Abstract
Gene knockout mice studies indicate that urokinase-type plasminogen activator (u-PA) is importantly involved in fibrinolysis, but its physiologic mechanism of action remains poorly understood. We postulated that platelets may be involved in this mechanism, as they carry a novel receptor for u-PA and a portion of the single-chain u-PA (scu-PA) intrinsic to blood is tightly associated with platelets. Therefore, plasminogen activation by platelet-associated u-PA was studied. When washed platelets were incubated with plasminogen, no plasmin was generated as detected by plasmin synthetic substrate (S2403) hydrolysis; however, after the addition of thrombin, but not other agonists, platelet-dependent plasminogen activation occurred. Plasminogen activation was surface-related, being inhibited by blocking platelet fibrinogen receptors or by preventing plasminogen binding to the thrombin-activated platelet surface. U-PA was identified as the only plasminogen activator responsible and enrichment of platelets with exogenous scu-PA significantly augmented plasminogen activation. These findings appeared paradoxical because thrombin inactivates scu-PA. Indeed, zymograms showed inactivation of scu-PA during the first hour of incubation with even the lowest dose of thrombin used (1 u/mL). However, this was followed by a thrombin dose-dependent (1 to 10 u/mL) partial return of u-PA activity. Reactivation of u-PA was not due to the direct action of thrombin, but required platelets and was found to be related to a platelet lysosomal thiol protease, consistent with cathepsin C. In conclusion, a new pathway of plasminogen activation by platelet-associated endogenous or exogenous scu-PA was demonstrated, which is specifically triggered by thrombin activation of platelets. These findings may help explain u-PA–mediated physiological fibrinolysis and have implications for therapeutic thrombolysis with scu-PA.
PLATELETS HAVE LONG been known to be important modulators of fibrinolysis. For example, the presence of antifibrinolytic activity in platelets was demonstrated more than 40 years ago1 and identified more recently to be due to the presence in platelet alpha granules of potent inhibitors, such as α2 -antiplasmin2 and plasminogen activator inhibitor-1 (PAI-1).3 Profibrinolytic properties have also been found, related to the assembly of plasminogen and tissue plasminogen activator (t-PA) on the platelet surface.4-6 Resting platelets induced an eightfold promotion of plasminogen activation by t-PA in a purified system,7 and thrombin stimulation of platelets increased plasminogen binding fivefold.4 However, in a plasma milieu, platelets have generally been found to confer resistance to fibrinolysis by t-PA,8 whereas they promoted clot lysis by single-chain urokinase-type plasminogen activator (scu-PA), which is resistant to inhibition by PAI-1.9
It was generally believed that u-PA, in contrast to t-PA, is involved principally in extravascular plasminogen activation rather than fibrinolysis, but recent gene inactivation studies have put this concept into question. Gene-targeted mice deficient in the u-PA gene developed spontaneous intravascular fibrin deposition, whereas those deficient in the t-PA gene did not, suggesting that u-PA has an important biologic role in fibrinolysis. At the same time, the u-PA knockout mice had unimpaired lysis of plasma clots injected into the jugular vein, indicating that platelet-poor clots were lysed by endogenous t-PA rather than u-PA.10 This is consistent with a previous study, showing that spontaneous clot lysis by endogenous u-PA was platelet-dependent in contrast to that by endogenous t-PA.11 In another recent study, fibrin deposition in endotoxin-treated mice correlated with “changes in u-PA mRNA, but not t-PA mRNA” and it was concluded that t-PA was not “essential for fibrin deposition/dissolution in this murine model” whereas u-PA was,12 further testifying to its biologic role in intravascular fibrinolysis.
Platelets are carriers of u-PA, as scu-PA was shown to be associated with the outer leaflet of the platelet membrane13 and it was estimated that about 20% of the u-PA intrinsic to blood was tightly associated with platelets.14 Platelet uptake of scu-PA from the ambient plasma or whole blood was also demonstrated12 and found to be related to a novel receptor on the platelet surface different from that on other cells.15 Based on these findings, we have postulated that platelets may help mediate fibrinolysis by scu-PA.
Single-chain u-PA is a proenzyme, which is activated by enzymatic cleavage at Lys158-Ile159 16 by plasmin17 or kallikrein.18 By contrast, thrombin inactivates scu-PA19 by cleavage at Arg156-Phe157,18 two residues from the activation site. This thrombin-generated two-chain u-PA, which has been referred to as thromb-UK,20 is relatively highly resistant to activation by plasmin,20 but has recently been shown by Nauland and Rijken21 to be sensitive to activation by lysosomal dipeptidyl-peptidase I or cathepsin C, which removes the N-terminal dipeptide from the B chain of thromb-UK by cleaving the Lys158-Ile159 bond.
In this study, we have evaluated whether platelet-associated scu-PA induced plasminogen activation in the presence of platelet agonists. It was observed that plasminogen activation occurred, but only when platelets were stimulated by thrombin. This paradoxical response to thrombin was shown to be largely related to activation of thromb-UK by a platelet lysosomal thiol protease consistent with cathepsin C, although cathepsin C has not been previously identified in human platelets.
MATERIALS AND METHODS
Reagents. Scu-PA, purified from Escherichia coli, was obtained from Farmitalia Carlo Erba (Milan, Italy). Thromb-UK was prepared by thrombin treatment of recombinant scu-PA as previously described.20 Glu-plasminogen was purified from human bank plasma essentially by the method of Castellino and Powell.22 Plasminogen, scu-PA, and thromb-UK were treated with diisopropylfluorophosphate (DFP) (1 mmol/L, 0°C, 16 hours or 5 mmol/L, 37°C, 1 hour) to inactivate trace contaminants of enzymatic plasmin or two-chain u-PA (tcu-PA). Human α-thrombin (3054 NIH U/mg) was obtained from Enzyme Research Laboratories (South Bend, IN). A murine monoclonal antibody against human u-PA and a rabbit polyclonal antibody against recombinant human t-PA were obtained from American Diagnostica (Greenwich, CT). Cellsep, a density gradient separation medium was purchased from Larex, Inc (St Paul, MN). Fibrinogen and synthetic chromogenic substrates for plasmin (S2403) and for u-PA (S2444) were obtained from Kabi Pharmacia, Inc (Franklin, OH). U46619, a structural analogue of PGH2 (9,11-dideoxy-9a,11,-methanoepoxy PGF2 ) was from Biomol Research Laboratory (Plymouth Meeting, PA) and the thrombin receptor-activating peptide (TRAP) SFL LRN PND KYEPF was from Calbiochem (LaJolla, CA). The peptidyl diazomethyl ketone, Gly-Phe-CHN2 , was from Enzyme Systems Products (Livermore, CA). Cystatin, ε-aminocaproic acid (EACA), amiloride, cytochalasins B and D, carboxypeptidase B (CpB), fibrinogen receptor antagonist N-Acetyl-Pen-Arg-Gly-Asp-Cys (Ac-pen-RGDC), Gly-Pro-Arg-Pro and all other chemicals were from Sigma (St Louis, MO).
Preparation of platelets. Venous blood was obtained from healthy volunteers and platelets were isolated via Cellsep (isotonic arabinogalactan) isopycnic centrifugation using the method of Corash et al23 as modified by Moon et al.24 This method is reported to yield a platelet preparation greater than 99% pure. Blood was collected into 19% sodium citrate (50/1, vol/vol) and platelet-rich plasma (PRP) was prepared by adding three parts of a buffered saline glucose-citrate solution (BSG-citrate; 0.08 mol/L NaCl, 0.0136 mol/L sodium citrate, 0.011 mol/L glucose, 0.008 mol/L Na2HPO4 , and 0.001 mol/L KH2PO4 , pH 7.4) to 10 parts blood and centrifuging at 850g for 5 minutes. For most experiments the PRP was used immediately, but for a few experiments, it was used after an overnight storage (room temperature with constant rocking and the addition of penicillin and streptomycin). Prostaglandin E1 (1 μmol/L) was added to the BSG-citrate–diluted PRP and 4 mL was layered over a discontinuous gradient consisting of 4 mL Cellsep and 2 mL diluted Cellsep (diluted 1:1 with BSG-citrate) in a 15-mL plastic conical centrifuge tube. After centrifugation for 20 minutes at 1,450g, the platelet layer was removed and washed free of Cellsep by centrifugation for 10 minutes at 2,000g in excess BSG-citrate. The platelet pellet was resuspended in HEPES-Tyrode's buffer containing albumin (HTA; 0.137 mol/L NaCl, 0.003 mol/L KCl, 0.012 mol/L NaHCO3 , 0.011 mol/L glucose, and 0.004 mol/L NaH2PO4 , 0.001 mol/L MgCl2 , 3.5 mg/mL bovine serum albumin, pH 7.4). All procedures were performed at room temperature unless otherwise specified. Platelet counts were performed with a Coulter counter (Coulter Electronics, Hialeah, FL).
For experiments comparing intact with detergent-lysed platelets, platelets were resuspended in 20 mmol/L HEPES, pH 7.4, 0.14 mol/L NaCl, 5 mmol/L glucose and the lysates were obtained by resuspending the cells in the same buffer (pH 6, 7.4 or 8.5) with the addition of 0.3% Triton X-100 followed by two freeze-thaw cycles. Acid lysates were also prepared by resuspending washed platelets in 0.1 mol/L phosphate buffer, pH 6, 20 mmol/L NaCl, 1.0 mmol/L EDTA, 0.3% Triton X-100. The lysates were aliquoted and stored at −70°C.
Enrichment of platelet u-PA. For certain experiments, platelets were enriched with u-PA as previously described12 by adding 1.0 μg/mL of scu-PA or thromb-UK to the PRP and incubating for 30 minutes at room temperature before isolation of the platelets.
Plasminogen activation assays. Platelets at 3.5 × 108/mL HTA (unless otherwise indicated) were premixed alone, or with additives as specified, in the presence of 1.2 μmol/L plasminogen and 1.2 mmol/L CaCl2 after which 0.175 mL aliquots of this mixture were added to 0.5 mL Eppendorf tubes containing 0.015 mL plasmin substrate S2403 (8 mmol/L). Finally, 0.01 mL of buffer, thrombin (1 to 40 u/mL) or other agonist was added, and the samples incubated at 37°C with continuous mixing in a Clay Adams Nutator (Becton Dickinson, Sparks, MD). At intervals, the platelets were pelleted by centrifugation (2 minutes, 3,000g ) in a microfuge (Beckman Instruments, Irvine, CA), and 75 μL of the supernatant was transferred to a microtiter plate well containing 25 μL 40% acetic acid for the measurement of absorbance at 405 nm (against a reference wavelength of 490 nm) using a microtiter plate reader (Molecular Devices, Menlo Park, CA). Identical incubation mixtures, but without platelets, were assayed to correct for the hydrolysis of S2403 by thrombin. This platelet-free control absorbance was subtracted from the absorbance measured in the platelet containing mixture to calculate the platelet related activity. Although thrombin itself is a weak plasminogen activator, the mixture of thrombin and plasminogen did not generate detectable plasmin under these experimental conditions.
Two additional variables were considered as possible modulators of the platelet-free control activity. First, because platelets and fibrin may compete with S2403 as substrates for thrombin, their absence in the control would give it a higher reading. Second, platelet associated plasminogen or thrombin could contribute to the S2403 activity and their absence in the control would give it a lower reading. The effect of the latter was measured and found to be negligible (see Fig 2). The influence of platelets on S2403 was measured in a two-stage assay described below.
The contribution of platelet-plasminogen and the effect of inhibitors of u-PA or t-PA on plasminogen activation by platelets. Platelets were incubated with S2403 either in the absence of added plasminogen1,2 or in the presence of 1 μmol/L plasminogen3-7 without1,3 or with 5 U/mL of thrombin.2,4-7 Some platelets5-7 were premixed with 25 μg/mL antihuman u-PA IgG, 1 mmol/L amiloride, or 25 μg/mL antihuman t-PA IgG before the addition of plasminogen, S2403, and thrombin. The samples were assayed for plasmin activity after a 5-hour incubation. The data shown are mean values of duplicate determinations from two experiments. The duplicate determinations were within 5% of each other.
The contribution of platelet-plasminogen and the effect of inhibitors of u-PA or t-PA on plasminogen activation by platelets. Platelets were incubated with S2403 either in the absence of added plasminogen1,2 or in the presence of 1 μmol/L plasminogen3-7 without1,3 or with 5 U/mL of thrombin.2,4-7 Some platelets5-7 were premixed with 25 μg/mL antihuman u-PA IgG, 1 mmol/L amiloride, or 25 μg/mL antihuman t-PA IgG before the addition of plasminogen, S2403, and thrombin. The samples were assayed for plasmin activity after a 5-hour incubation. The data shown are mean values of duplicate determinations from two experiments. The duplicate determinations were within 5% of each other.
A two-stage assay was also used in which platelets were incubated with thrombin for 30 minutes after which hirudin (10-fold molar excess) was added to inactivate thrombin, after which S2403 was then added, the incubation continued, and samples were analyzed as described above.
Zymography. After removal of 75 μL of the supernatant from the platelet incubations for plasmin S2403 analysis as noted above, sodium dodecyl sulfate (SDS) (2% final concentration) was added to the platelet pellet and remaining supernatant. The samples were heated to 60°C for 5 minutes for subsequent SDS-polyacrylamide gel electrophoresis (PAGE), as described by Laemmli25 using 8% slab gels. After electrophoresis, the gels were washed by agitation for 1 hour in 2.5% Triton X-100 in water, followed by 40 minutes in 0.1 mol/L Tris-HCl (pH 8.0), layered over an underlay consisting of 0.8% agarose (agarose low melting, Fisher Biotech, Fair Lawn, NJ), 2% casein (Carnation Non-fat Dry Milk; Carnation Co, Los Angeles, CA) and plasminogen (20 μg/mL) in 0.1 mol/L Tris-HCl (pH 8.0) and incubated at 37°C overnight.
Platelet membrane preparation. Washed platelets were suspended in 0.05 mol/L HEPES, pH 7.2 containing 0.01 mol/L EDTA, 1 mmol/L phenylmethylsulfonyl chloride and 150 KIU/mL aprotinin. After five cycles of freezing and thawing at −80°C and 37°C, the suspension was sonicated with three 15-second bursts and centrifuged for 10 minutes at 12,000g at 4°C to remove intact platelets and large fragments. The supernatant was centrifuged at 100,000g for 1 hour at 4°C, and the pellet was resuspended in HTA. When membranes were isolated from activated platelets, the washed platelets were first resuspended in HEPES-Tyrode's buffer and treated with 0.4 mmol/L Gly-Pro-Arg-Pro to prevent fibrin polymerization. Thrombin (1 U/mL) was then added and incubated for 10 minutes at 37°C without stirring after which excess HEPES-Tyrode's buffer was added and the platelets pelleted and resuspended in the buffer described above for membrane preparation.
Studies of the activation of thrombin-inactivated scu-PA (thromb-UK). Partial reactivation of u-PA during plasminogen activation was identified by zymography of the platelet reaction mixtures. Therefore, the mechanism for this was studied. The role of plasmin as a potential activator of thromb-UK was evaluated by omitting plasminogen from the platelet reaction mixture and by adding aprotinin.
To directly examine thromb-UK activation, thromb-UK (25 μg/mL) was incubated with either intact- or detergent-lysed platelets (3 to 5 × 108 plt/mL) in the presence or absence of a platelet agonist for 1 hour at 37°C. Determination of u-PA activity was done by adding an equal volume of 50 mmol/L Tris/HCl, pH 8.8, 0.12 mol/L NaCl, 0.01% Tween 80 to the incubation and S2444 (0.75 mmol/L final concentration). Thrombin was inactivated with hirudin (10-fold molar excess) before the addition of synthetic substrate. Absorbance at 405/490 nm was measured at time intervals and expressed as ΔA/minute.
The inhibition of thromb-UK activation by aprotinin and thiol protease inhibitors, such as cystatin and the peptidyl diazomethyl ketone, Gly-Phe-CHN2 , an inhibitor specific for cathepsin C,26 was tested.
RESULTS
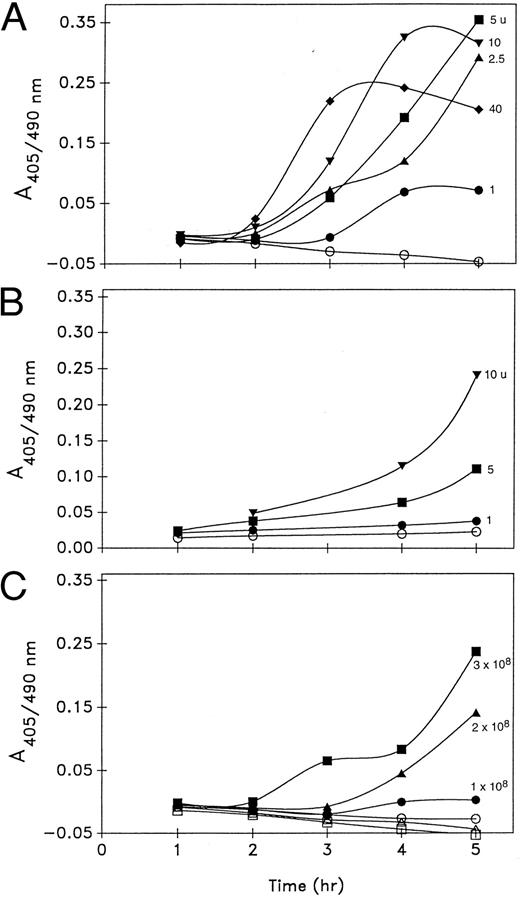
Thrombin and platelet dependence of plasminogen activation by platelets. When unstimulated platelets (3 × 108/mL) were incubated with Glu-plasminogen (1 μmol/L) in the presence of synthetic substrate S2403, no detectable plasmin was generated over the course of 5 hours. In fact, less S2403 activity was measured in the presence of unstimulated platelets than in the cell-free control indicating that the platelets were competing with the substrate for the trace amidolytic activity in the plasminogen preparation or releasing some inhibitors. By contrast, when thrombin (1 to 40 U/mL) was added to the platelet incubations, progressive S2403 hydrolysis occurred, which was thrombin-dose dependent during the initial 3 hours of incubation. After longer incubations (5 to 7 hours), the activity plateaued and eventually declined in the platelet incubation mixtures. This may be related to the inhibition of plasmin by α2 -antiplasmin released from platelets during activation (Fig 1A).
Plasminogen activation (A405/490 nm ) by platelets. Thrombin dose dependence by a one-stage assay (A) and two-stage assay (B), and platelet concentration dependence (C) of plasminogen activation. (A) Platelets (3 × 108/mL) were incubated at 37°C with 1 μmol/L plasminogen, 0.6 mmol/L S2403, and thrombin at 0 (○), 1 U/mL (•), 2.5 U/mL (▴), 5 U/mL (▪), 10 U/mL (▾), or 40 U/mL (♦). (B) Platelets (3 × 108/mL) were incubated with plasminogen and thrombin at 0 (○), 1 U/mL (•), 5 U/mL (▪), and 10 U/mL (▾) for 0.5 hours at 37°C. Hirudin (10 μg/mL) was then added, and 10 minutes later S2403 (0.6 mmol/L) was added and the incubation continued. (C) Platelets at 1 × 108/mL (circles), 2 × 108/mL (triangles), and 3 × 108/mL (squares) were incubated with plasminogen and S2403 in the absence (open symbols) or presence (closed symbols) of 5 U/mL thrombin. Samples were assayed for plasmin activity as described in Materials and Methods. The data shown in (A) are from six experiments, and in (B and C) from three experiments each.
Plasminogen activation (A405/490 nm ) by platelets. Thrombin dose dependence by a one-stage assay (A) and two-stage assay (B), and platelet concentration dependence (C) of plasminogen activation. (A) Platelets (3 × 108/mL) were incubated at 37°C with 1 μmol/L plasminogen, 0.6 mmol/L S2403, and thrombin at 0 (○), 1 U/mL (•), 2.5 U/mL (▴), 5 U/mL (▪), 10 U/mL (▾), or 40 U/mL (♦). (B) Platelets (3 × 108/mL) were incubated with plasminogen and thrombin at 0 (○), 1 U/mL (•), 5 U/mL (▪), and 10 U/mL (▾) for 0.5 hours at 37°C. Hirudin (10 μg/mL) was then added, and 10 minutes later S2403 (0.6 mmol/L) was added and the incubation continued. (C) Platelets at 1 × 108/mL (circles), 2 × 108/mL (triangles), and 3 × 108/mL (squares) were incubated with plasminogen and S2403 in the absence (open symbols) or presence (closed symbols) of 5 U/mL thrombin. Samples were assayed for plasmin activity as described in Materials and Methods. The data shown in (A) are from six experiments, and in (B and C) from three experiments each.
In the two-stage assay of plasminogen activation, platelets were first incubated with thrombin (1 to 10 u/mL) for 30 minutes after which hirudin was added to inactivate thrombin. S2403 was then added to the platelet mixture and the incubation continued. Under these conditions, a thrombin dose-dependent promotion of plasmin generation was again observed, but the rate of plasmin generation was significantly reduced (Fig 1B). Because limiting the exposure time to active thrombin significantly attenuated the platelet response measured, subsequent experiments were done without the addition of hirudin where cell-free controls were used to correct the S2403 activity of the added thrombin.
The promotion of plasminogen activation by thrombin (5 u/mL), as measured by S2403 hydrolysis, was also platelet concentration-dependent over a range of concentrations of 1 to 3 × 108/mL (Fig 1C).
The thrombin-stimulated induction of S2403 hydrolysis by platelets was shown to be related specifically to the activation of plasminogen added to the incubation mixture. When platelets were stimulated by thrombin in the absence of added plasminogen, S2403 hydrolysis was significantly reduced (Fig 2) and this remaining activity was completely inhibited by the plasmin inhibitor aprotinin (100 KIU/mL). Therefore, generation of thrombin from prothrombin on the platelet surface did not contribute to the activity measured.
Stimulation of platelets by other agonists. Adenine diphosphate (ADP) (50 μmol/L), the thromboxane analogue U46619 (10 μg/mL), and TRAP (50 μmol/L) failed to generate S2403 activity, indicating that the platelet response was specific for active thrombin.
Some variability in the level of plasmin activity generated was observed among different platelet preparations obtained from different donors. Freshly prepared platelets also tended to generate more plasmin activity than those isolated from PRP, which had been stored overnight.
Identification of the platelet-associated plasminogen activator. Thrombin-induced plasminogen activation by platelets was completely inhibited by both an inhibitory antibody against u-PA and by the specific inhibitor of u-PA activity, amiloride. In contrast, an inhibitory antibody against t-PA did not significantly affect the reaction (Fig 2).
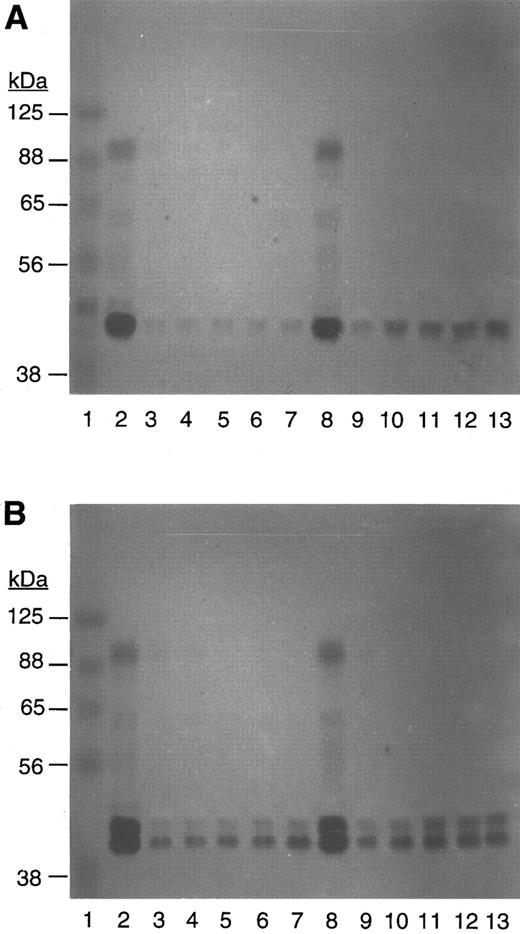
The above finding was consistent with the zymograms of the platelets (see Fig 5A), which showed u-PA activity at its molecular weight of ≈50 kD and in a complex with PAI-1 at ≈100 kD, but no activity corresponding to t-PA was seen (even when fibrin was used in place of casein). The absence of t-PA was probably related to its removal during the platelet isolation procedure. These findings indicated that platelet-associated u-PA was the plasminogen activator responsible for the thrombin-induced plasmin activity.
Zymogram of native platelets (A) and scu-PA–enriched platelets (B) after incubation with plasminogen and S2403 in the absence or presence of thrombin. Lane 1 contains MW standards; lanes 2 to 7 are platelets after 1 hour; and lanes 8 to 13 are platelets after a 5-hour incubation with thrombin, 0,2,8 1 U/mL,3,9 2.5 U/mL,4,10 5 U/mL,5,11 10 U/mL,6,12 or 40 U/mL.7 13
Zymogram of native platelets (A) and scu-PA–enriched platelets (B) after incubation with plasminogen and S2403 in the absence or presence of thrombin. Lane 1 contains MW standards; lanes 2 to 7 are platelets after 1 hour; and lanes 8 to 13 are platelets after a 5-hour incubation with thrombin, 0,2,8 1 U/mL,3,9 2.5 U/mL,4,10 5 U/mL,5,11 10 U/mL,6,12 or 40 U/mL.7 13
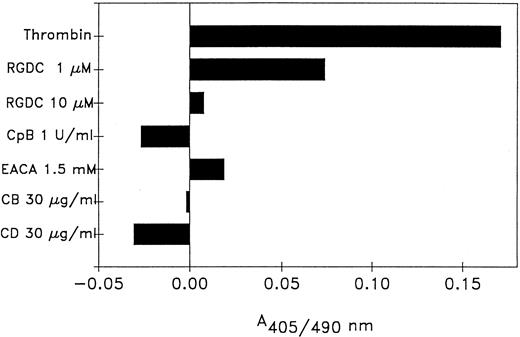
Role of the platelet-fibrin surface. Because the u-PA in this system was platelet-bound, experiments were performed to determine the role of plasminogen binding to the thrombin-stimulated platelet surface. Plasminogen interacts with platelet-bound fibrin via lysine-binding sites,4,27 therefore, the lysine analogue EACA, which acts as a competitor, and CpB, which removes carboxyterminal lysine residues, were used. EACA, at a concentration (1.5 mmol/L) previously shown to reduce plasminogen binding to thrombin-stimulated platelets,4 inhibited thrombin-stimulated plasminogen activation by 90% and pretreatment of platelets with CpB (1 U/mL) abolished the thrombin-induced reaction in these experiments. The role of platelet-bound fibrin was evaluated using the fibrinogen receptor antagonist peptide, Ac-pen-RGDC, which inhibited thrombin-stimulated plasminogen activation. Additionally, cytochalasins B and D, which reduces fibrinogen binding to activated platelets by disrupting platelet cytoskeletal proteins,28 also inhibited activation (Fig 3).
Effect of reagents that interfere with plasminogen or fibrinogen binding to platelets. Platelets were preincubated 30 minutes with: Ac-pen-RGDC, CpB, EACA, cytochalasin B (CB) or cytochalasin D (CD) before the addition of plasminogen, S2403 and 5 U/mL thrombin. The samples were assayed for plasmin activity after a 5-hour incubation. The reagents had no effect on plasmin generation by unstimulated platelets. The data shown are the mean values of duplicate determinations from two experiments. The duplicate determinations were within 5% of each other.
Effect of reagents that interfere with plasminogen or fibrinogen binding to platelets. Platelets were preincubated 30 minutes with: Ac-pen-RGDC, CpB, EACA, cytochalasin B (CB) or cytochalasin D (CD) before the addition of plasminogen, S2403 and 5 U/mL thrombin. The samples were assayed for plasmin activity after a 5-hour incubation. The reagents had no effect on plasmin generation by unstimulated platelets. The data shown are the mean values of duplicate determinations from two experiments. The duplicate determinations were within 5% of each other.
The observation that only thrombin and not other platelet agonists, including ADP, TRAP, and U46619 induced plasminogen activation additionally implicates fibrin in this reaction, as only thrombin catalyzes fibrin formation. Because isolated platelet membranes also contain u-PA,12 13 they were tested for plasminogen activator activity. Platelet membranes from either unstimulated or thrombin-stimulated (1 U/mL) platelets were prepared and a range of concentrations equivalent to 3 to 8 × 108 platelets/mL were incubated with 1 μmol/L plasminogen and S2403. However, no plasmin activity was detected during a 6-hour incubation of the platelet membranes either in the absence or presence of thrombin (1 to 10 U/mL) with or without the addition of fibrinogen (0.5 μmol/L).
Taken together, these findings indicated that thrombin-induced activation of plasminogen by platelets involved the fibrinogen receptor, platelet-bound plasminogen, and whole platelets.
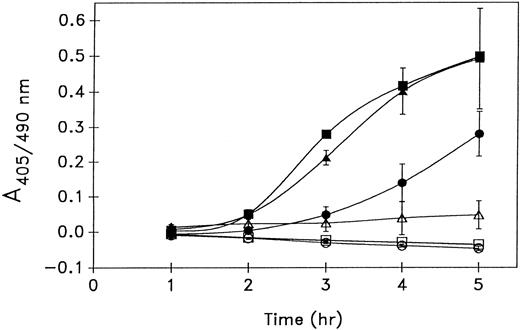
Plasminogen activation by platelets enriched with u-PA. When PRP was incubated with 1 μg/mL of recombinant scu-PA, an amount well within the therapeutic concentration, for 30 minutes before the isolation of platelets, the quantity of platelet-associated u-PA increased substantially (see Fig 5B) and as previously described.12 On SDS-PAGE, the exogenous u-PA can be seen migrating ahead of the endogenous u-PA, as nonglycosylated recombinant scu-PA from E coli was used in these experiments. After thrombin stimulation, the scu-PA enriched platelets generated threefold more plasmin activity than the nonenriched platelets. When platelets were enriched with thromb-UK in place of scu-PA, a comparable augmentation of plasminogen activation was observed, although thromb-UK has only about 0.4% of the activity of enzymatic two-chain (tc) u-PA 20 (Fig 4). The equivalent induction of plasmin generation by either scu-PA or its thrombin-cleaved form suggested that thromb-UK was importantly involved in the plasminogen activation.
Effect of u-PA enrichment of platelets on thrombin-stimulated plasminogen activation. Platelets were isolated from PRP without (circles) or with a 30-minute preincubation with either 1 μg/mL of scu-PA (triangles) or thromb-UK (squares) and incubated with plasminogen and S2403 in the absence (open symbols) or presence (closed symbols) of 5 U/mL thrombin. Mean ± standard deviation (SD) of three experiments.
Effect of u-PA enrichment of platelets on thrombin-stimulated plasminogen activation. Platelets were isolated from PRP without (circles) or with a 30-minute preincubation with either 1 μg/mL of scu-PA (triangles) or thromb-UK (squares) and incubated with plasminogen and S2403 in the absence (open symbols) or presence (closed symbols) of 5 U/mL thrombin. Mean ± standard deviation (SD) of three experiments.
Inactivation and reactivation of platelet u-PA. Examination of the zymograms prepared from native platelets (Fig 5A) and scu-PA enriched platelets (Fig 5B), which had been incubated without or with thrombin (1 to 40 U/mL) showed that during the first hour of incubation with thrombin, all of the ≈100 kD u-PA activity and most of the ≈50 kD u-PA activity was inactivated, consistent with the conversion of scu-PA to the thrombin-cleaved form, or thromb-UK. It also indicated that the ≈100 kD activity represented a scu-PA:inhibitor complex because scu-PA is the only form of u-PA inactivated by thrombin. A similar SDS-stable complex of PAI-1 and scu-PA was recently described by Manchanda and Schwartz.29 Analysis of the platelet incubations after a 5-hour incubation showed a partial return of activity at 50 kD indicating that some reactivation of u-PA had occurred. The degree of reactivation increased with higher doses of thrombin up to ≈10 u/mL (Fig 5A). The same pattern of inactivation and reactivation was seen with scu-PA enriched platelets (Fig 5B). When plasminogen was excluded from the platelet incubation mixture or aprotinin was added, it did not affect the reactivation phenomenon, indicating that it was not due to plasmin.
The zymograms from the two-stage assay experiments (Fig 1B), in which thrombin was inactivated after 30 minutes by hirudin, showed a similar complete inactivation of u-PA. However, the degree of reactivation after a subsequent 5-hour incubation was attenuated, indicating that thromb-UK activation was promoted by a longer exposure of platelets to thrombin (zymograms not shown).
Activation of thromb-UK by platelets. Because a partial restoration of u-PA activity was identified on the zymograms, consistent with activation of thromb-UK, experiments were performed to characterize this reaction. Thromb-UK (25 μg/mL), generated from recombinant scu-PA, was incubated with platelet agonists and platelets using u-PA synthetic substrate, S2444 to measure u-PA activity. Incubation mixtures of thrombin and platelets, but neither one alone, generated u-PA activity. Other agonists, such as TRAP or ADP, had no effect. Detergent-lysed platelets also induced thromb-UK activation, but this was not potentiated by thrombin. The platelet lysate was more active at an acid pH, consistent with a platelet lysosomal enzyme being responsible for the activation of thromb-UK (Table 1). Thromb-UK activation by thrombin stimulated platelets or platelet lysate was also demonstrated on zymograms (data not shown).
Activation of Thromb-UK by Intact- or Detergent-Lysed Platelets
| Agonist . | S2444 Activity (ΔA/min) . | ||
|---|---|---|---|
| . | Intact pH 7.4 . | Lysate pH 7.4 . | Lysate pH 6.0 . |
| None | 0.001 | 0.030 | 0.116 |
| Thrombin (2.5 to 10 U/mL) | 0.042 | 0.036 | 0.115 |
| ADP (50 μmol/L) | 0.002 | — | — |
| TRAP (60 μmol/L) | 0.008 | — | — |
| Agonist . | S2444 Activity (ΔA/min) . | ||
|---|---|---|---|
| . | Intact pH 7.4 . | Lysate pH 7.4 . | Lysate pH 6.0 . |
| None | 0.001 | 0.030 | 0.116 |
| Thrombin (2.5 to 10 U/mL) | 0.042 | 0.036 | 0.115 |
| ADP (50 μmol/L) | 0.002 | — | — |
| TRAP (60 μmol/L) | 0.008 | — | — |
Intact platelets (5 × 108/mL) at pH 7.4, or Triton X-100 lysates (of equivalent platelet number) at pH 6.0 or 7.4 were mixed with thromb-UK (0.45 μmol/L) in the absence or presence of the indicated agonist for 1 hour at 37°C after which the platelets were pelleted and the supernatant or lysate assayed for S2444 activity in buffer at pH 8.0.
A lysosomal thiol protease, cathepsin C, was recently reported to efficiently activate thromb-UK.21 Although cathepsin C has not been reported in platelet lysosomes, the present findings were consistent with the involvement of such a protease, as the thiol protease inhibitor cystatin (20 nmol/L) greatly delayed both thrombin-induced activation of plasminogen by platelets (Fig 6A) and the reactivation of platelet u-PA (Fig 6B). In separate experiments, it was determined that cystatin did not effect either plasminogen activation by u-PA or plasmin S2403 hydrolysis.
Effect of cystatin on thrombin-stimulated plasminogen activation (A) and on the reactivation of platelet u-PA (B). (A) Platelets (3.5 × 108/mL) were incubated at 37°C with plasminogen, S2403, and thrombin at 2.5 U/mL (circles), 5 U/mL (triangles), or 10 U/mL (squares) in the absence (open symbols) or presence of 20 nmol/L cystatin (closed symbols) and assayed for plasmin activity. (B) Zymogram of the platelet incubation mixtures containing thrombin at 0,1,5 2.5 u/mL,2,6 5 u/mL,3,7 10 u/mL4,8 after 1, 3, or 5 hours of incubation in the absence 1-4 or presence5-8 of cystatin.
Effect of cystatin on thrombin-stimulated plasminogen activation (A) and on the reactivation of platelet u-PA (B). (A) Platelets (3.5 × 108/mL) were incubated at 37°C with plasminogen, S2403, and thrombin at 2.5 U/mL (circles), 5 U/mL (triangles), or 10 U/mL (squares) in the absence (open symbols) or presence of 20 nmol/L cystatin (closed symbols) and assayed for plasmin activity. (B) Zymogram of the platelet incubation mixtures containing thrombin at 0,1,5 2.5 u/mL,2,6 5 u/mL,3,7 10 u/mL4,8 after 1, 3, or 5 hours of incubation in the absence 1-4 or presence5-8 of cystatin.
Furthermore, thromb-UK activation by detergent-lysed platelets was also inhibited 96% by cystatin (10 nmol/L) and 77% by Gly-phe-CHN2 (10 μmol/L), a peptidyl diazomethyl ketone reported to specifically inactivate cathepsin C.26 By contrast, aprotinin (100 KIU/mL) induced no inhibition of thromb-UK activation.
DISCUSSION
Single-chain u-PA has recently been found to be more important to physiologic fibrinolysis than was previously believed, and yet its mechanism of action remains poorly understood. The reports that u-PA is associated with platelets,12-14 and that platelets contain a novel u-PA receptor,15 suggested that platelets may help mediate scu-PA–induced fibrinolysis. Moreover, because the scu-PA content of platelets can be enriched by scu-PA in the ambient whole blood,12 this hypothesis has implications for therapeutic thrombolysis. However, scu-PA is inactivated by thrombin,19 20 which would be expected to neutralize platelet-associated scu-PA during thrombosis. To better understand this apparent paradox, plasminogen activation by platelet-associated scu-PA was measured and characterized.
The findings showed that plasminogen activation by an endogenous activator was specifically and selectively triggered by thrombin stimulation of platelets. The thrombin-induced plasminogen activation was time, platelet concentration, and thrombin dose-dependent. No plasminogen activation occurred in platelet-free controls or with unstimulated platelets (Fig 1). Other platelet agonists such as ADP, TRAP, or a PGH2 analogue had no effect. The plasminogen activator responsible was u-PA as evidenced by inhibition of activity by either amiloride or antibodies to u-PA (Fig 2), and by its presence on zymograms of platelets (Fig 5). No t-PA was detectable on the zymograms and anti-t–PA antibodies did not significantly inhibit plasminogen activation.
In a previous study, Miles et al27 showed that thrombin stimulation of platelets promoted plasminogen binding and activation, and that this was related to fibrin on the platelet surface. Similarly, in the present study, plasminogen activation was prevented by agents that interfered with fibrinogen binding to the platelet receptor or agents that interfered with plasminogen binding to the platelet-fibrin surface, like EACA or CpB (Fig 3). In contrast to intact platelets, isolated platelet membrane, which also contains u-PA,12 13 prepared from unstimulated or thrombin stimulated platelets, induced no plasminogen activation even in the presence of fibrinogen. Therefore, plasminogen activation was surface-related, but required thrombin-stimulated, intact platelets. The inhibition by CpB treatment, which cleaves carboxyterminal lysines, is also consistent with previous studies showing that both scu-PA30 and thromb-UK20 selectively activate plasminogen bound to fibrin via carboxyterminal lysines.
When therapeutic concentrations of exogenous scu-PA was added to platelet-rich plasma before the platelets were isolated, the platelet content of u-PA was enriched more than twofold as previously reported.12 Thrombin activation of these platelets induced significantly more plasminogen activation (Fig 4). Enrichment of the u-PA content of platelets in vivo by uptake of scu-PA infused intravenously for therapeutic coronary thrombolysis, has been previously postulated to explain the exceptionally low rate of reocclusions observed with scu-PA compared with other plasminogen activators.31 The present findings that u-PA enrichment of platelets promoted plasminogen activation are consistent with this hypothesis.
The essential role of thrombin in platelet-associated scu-PA-induced plasminogen activation seems paradoxical because thrombin inactivates scu-PA,19,20 by hydrolysis of the Arg156-Phe157 peptide bond.18 That scu-PA had indeed been inactivated was apparent from the zymograms, which showed a loss of u-PA activity (Fig 5). Although thromb-UK has only 0.4% the activity of tcu-PA, its activity is promoted >150-fold against fibrin fragment E-bound plasminogen.20 A similar promotion of plasminogen activator activity has also been described for scu-PA.30 Therefore, it was possible that fragment E domains on the platelet-fibrin surface promoted plasminogen activation by platelet associated scu-PA or thromb-UK. The inhibition by CpB was consistent with this hypothesis, as it prevents plasminogen binding to the E-domain of fibrin. However, this would leave unexplained how plasminogen activation was initiated because fibrin fragment E is absent in intact fibrin and is formed by plasmin degradation.
Examination of serial zymograms of the platelet incubation mixtures showed a partial return of u-PA activity, indicative of activation of thromb-UK. Activation was not accompanied by any change in electrophoretic mobility and was time and thrombin dose-dependent (Figs 5 and 6). This reactivation of u-PA contributed significantly to the plasminogen activation because when it was blocked by the inhibitor, cystatin, plasmin generation was substantially delayed (Fig 6). Moreover, a good correlation was observed between plasminogen activation and u-PA reactivation on the zymograms (Figs 5 and 6) indicating that thromb-UK activation was functionally important. It has been previously shown that thromb-UK is a surprisingly efficient and highly fibrin specific fibrinolytic agent in vivo when administered to rabbits.32 The present study supports the suggestion by Nauland and Rijken21 that activation of thromb-UK may explain its fibrinolytic effect, although a contribution by the promoting effect of fibrin fragment E-bound plasminogen on thromb-UK itself remains an additional possibility.
The mechanism of thromb-UK activation was investigated. Although thromb-UK activation by plasmin has been demonstrated, it is more than 90-fold less sensitive to activation than scu-PA.20 The contribution of plasmin in this system was negligible, as the omission of plasminogen from the platelet incubations or the addition of aprotinin did not affect u-PA reactivation. The activation of thromb-UK was studied in incubation mixtures of platelets and various agonists. Similar to plasminogen activation in this system, thromb-UK activation was limited to thrombin activated whole platelets. However, detergent-lysed platelets also induced thromb-UK activation and this reaction was not promoted by thrombin, but was augmented by adjusting the pH to 6.0, indicative of a lysosomal enzyme. Moreover, the activity was inhibited by the thiol protease inhibitor cystatin and by the peptidyl diazomethyl ketone reported to be selective and specific for cathepsin C.26 Recently, Nauland and Rijken21 showed that cathepsin C efficiently activates thromb-UK by cleavage of the activation site, Lys158-Ile159, removing the two N-terminal residues from the B-chain of thromb-UK. Cathepsin C is an exopeptidase, which removes dipeptides from the N-termini of proteins. It is found in the lysosomes of leukocytes and a variety of tissues, but has not previously been identified in human platelets to our knowledge. The isolation of platelets by the method used in the present study has been shown to be essentially free (<1%) of leukocytes. Release of this platelet lysosomal enzyme appeared to require thrombin itself, as no thromb-UK activation occurred when the platelet thrombin receptor was stimulated by TRAP.
A physiologic role for a platelet lysosomal enzyme has not previously been suggested, to our knowledge, but is supported by two other observations. First, Silverstein and Febbraio33 reported that lysosome-associated membrane protein (LAMP-2) translocates to the surface membrane when platelets are activated by thrombin. Second, Ciferri et al34 showed that platelet lysosomal enzymes are released in vivo at the site of a standard bleeding time skin wound.
Plasmin generation by the sequence of reactions observed may allow u-PA to retain activity on the activated platelet surface despite the high concentration of PAI-1 released into this environment because both scu-PA and thromb-UK are resistant to PAI-1 inactivation. The subsequent activation of thromb-UK to enzymatic tcu-PA provides time for PAI-1 to be neutralized by thrombin, a reaction which was described by Ehrlich et al.35 This delay in plasminogen activation also allows early hemostasis to proceed unchallenged until growth of the platelet-fibrin plug needs to be curtailed.
In conclusion, the present findings describe a new pathway of plasminogen activation by u-PA, which is based on its association with platelets, and which is specifically triggered by thrombin, is surface related, dependent on the formation of platelet-bound fibrin, and involves a platelet lysosomal enzyme consistent with cathepsin C. This pathway targets u-PA to a platelet thrombus and may help explain the mechanism by which u-PA induces physiologic fibrinolysis, the biologic importance of which has only recently been recognized.10 36 Because the u-PA content of platelets can be enriched from the ambient plasma, which substantially augmented plasminogen activation, this pathway may also have implications for the use of scu-PA in therapeutic thrombolysis.
ACKNOWLEDGMENT
The authors thank Dr Ralph Pannell for his advice and help, Dr Stephen Mayer for his assistance, Karen Pichette for her technical assistance, and Joyce J. Lloyd for the preparation of the manuscript.
Supported by a grant from Neurex Corporation, Menlo Park, CA.
Address reprint requests to Victor Gurewich, MD, Vascular Research Laboratory, Burlington Bldg, Room 554G, Beth Israel Deaconess Medical Center, One Deaconess Rd, Boston, MA 02215.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal