. Most interestingly, B cells stimulated with CD70 transfectants in the presence of IL-10 for 8 days began to display the typical morphology of plasma cells: they had a basophilic cytoplasm with a pale Golgi zone and an eccentric nucleus. In contrast, IL-10 without CD27 signaling induced a slight increase in the number of plasma cells. The increase in differentiation to plasma cells was completely blocked by initial addition of anti-CD70 MoAb (Fig 1A and B). Based on the evidence that IL-10 plus IL-2 is the most efficient cytokine combination allowing differentiation of normal B cells into plasma cells9 and that IL-2 inhibits IL-10–induced apoptosis of B cells by affecting the expression of bcl-2 oncoprotein,26 we also conducted an experiment in which IL-2 was added. Greater induction of plasma cells by CD70 transfectants was seen in the presence of IL-2 and IL-10 (Fig 1B). Induction of plasma cells by CD70 transfectants was enhanced by IL-10 in a dose-dependent manner (Fig 1C).
Enhancement of IgG Production Via CD27 Triggering in the Presence of IL-10
| Culture Condition . | IgG (ng/mL) . | ||
|---|---|---|---|
| UF B Cells . | CD27+B Cells . | CD27− B Cells . | |
| Medium alone | ND | ND | ND |
| IL-10 | 286 ± 12 | 364 ± 17 | ND |
| IL-10 + mock transfectants | 247 ± 8 | 376 ± 23 | ND |
| IL-10 + CD70 transfectants | 1,139 ± 89 | 1,654 ± 98 | ND |
| IL-10 + IL-2 | 892 ± 67 | 1,267 ± 113 | ND |
| IL-10 + IL-2 + mock transfectants | 763 ± 104 | 1,192 ± 84 | ND |
| IL-10 + IL-2 + CD70 transfectants | 4,693 ± 236 | 7,975 ± 846 | ND |
| Culture Condition . | IgG (ng/mL) . | ||
|---|---|---|---|
| UF B Cells . | CD27+B Cells . | CD27− B Cells . | |
| Medium alone | ND | ND | ND |
| IL-10 | 286 ± 12 | 364 ± 17 | ND |
| IL-10 + mock transfectants | 247 ± 8 | 376 ± 23 | ND |
| IL-10 + CD70 transfectants | 1,139 ± 89 | 1,654 ± 98 | ND |
| IL-10 + IL-2 | 892 ± 67 | 1,267 ± 113 | ND |
| IL-10 + IL-2 + mock transfectants | 763 ± 104 | 1,192 ± 84 | ND |
| IL-10 + IL-2 + CD70 transfectants | 4,693 ± 236 | 7,975 ± 846 | ND |
Data are expressed as the mean ± SD for triplicate wells and are representative of 4 experiments using different donors. B cells were cultured at 1.25 × 105/mL for 8 days in the indicated culture conditions.
Abbreviations: UF, unfractionated; ND, not detected.
Induction of plasma cells by triggering via CD27 on B cells. (A) Highly purified peripheral blood B cells were cultured with 100 ng/mL IL-10 in the presence of mock transfectants (a), CD70 transfectants (b), or CD70 transfectants plus anti-CD70 MoAb 2 μg/mL (c) for 8 days, cytospun, and stained with May-Giemsa staining. The results are representative of 12 experiments using different donors. (B) Cultured B cells in the presence of 100 ng/mL IL-10 (○) or IL-10 + IL-2 50 U/mL (•) were stained with May-Giemsa staining and counted for plasma cells and B cells. Transfectants and apparent dead cells were eliminated from the cell count. Horizontal bars represent mean values. (C) Percentage of plasma cells cultured with indicated various concentrations of IL-10 in the presence of mock transfectants (○) or CD70 transfectants (•). Values are the mean ± SD of three experiments.
Induction of plasma cells by triggering via CD27 on B cells. (A) Highly purified peripheral blood B cells were cultured with 100 ng/mL IL-10 in the presence of mock transfectants (a), CD70 transfectants (b), or CD70 transfectants plus anti-CD70 MoAb 2 μg/mL (c) for 8 days, cytospun, and stained with May-Giemsa staining. The results are representative of 12 experiments using different donors. (B) Cultured B cells in the presence of 100 ng/mL IL-10 (○) or IL-10 + IL-2 50 U/mL (•) were stained with May-Giemsa staining and counted for plasma cells and B cells. Transfectants and apparent dead cells were eliminated from the cell count. Horizontal bars represent mean values. (C) Percentage of plasma cells cultured with indicated various concentrations of IL-10 in the presence of mock transfectants (○) or CD70 transfectants (•). Values are the mean ± SD of three experiments.
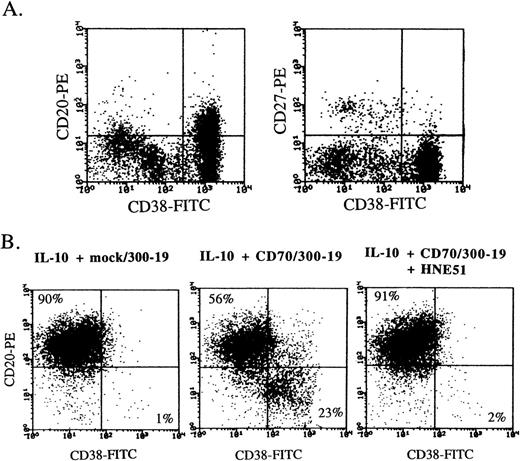
Human peripheral blood B cells express a high density of CD20 and CD40 and a low density of CD38, and plasma cells express CD38 greatly. Multiple myeloma cells strongly expressed CD38 but not CD27, and mildly expressed CD20 (Fig 2A). Flow-cytometric analysis also indicated that the differentiation into the CD38+ plasma phenotype was greatly induced by addition of CD70 transfectants, and the enhancement of differentiation completely disappeared by the blockage between CD27 and CD70 interaction with anti-CD70 MoAb (Fig 2B). Induction of plasma cells by CD27 triggering was also recognized in the presence of SAC plus IL-2, whereas the plasma phenotype did not increase by addition of CD70 transfectants in the presence of only IL-2, IL-4, or IL-6 (data not shown).
CD38 expression on multiple myeloma cells and plasma cells induced by CD27 signaling and IL-10. (A) Freshly isolated bone marrow cells from a multiple myeloma patient were stained by the combination of anti-CD38-FITC, anti-CD20-PE, and anti-CD27-PE. (B) Highly purified B cells were cultured with 100 ng/mL IL-10 for 8 days in the presence of mock transfectants, CD70 transfectants, or CD70 transfectants + anti-CD70 MoAb. Then, the cells were stained with anti-CD38-FITC and anti-CD20-PE. Antibody-coated cells, gated on live cells by cell size and granularity, and removed dead cells by propidium iodide staining, were enumerated by flow-cytometric analysis.
CD38 expression on multiple myeloma cells and plasma cells induced by CD27 signaling and IL-10. (A) Freshly isolated bone marrow cells from a multiple myeloma patient were stained by the combination of anti-CD38-FITC, anti-CD20-PE, and anti-CD27-PE. (B) Highly purified B cells were cultured with 100 ng/mL IL-10 for 8 days in the presence of mock transfectants, CD70 transfectants, or CD70 transfectants + anti-CD70 MoAb. Then, the cells were stained with anti-CD38-FITC and anti-CD20-PE. Antibody-coated cells, gated on live cells by cell size and granularity, and removed dead cells by propidium iodide staining, were enumerated by flow-cytometric analysis.
CD27+ B cells, but not CD27− B cells, differentiate into plasma cells by CD70 transfectants.
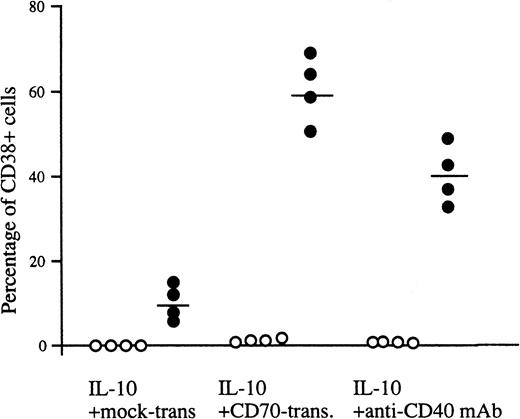
Inasmuch as peripheral blood B cells are composed of CD27− naive B cells and IgD− memory B cells expressing CD27, CD27+ B cells produced IgG, but CD27− B cells did not (Table 1). In addition, CD20+ CD38− B cells remained after stimulation with IL-10 and CD70 transfectants (Fig 2A). Accordingly, we purified CD27+ and CD27− B cells from human peripheral blood and investigated the effect of CD27/CD70 interaction on the differentiation of the two populations into plasma cells. The majority of CD27+ B cells differentiated into CD38+ plasma cells in the presence of IL-10 and CD70 transfectants, whereas IL-10 alone promoted very low levels of differentiation into plasma cells. In parallel with the increase in the number of plasma cells, IL-10 and CD27 triggering induced CD27+ memory B cells to secrete large amounts of IgG (Table 1). In contrast, apparent induction of plasma cells was not recognized in CD27− naive B cells in the presence of IL-10 and CD70 transfectants (Fig 3). Similar results were obtained when naive B cells were stimulated with IL-2, IL-10, and CD70 transfectants. These results demonstrate that IgD+CD27− naive B cells do not have the ability to differentiate into the Ig-producing cells, plasma cells, in the IL-2 plus IL-10 system, at least not with short-term culture up to 8 days, and CD70 in cooperation with IL-10 was involved in the differentiation of CD27+ memory B cells into plasma cells.
Generation of plasma cells from CD27+ B cells. Peripheral blood B cells were separated into CD27+(•) and CD27− B cells (○) by a cell sorter and then incubated with IL-10 in the presence of mock transfectants, CD70 transfectants, or anti-CD40 MoAb for 8 days, and two-color analysis using anti-CD38-FITC and anti-CD20-PE was performed by flow cytometry. Percentages represent a ratio of highly expressed CD38+cells. The results are representative of four experiments. Horizontal bars represent the mean values.
Generation of plasma cells from CD27+ B cells. Peripheral blood B cells were separated into CD27+(•) and CD27− B cells (○) by a cell sorter and then incubated with IL-10 in the presence of mock transfectants, CD70 transfectants, or anti-CD40 MoAb for 8 days, and two-color analysis using anti-CD38-FITC and anti-CD20-PE was performed by flow cytometry. Percentages represent a ratio of highly expressed CD38+cells. The results are representative of four experiments. Horizontal bars represent the mean values.
Different effects of CD154 and CD70 on B-cell differentiation.
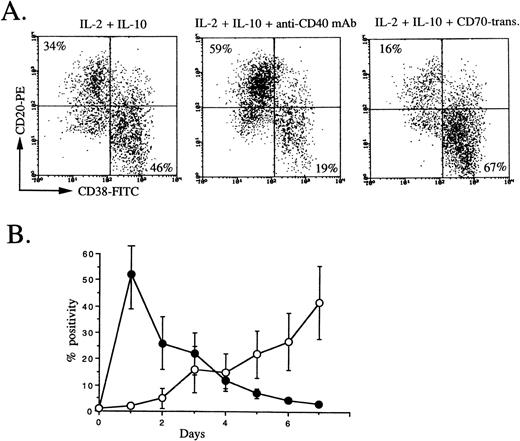
Since IL-2 plus IL-10 strongly induce B-cell proliferation, especially anti-CD40–activated B cells,27 and CD40 is equally expressed on peripheral blood memory and naive B cells, a primary culture was used to stimulate whole peripheral blood B cells by IL-2, IL-10, and anti-CD40 MoAb. The second culture condition, IL-2 + IL-10, IL-2 + IL-10 + anti-CD40 MoAb, or IL-2 + IL-10 + CD70 transfectants, was used on the basis of two considerations (Fig 4A). First, it has been shown that CD154 expression on T cells occurs transiently only in an early phase of activation28; on the other hand, CD70 expression on T cells occurs in a late phase of activation17 (Fig 4B). Second, CD40 signaling prevents differentiation into plasma cells from germinal-center B cells primarily stimulated with IL-2, IL-10, and triggering via CD40,10 suggesting that CD40 signaling acts on an early phase of B-cell activation and CD27 signaling acts on a late phase. It was evident that the percentage of cells with the CD38+plasma phenotype increased by the second culture with IL-2 plus IL-10, while, in agreement with Arpin et al,10 using tonsillar germinal-center B cells, the percentage of cells with the plasma phenotype decreased by triggering via CD40 by anti-CD40 MoAb in the second culture. Most strikingly, plasma phenotypes remarkably increased by triggering via CD27 by CD70 transfectants in the second culture (Fig4A). These findings support a view that CD27 on B cells is a key molecule to promote the differentiation into plasma cells by activated helper T cells with CD70, indicating that the differentiation of plasma cells is largely controlled by CD27/CD70 interaction.
CD38 and CD20 expression by peripheral blood B cells in secondary culture, and CD154 and CD70 expression on activated T cells. (A) Isolated peripheral blood B cells were cultured with IL-2 50 U/mL + IL-10 50 ng/mL + anti-CD40 MoAb 1 μg/mL. At day 3, cells were restimulated with IL-2 + IL-10, IL-2 + IL-10 + anti-CD40 MoAb, or IL-2 + IL-10 + CD70 transfectants. At the end of secondary culture (5 days), flow-cytometric analysis was performed following removal of dead cells by propidium iodide. The results are representative of 4 experiments. (B) CD154 (•) and CD70 (○) expression on E+ cells stimulated with PHA at indicated days were analyzed by flow cytometry. Values are the mean ± SD of three experiments using different donors.
CD38 and CD20 expression by peripheral blood B cells in secondary culture, and CD154 and CD70 expression on activated T cells. (A) Isolated peripheral blood B cells were cultured with IL-2 50 U/mL + IL-10 50 ng/mL + anti-CD40 MoAb 1 μg/mL. At day 3, cells were restimulated with IL-2 + IL-10, IL-2 + IL-10 + anti-CD40 MoAb, or IL-2 + IL-10 + CD70 transfectants. At the end of secondary culture (5 days), flow-cytometric analysis was performed following removal of dead cells by propidium iodide. The results are representative of 4 experiments. (B) CD154 (•) and CD70 (○) expression on E+ cells stimulated with PHA at indicated days were analyzed by flow cytometry. Values are the mean ± SD of three experiments using different donors.
CD27 stimulation prevents IL-10–induced apoptosis.
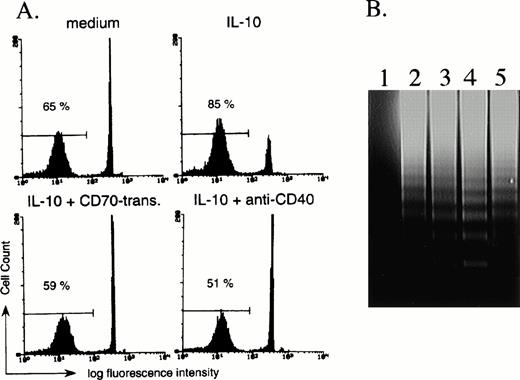
Since it has been reported that IL-10 induces apoptosis of activated B cells,29 we finally investigated the effect of CD27 signaling on IL-10–induced apoptosis. According to cell size and granularity by flow-cytometric analysis, we had the impression that dead B cells increased in the presence of IL-10 and that the addition of CD70 transfectants increased the number of viable cells as compared with IL-10 alone. Experiments were therefore conducted in which B cells were stimulated with IL-10 in the presence of mock transfectants or CD70 transfectants, and were analyzed by flow cytometry following staining of the DNA with propidium iodide. After 3 days in the presence of IL-10, the number of B cells with reduced levels of DNA increased, indicating increased numbers of B cells unde βrgoing apoptosis as compared with medium alone (Fig 5A). In contrast, the initial addition of CD70 transfectants in the presence of IL-10 decreased the number of B cells undergoing apoptosis (Fig 5), but addition of mock transfectants had no such effect (data not shown). The decrease of B cells undergoing apoptosis by CD70 transfectants was completely blocked by the initial addition of anti-CD70 MoAb (data not shown). The percentage (mean ± SD) of viable B cells as determined by staining with trypan blue after 72 hours of incubation with medium alone, IL-10 (100 ng/mL) plus mock transfectants, or IL-10 (100 ng/mL) plus CD70 transfectants was 67% ± 7.6%, 53.5% ± 6.2%, and 69.1% ± 3.8%, respectively (n = 3). Increased DNA fragmentation by IL-10 and its blockage by the triggering via CD27 by CD70 transfectants were also observed following 3-day culture of purified B cells (Fig 5B). These results indicate that CD27 signaling rescues B cells from IL-10–mediated apoptosis. Since IL-10 and CD40 triggering increased the number of B cells, CD40 signaling also decreased the ratio of B cells undergoing apoptosis induced by IL-10 (Fig 5A).
CD27 signaling prevents IL-10–induced apoptosis of B cells. (A) B cells were cultured with medium alone, 100 ng/mL IL-10, IL-10 + 20% CD70 transfectants, or IL-10 + anti-CD40 MoAb (1 μg/mL). After 72 hours of incubation, the number of B cells undergoing apoptosis was determined by staining with propidium iodide, followed by analysis with flow cytometry. The percentage of apoptotic B cells is indicated. (B) DNA fragmentation was assessed by agarose gel electrophoresis. CD70 transfectants only (lane 1) and purified 2 × 106 B cells (lanes 2 to 5) were incubated in the presence of medium alone (lane 2), 100 ng/mL IL-10 (lane 3), IL-10 + mock transfectants (lane 4), or IL-10 + CD70 transfectants (lane 5). After 3-day culture, the DNA fragmentation ladder was detected. A quantity of 4 × 105 fixed transfectants were used for 1 line.
CD27 signaling prevents IL-10–induced apoptosis of B cells. (A) B cells were cultured with medium alone, 100 ng/mL IL-10, IL-10 + 20% CD70 transfectants, or IL-10 + anti-CD40 MoAb (1 μg/mL). After 72 hours of incubation, the number of B cells undergoing apoptosis was determined by staining with propidium iodide, followed by analysis with flow cytometry. The percentage of apoptotic B cells is indicated. (B) DNA fragmentation was assessed by agarose gel electrophoresis. CD70 transfectants only (lane 1) and purified 2 × 106 B cells (lanes 2 to 5) were incubated in the presence of medium alone (lane 2), 100 ng/mL IL-10 (lane 3), IL-10 + mock transfectants (lane 4), or IL-10 + CD70 transfectants (lane 5). After 3-day culture, the DNA fragmentation ladder was detected. A quantity of 4 × 105 fixed transfectants were used for 1 line.
DISCUSSION
Plasma cells are the finally differentiated cells of the B-cell lineage, but the exact pathway and regulation of their differentiation have not been clarified in detail. As shown here, the CD27 signal resulted in the terminal differentiation of peripheral blood memory B cells into plasma cells in B-cell activation systems by IL-10, which was augmented by addition of IL-2. In contrast, the CD40 signal after 3 days of primary culture increased the number of B cells. Thus, CD27 is crucial in controlling the differentiation of memory B cells or activated B cells into plasma cells.
According to the steps of differentiation of plasma cells, several phenotypes of plasma cells are found, such as an early type (CD38++CD19+) and a mature type (CD38+++CD19+).29 In our experiments, morphologic and flow-cytometric analyses demonstrated that cells induced by CD27 triggering in the presence of IL-10 or IL-2 plus IL-10 were typical plasma cells, including probably early to mature types. To date, the importance of IL-6 for differentiation into plasma cells has been reported. Splawski et al7 demonstrated that IL-6 alone did not support generation of Ig-secreting cells or induction of Ig production, but IL-6 augmented the generation of Ig-secreting cells and secretion of all isotypes of Ig induced by SAC plus IL-2. In our experiments, since differentiation into plasma cells from freshly isolated peripheral blood B cells by CD27 triggering was not observed in the presence of IL-6, this effect may be specific for IL-10 stimulation. As reported previously,8 IL-10 alone had a function to promote B-cell differentiation into plasma cells, even though the induction was very mild. In agreement with Itoh and Hirohata,26 the initial addition of IL-10 promoted apoptosis of resting B cells, and IL-10–induced apoptosis was reversed by addition of CD70 transfectants. Accordingly, CD27 signaling on CD27+ B cells in the presence of IL-10 may induce plasma cells, at least in part, by blocking B cells from undergoing apoptosis. Ig production was also enhanced by CD70 transfectants in the presence of SAC plus IL-2.18 Only SAC plus IL-2 considerably increased the number of plasma cells, and enhancement of plasma cells by CD70 transfectants was not so remarkable under these conditions (data not shown). Recently, Stüber et al30 31demonstrated that the OX40-OX40 ligand pair belonging to the NGFR/TNFR superfamily promotes B-cell proliferation and differentiation and increases Ig secretion by increasing Ig heavy-chain mRNA levels. In addition, OX40 ligand cross-linking results in downregulation of the transcription factor BSAP. They also showed that blocking the OX40-OX40 ligand interaction in vivo results in a profound decrease of the anti-hapten IgG response and inhibition of the development of periarteriolar lymphoid sheath–associated B-cell foci, indicating that the OX40-OX40 ligand interaction in vivo is necessary for differentiation of activated B cells into highly Ig-producing cells. Further investigations such as the study of transcription factor BSAP and Blimp-1 involved in multiple steps of B-cell differentiation leading to plasma cell development are necessary to define the mechanism of induction to plasma cells by CD27/CD70 interaction.
To define memory B cells, cell-surface analyses on B cells have been performed. Human peripheral blood B cells can be separated into IgD+ and IgD− subpopulations with different functions: IgD+ B cells are primary, and IgD−B cells are postswitch memory cells.32 As reported recently,33 adult peripheral blood B cells can be further subdivided into three discrete subtypes: IgD+CD27−, IgD+CD27+, and IgD−CD27+. IgD−CD27+ B cells produce IgG, IgM, and IgA, IgD+CD27− B cells do not produce Ig, and IgD+CD27+ B cells predominantly produce IgM. CD27− B cells were CD38low, and a majority of CD27+ B cells were composed of CD38− cells (data not shown). Morphologically, CD27+ B cells are larger cells with abundant cytoplasm, and IgD+CD27−B cells are smaller with scant cytoplasm. These findings support a view that IgD− B cells that express CD27 at least include memory B cells. In further support of this concept, bcl-2 expression in CD27+ B cells was higher than in CD27− B cells (data not shown).
In the immune response, activated helper T cells not only secrete cytokines but also express molecules on the surface such as CD154 and CD70. The cell-to-cell interaction between T and B cells via two signals, CD40/CD154 and CD27/CD70, strictly regulates B-cell activation, proliferation, differentiation, and cell death.18,34,35 It is reported that CD40/CD154 interaction greatly enhances B-cell proliferation.27,28 In our experiments, CD27/CD70 interaction enhanced B-cell proliferation in the presence of SAC plus IL-2,18 and triggering via CD27 by CD70 transfectants in the presence of IL-4 enhanced B-cell proliferation about two to three times (data not shown). CD40 is expressed on most B cells, including naive and memory B cells. On the other hand, CD27 is expressed only on memory B cells, not on naive B cells, and CD27 expression on B cells was upregulated by the stimulation of SAC plus IL-2 or IL-2, IL-10, plus CD40 signaling (data not shown). We have determined that CD27− B cells are induced to express CD27 on the surface in the presence of SAC plus IL-2 or IL-2 plus IL-10 plus anti-CD40 MoAb, but not to produce Igs by SAC plus IL-2, and to express small amounts of Ig by IL-2 plus IL-10 plus anti-CD40 MoAb (Nagumo H, Agematsu K, Komiyama A, manuscript in preparation), suggesting that antigen receptor triggering or CD40 signaling in combination with the cytokines interferes with CD27/CD70-mediated B-cell differentiation by inducing CD27 expression. CD154 was expressed on T cells soon after activation, and CD70 was expressed on T cells later after activation (Fig 4B). On the basis of these findings, mature B-cell differentiation and Ig production are regulated by two of the major cell-to-cell signaling mechanisms. CD40/CD154 interaction acts on an early phase of B-cell activation and induces the expansion of a memory B-cell pool. Memory B cells differentiated into plasma cells by activated helper T cells via CD27/CD70 in the presence of several cytokines such as IL-10 and IL-2.
Interestingly, molecules belonging to the NGFR/TNFR family, CD30, CD40, OX40, CD95, and CD27, are involved in preventing or mediating apoptosis.36 As for the effects of NGF on immune systems, it has recently been demonstrated that NGF is constitutively produced by B cells and maintains the viability of cells with the surface phenotype of memory B cells, indicating that NGF is an autocrine survival factor for memory B lymphocytes.37 The interactions between many members of the NGFR/TNFR family and its ligands may serve important roles in B-cell activation, proliferation, differentiation, and cell death. Our data that the CD27 molecule is a novel inducer of plasma cells are not only important to explain an antibody immune response initiation by antigen exposure to antibody-producing cells, but also are of interest in considering the occurrence of such neoplasms as multiple myeloma.
ACKNOWLEDGMENT
The authors thank Drs S.F. Schlossman and Y. Kitano for support.
Address reprint requests to Kazunaga Agematsu, MD, Department of Pediatrics, Shinshu University School of Medicine, Asahi 3-1-1, Matsumoto 390, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal