Abstract
Tissue factor pathway inhibitor (TFPI) is a serine protease inhibitor of the extrinsic coagulation system, synthesized in endothelial cells, which has recently been shown to play an important role in the regulation of activated coagulation factors at the endothelial cell surface. In the present study we investigated the subcellular localization and metabolism of TFPI in human umbilical vein endothelial cells (HUVEC). Immunocytochemical labeling of HUVEC with anti-TFPI showed specific labeling associated with the cell surface and with many intracellular organelles including the Golgi complex. Further characterization of these organelles was performed by colocalizing the anti-TFPI with 3-(2,4-dinitroanilino)′-amino-N-methyldipropylamine (DAMP; to demonstrate low pH), mannose phosphate receptor (endosomes), and LAMP 1 (late endocytic compartments). TFPI also colocalized with antibodies to the human transferrin receptor, a marker for early endocytic, recycling compartment. Endogenous TFPI colocalized with biotin in intracellular vesicles during endocytosis after biotinylation of the cell surface, which indicated that TFPI was being co-internalized with the surface biotin. The binding of exogenously added 125I-TFPI increased linearly to HUVEC over the concentration range of 0 to 32 nmol/L without saturation, the binding was not affected by up to a thousand-fold molar excess of unlabeled TFPI, and heparin inhibited the binding dose dependently. An intact C-terminal domain was important for the interaction between TFPI and the cell surface of HUVEC, because less than 10% of a C-terminal truncated form of TFPI (TFPI1-161 ) was bound after addition of equimolar concentrations of full-length TFPI. Exogenously added 125I-TFPI was not degraded in HUVEC during 4 hours at 37°C. The presence of TFPI in endocytic and recycling compartments support the hypothesis that endogenous, membrane-anchored TFPI could be internalized for subsequent recycling back to the cell surface.
TISSUE FACTOR pathway inhibitor (TFPI) is a serine protease inhibitor which regulates TF-induced blood coagulation, initiated when factor VII complexes with TF exposed in the vascular system.1 The factor VIIa-TF complex has the ability to activate factor IX and X by proteolysis.2 TFPI exerts its function by neutralizing the catalytic activity of factor Xa directly and by feedback inhibition of the factor VIIa-TF complex in the presence of factor Xa.3,4 TFPI contains three Kunitz-type domains, the first and second of which are responsible for binding of factor VIIa and factor Xa, respectively.5 The third and C-terminally located domain is probably involved in the association with lipoproteins6 and is mandatory for the anticoagulant function of TFPI in TF-induced coagulation in vitro.7 8
TFPI is the major inhibitor of the factor VIIa complexed to TF expressed on the surface membranes of perturbed endothelial cells.9 In an umbilical vein model, Almus et al10 showed that reduction of TFPI by 50% causes a similar increase of the factor VIIa-TF catalytic activity. Studies in rabbits indicate that TFPI functions as a natural anticoagulant by inhibiting the factor VIIa-TF catalytic activity formed in circulating blood exposed to low concentrations of TF.11 In baboons, TFPI treatment has been shown to prevent septic shock and improve survival after administration of lethal doses of Escherichia coli.12 Furthermore, infusion of TFPI prevented arterial thrombosis formation after endothelial cell injury in a baboon shunt model.13
In humans, TFPI exists in four intravascular pools.14 In plasma, more than 80% is bound to lipoproteins (pool I), primarly low-density lipoproteins (LDL), whereas 10% to 20% is carrier free (pool II). A small amount of TFPI (pool III), representing about 8% of the plasma pool, is found associated with platelets. Finally, a major pool is probably associated with the surface of the endothelium (pool IV), which is released into the circulation by heparin.15 Under normal conditions, the endothelium is the primary site of TFPI synthesis16 and may also have a crucial regulatory role for maintainance of vascular patency. The endothelial surface serves as a binding site for factor VII/VIIa, mediating the activation of the extrinsic coagulation system.17
Animal studies have shown that infused TFPI is taken up by the liver and kidney18 and that the disappearance of full-length TFPI (rTFPI) from plasma is more rapid than that of a carboxy-terminal truncated form.19 In baboons, the plasma level of rTFPI in the initial 7.5-minute sample was less than 3% of the anticipated value and continued to decline rapidly thereafter.20 Recently, the low-density lipoprotein receptor-related protein (LRP) has been shown to mediate the cellular degradation of TFPI after TFPI binding to hepatoma cells.21 Firstly, TFPI binds to an unrelated receptor(s), possibly cell-surface GAG, before being transfered to LRP for subsequent internalization,22 presumably by endocytosis. This initial hepatic cell-surface binding requires the carboxy-terminus of TFPI and is inhibited by clinically achievable levels of heparin and other polyanions.22
The vascular endothelium has well-developed endocytic processes23-25 and also expresses LDL receptors.26 These observations suggest that endothelial cells could play a key role in synthesis, storage, and perhaps degradation of intravascular TFPI. The purpose of the present study was to investigate the subcellular localization and metabolism of TFPI in human umbilical vein endothelial cells (HUVEC).
MATERIALS AND METHODS
Materials. Iodogen (T0656) and cold-water fish skin gelatin were purchased from Sigma Chemical Co (St Louis, MO); Carrier-free sodium (125I)iodide from the Institute for Energy Technology (Kjeller, Norway) in aliqouts of 2 mCi; Medium 199 E-11 and L-glutamine from GIBCO Ltd (Middlesex, UK) and fetal calf serum (FCS) was purchased from HyClone Laboratories (Logan, UT) and heat inactivated. Recombinant full-length TFPI (TFPI) was obtained from American Diagnostica Inc (Greenwich, CT). Recombinant C-terminally truncated TFPI (TFPI1-161 ),27 rabbit C-terminal directed anti-TFPI IgG,7 and affinity-purified inhibitory goat anti-TFPI IgG28 were all kind gifts from Novo Nordisk AS (Biopharmaceutical Division, Gentofte, Denmark). Bridging antibodies for anti-TFPI IgG and for monoclonal antibiotin were purchased from DAKO AS (Glostrup, Denmark) and from Dakopatts AS (Glostrup, Denmark) (rabbit-antimouse IgG, Z259), respectively. Protein A-gold was purchased from the Department of Cell Biology at the University of Utrecht (The Netherlands), 3-(2,4-dinitroanilino)3′-amino-N-methyldipropylamine (DAMP) from Molecular Probes Inc (Eugene, OR), and antibodies to human lysosomal membrane protein 1 (LAMP-1) from Developmental Studies (Hybridoma Bank, Iowa City, IA). Antibodies to dinitro-phenol (DNP) and to the mannose-6-phosphate receptor (MPR) were kind gifts from Prof Ira Mellman (Department of Cell Biology, Yale University School of Medicine, New Haven, CT) and Dr Bernard Hoflack (EMBL, Heidelberg, Germany), respectively. Antihuman transferrin receptor (cat. no. 1118048) was purchased from Boehringer Mannheim (Mannheim, Germany). Biotin (Immunopure NHS-LC-Biotin) was purchased from Pierce Chemical Company (Rockford, IL) and monoclonal mouse antibiotin IgG (B-7653) was obtained from Sigma Chemical Co.
Endothelial cell cultures. Primary cultures of human umbilical vein endothelial cells were prepared according to the method of Jaffe et al,29 with slight modifications.30 The cells were seeded on 24-well polystyrene culture plates (Falcon 3047; Becton Dickinson Labware, Lincoln Park, NJ) in Medium 199 E-11 (GIBCO Ltd) with 20% heat-inactivated FCS and 0.2 mol/L L-glutamine (GIBCO) (cell medium [CM]) at a density of 2.25 × 105/dish. The cells were incubated in a 5% CO2 atmosphere and 100% relative humidity at 37°C for 24 hours, washed twice with fresh CM, and relayered by CM and grown for another 48 to 72 hours until confluence. Endothelial cells were identified by their characteristic appearance of uniform polygonal shape by light microscopy.
Immunocytochemistry. Endothelial cells in primary culture were washed twice in phosphate-buffered saline (PBS) and chemically fixed in 100 mmol/L HEPES buffer (pH 7.4) containing 0.5% gluteraldehyde, scraped from the plastic dish with teflon, and pelleted. The cell pellets were infused with 2.3 mol/L sucrose containing 20% poly(vinyl) pyrrolidone (PVP-sucrose),31 frozen on aluminum specimen pins by immersion in liquid nitrogen, and sectioned using an Ultracut S ultramicrotome with FCS cryochamber (Leica, Deerfield, IL). Thin sections (approximately 30 to 60 nm), cut with glass or diamond (Diatome, Biel, Switzerland) knives, were flattened using a stream of electrons from an anti-static line (Diatome) placed in the cryochamber. Sections retrieved from the knife surface with drops of methyl cellulose or methyl cellulose mixed with sucrose32 were mounted on coated specimen grids, and labeled with primary antibody and then with protein A-gold. When primary antibodies that did not bind to protein A were used, a bridging antibody was applied to the sections before incubation with protein A-gold. Specific binding of the TFPI antibodies to the sections was determined using an adsorption control. Briefly, purified rTFPI was incubated with diluted anti-TFPI antibodies for 30 minutes at 20°C, and the antibody solution was centrifuged (13,500g for 3 minutes) and then applied to sections. After washing, the sections were incubated with protein A-gold and examined by transmission electron microscope (TEM).
Double-label experiments followed previously described protocols33 where the antibodies and gold probes were applied sequentially. A 1% gluteraldehyde blocking step was included to inhibit the binding of the second protein A-gold probe to the first antibody.34 After immunolabeling, the sections were dried in the presence of 0.3% uranyl acetate in 2% methyl cellulose35 and examined using a Jeol JEM 1010 transmission electron microscope (Jeol, Tokyo, Japan) operating at 80 kVa. Care was taken to check that the labeling patterns in the double-label experiments followed the patterns seen in single-label experiments, and also were the same in cross-over controls.33
Blocking of nonspecific binding of the antibodies and colloidal gold probes, normally achieved by treating sections, and diluting the antibodies in serum proteins (eg, bovine serum albumin [BSA] or FCS) was not possible for this antigen, which is present in serum. In our preliminary experiments, labeling was completely abolished if bovine serum was used to dilute the antibodies. To overcome this, we incubated sections, before application of antibodies, in PBS containing 1% cold-water fish skin gelatin. Antibodies and colliodal gold probes were also diluted in this solution.
Visualization of intracellular acidic compartments. For electron microscopy, it is possible to incubate living cells in the presence of 3-(2,4-dinitroanilino)3′-amino-N-methyldipropylamine (DAMP),36 a compound that will pass freely through living membranes unless protonated, which occurs when the compound encounters low pH.36 If the cells are then rapidly washed and fixed for electron microscopy, the DAMP is crosslinked to primary amines by the glutaraldehyde. The DAMP can be easily detected on cryosections using anti-dinitrophenol antibodies, which bind to DAMP. The endothelial cells were incubated with 30 μg/mL of DAMP dissolved in Medium 199E-11 for 30 minutes at 37°C, washed in fresh ice-cold Medium 199E-11 (without DAMP), and fixed for immunocytochemistry. The DAMP was visualized on cryosections through gluteraldehyde-fixed cells using rabbit antibodies to dinitrophenol.
Biotinylation and endocytosis. HUVEC were cultured as previously described and the monolayers were used at confluence after 72 to 96 hours in culture. The cells were washed twice with PBS, gradually cooled to 4°C, and incubated with 1 mg/mL biotin dissolved in PBS with 0.17 mmol/L Ca2+ and 0.09 mmol/L Mg2+. After incubation for 10 minutes at 4°C, the cells were washed twice in PBS at 4°C to remove unbound biotin. Cells were relayered with CM containing 20% FCS, warmed rapidly to 37°C, and fixed at selected time intervals with 8% formaldehyde for immunocytochemistry. The biotin was visualized on cryosections using monoclonal mouse antibiotin IgG, and double labeling with TFPI was conducted sequantially as previously described.
Stereology. Thin cryosections of endothelial cells, labeled after sectioning, were sampled using systematic uniform sampling methods.37 The micrographs obtained were used to estimate the mean surface density of gold labeling on the plasma membrane38 and the number of organelle profiles labeled with antibodies.
Cell binding and degradation assays. Full-length TFPI and C-terminally truncated TFPI (TFPI1-161 ) were iodinated using the Iodogen method39 and the specific radioactivities were typically 5.0 to 7.0 × 107 cpm/μg protein and 0.7 to 1.0 × 107 cpm/μg protein, respectively. The unincorporated 125I, after purification over a PD-10 column (Pharmacia) was less than 5% of the total radioactivity. 125I-TFPI retained approximately 90% of its functional activity when assessed by a diluted thromboplastin time assay.40
HUVEC were cultured as previously described and the monolayers were used at confluence after 72 to 96 hours in culture. The cells were gradually cooled to 4°C, washed twice with 4°C CM (without FCS), and binding was initiated by adding 0.5 mL of CM with 20 mg/mL human serum albumin (HSA; Octapharma AG, Ziegelbrücke, Switzerland) containing indicated concentrations of 125I-TFPI or 125I-TFPI1-161 in the absence or presence of competitor protein (unlabeled TFPI) or heparin. HSA was added to the CM layered over the cells before the radioactive ligand to avoid nonspecific binding. After incubation for 2 hours at 4°C, the cells were washed three times with cold CM and lysed in 62.0 mmol/L Tris-HCl, pH 6.8, containing 0.2% (wt/vol) sodium dodecyl sulfate (SDS) and 10% (vol/vol) glycerol. Radioactivity of cell lysates was determined in a LKB-Wallac gamma counter (Turku, Finland). Degradation assays were performed after binding of 1.0 nm 125I-TFPI to the cell surface for 2 hours at 4°C as previously described. The cell monolayers were washed three times with 4°C CM to remove unbound ligand. Cells were relayered with CM containing 20 mg/mL HSA, warmed rapidly to 37°C, and the overlying medium was removed at selected time intervals. Proteins in the CM were precipitated by 20% trichloroacetic acid. Cells were lysed as previously described. Degradation of ligand was defined as the appearance of radioactive ligand fragments in the overlying medium that were soluble in trichloracetic acid. Degradation of 125I-ligand in parallel dishes that did not contain cells was subtracted from the total degradation value. Dissociation of cell-surface bound 125I-TFPI to the medium was expressed as percent radioactivity precipitable with trichloroacetic acid in the medium relative to the total radioactivity associated with the cells and in the medium.
RESULTS
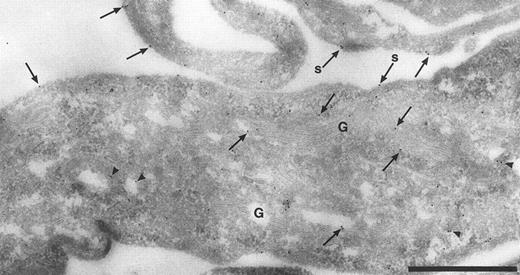
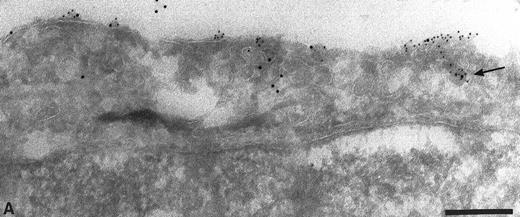
Intracellular localization of TFPI. The normal distribution of TFPI within endothelial cells, maintained in vitro, was examined on thin cryosections labeled with specific antibodies and colloidal gold probes. Label was seen associated with the cell surface and with many intracellular structures including the Golgi complex (Fig 1) and many organelles with vesicular profiles (data not shown). The surface label was irregular with noticable variation of signal intensity between cells and different parts of the same cell (data not shown). The signal appeared to concentrate over the thin cellular processes of the outer perimeter of these flat endothelial cells (Fig 1). The frequency of anti-TFPI labeling was 3.5 ± 0.5 gold particles per 10-μm length of plasma membrane (data not shown). If the anti-TFPI IgG was preadsorbed with recombinant TFPI before labeling, the frequency of TFPI labeling was 0.7 ± 0.3 gold particles per 10-μm length of plasma membrane (data not shown) and also resulted in an almost total abolition of signal over the Golgi complex (Fig 2A) and cytoplasmic vesicles (Fig 2B). Similar labeling patterns on sections through intact veins of umbilical cords were observed using αTFPI and rTFPI plus αTFPI, respectively (data not shown).
Cryosection through HUVEC labeled with anti-TFPI antibodies. Antibody binding was visualized using protein A-gold particles, some of which are indicated with arrows. This micrograph shows anti-TFPI label over the Golgi complex (G) and associated with cytoplasmic vesicles (arrowheads). Surface label (s) is concentrated on the thin cellular processes. Bar = 1 μm.
Cryosection through HUVEC labeled with anti-TFPI antibodies. Antibody binding was visualized using protein A-gold particles, some of which are indicated with arrows. This micrograph shows anti-TFPI label over the Golgi complex (G) and associated with cytoplasmic vesicles (arrowheads). Surface label (s) is concentrated on the thin cellular processes. Bar = 1 μm.
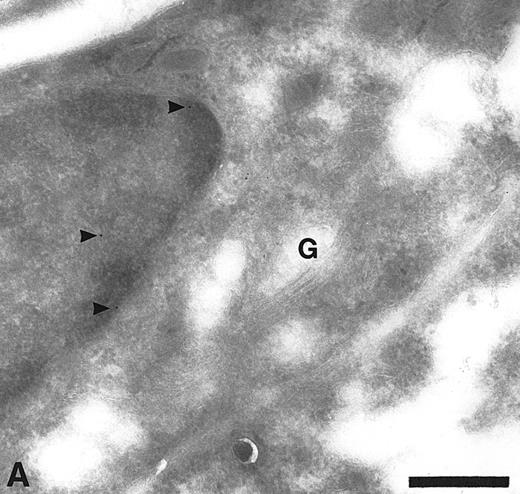
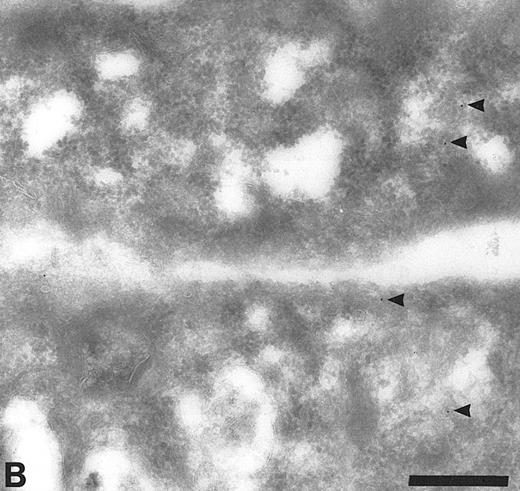
Cryosections of HUVEC showing adsorption control results. The sections were incubated with anti-TFPI diluted in medium containing rTFPI, and then with protein A-gold. (A) There is no label over the region of the Golgi complex (G) even though some nonspecific label (arrowheads) is seen over the nucleus. Bar = 0.5 μm. (B) Although the adsorption control did not completely abolish labeling (arrowheads), the amount of label is reduced and no label is observed over the many intracellular vacuoles (v) characteristic of these cells. Bar = 0.5 μm.
Cryosections of HUVEC showing adsorption control results. The sections were incubated with anti-TFPI diluted in medium containing rTFPI, and then with protein A-gold. (A) There is no label over the region of the Golgi complex (G) even though some nonspecific label (arrowheads) is seen over the nucleus. Bar = 0.5 μm. (B) Although the adsorption control did not completely abolish labeling (arrowheads), the amount of label is reduced and no label is observed over the many intracellular vacuoles (v) characteristic of these cells. Bar = 0.5 μm.
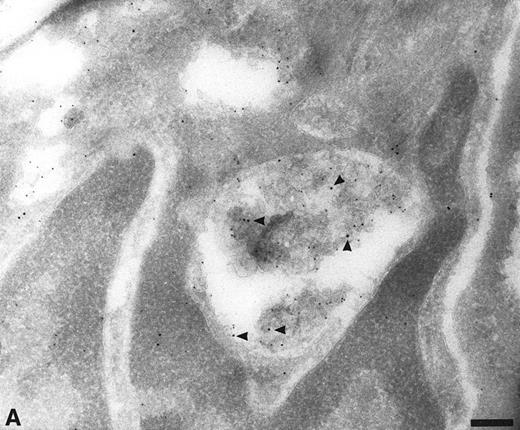
Apart from the labeling over the Golgi complex, it was difficult to identify most of the TFPI-labeling structures. We speculated that some of these TFPI-positive organelles were part of the endocytic pathway. To demonstrate this, we labeled sections with antibodies to TFPI and with antibodies to known markers of endocytic organelles. The organelles of the endocytic pathway are known to have low luminal pH and will accumulate DAMP in their lumen. On cryosections through endothelial cells incubated with DAMP before fixation, anti-TFPI antibodies were seen over organelles which also contained DAMP, as visualized using anti-DNP antibodies (Fig 3). From the total population of labeled organelles, 61.9% ± 7.6% were labeled with both TFPI and DAMP antibodies (Table 1). Structures that contained exclusively TFPI (9.5%) or DAMP (28.6%) were also seen in these sections (data not shown), suggesting that TFPI did not enter all endocytic organelles and was not exclusively localized to low pH compartments.
Section through HUVEC incubated with DAMP before fixation. The sections were double labeled with antibodies to dinitrophenol (DNP, small gold) and with anti-TFPI (large gold, arrowheads). The anti-DNP antibodies labeled the lumen of many intracellular organelles, indicating the presence of low pH. These organelles also labeled with anti-TFPI. Bar = 0.2 μm.
Section through HUVEC incubated with DAMP before fixation. The sections were double labeled with antibodies to dinitrophenol (DNP, small gold) and with anti-TFPI (large gold, arrowheads). The anti-DNP antibodies labeled the lumen of many intracellular organelles, indicating the presence of low pH. These organelles also labeled with anti-TFPI. Bar = 0.2 μm.
Percent Distribution of Labeled TFPI and Respective Markers of the Endocytic Compartments Among Total Labeled Organelles
| Labeled Organelles . | Percent Labeling . |
|---|---|
| TFPI + DAMP | 61.9 ± 7.6 |
| TFPI alone | 9.5 ± 5.2 |
| DAMP alone | 28.6 ± 6.1 |
| TFPI + MPR | 55.2 ± 8.4 |
| TFPI alone | 29.3 ± 29.3 |
| MPR alone | 15.5 ± 4.3 |
| TFPI + HTfR | 38.4 ± 3.8 |
| TFPI alone | 35.3 ± 4.2 |
| HTfR alone | 26.3 ± 1.2 |
| TFPI + LAMP 1 | 68.7 ± 5.4 |
| TFPI alone | 15.3 ± 2.2 |
| LAMP 1 alone | 16.0 ± 5.6 |
| Labeled Organelles . | Percent Labeling . |
|---|---|
| TFPI + DAMP | 61.9 ± 7.6 |
| TFPI alone | 9.5 ± 5.2 |
| DAMP alone | 28.6 ± 6.1 |
| TFPI + MPR | 55.2 ± 8.4 |
| TFPI alone | 29.3 ± 29.3 |
| MPR alone | 15.5 ± 4.3 |
| TFPI + HTfR | 38.4 ± 3.8 |
| TFPI alone | 35.3 ± 4.2 |
| HTfR alone | 26.3 ± 1.2 |
| TFPI + LAMP 1 | 68.7 ± 5.4 |
| TFPI alone | 15.3 ± 2.2 |
| LAMP 1 alone | 16.0 ± 5.6 |
Abbreviations: DAMP, 3-(2,4-dinitroanilino)3′-amino-N-methylpropulamine (low pH compartments); MPR, mannose-6-phosphate receptor (late endosomes); HTfR, human transferrin receptor (early endosomes); LAMP 1, lysosomal membrane protein 1 (lysosomes).
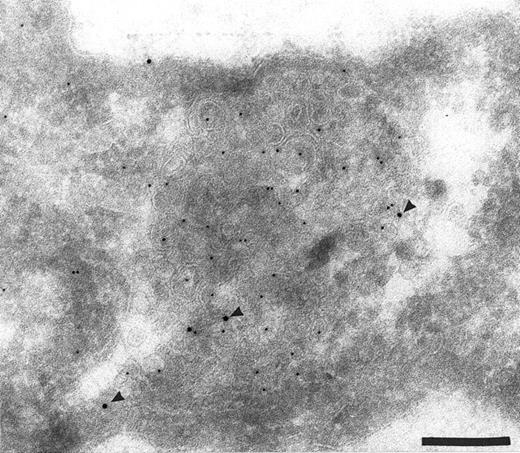
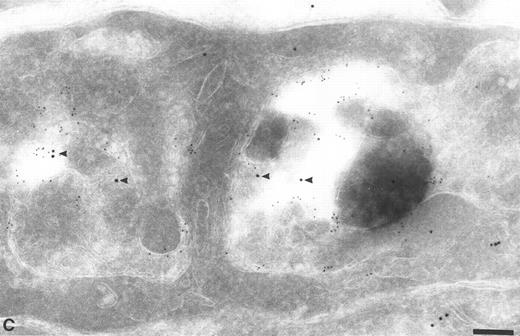
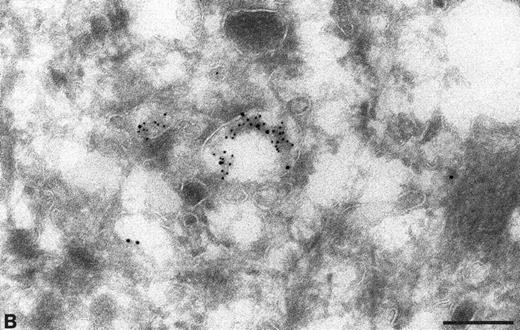
To confirm the presence of TFPI in endocytic compartments, the sections were labeled with specific antibodies directed against known markers of endosomes (MPR) and lysosomes (LAMP 1). The TFPI antibodies colocalized with antibodies to both MPR (Fig 4A and B) and LAMP 1 (Fig 4C) (Table 1), showing the presence of TFPI in late endocytic compartments. When anti-TFPI antibodies were colocalized with anti-MPR, approximately 55.2% of the labeled vesicles contained both antigens (Table 1), whereas only 15.5% labeled with MPR alone and 29.3% had only anti-TFPI associated with them (Table 1). When used with anti-LAMP 1, the TFPI was found colocalized in 67.8% of the labeled vesicles (Table 1). The TFPI and LAMP 1 antibodies each exclusively labeled only approximately 16% of the vesicles (Table 1).
Colocalization of anti-TFPI antibodies with endosomes and lysosome marker proteins on cryosections of HUVEC. (A) Cell labeled with anti-mannose-6-phosphate receptor (MPR, small gold), considered to be a marker for late endosomes. The anti-TFPI (large gold, arrowheads) labeling is present in MPR-positive structures. Bar = 0.2 μm. (B) Smaller, MPR-positive vesicles (small gold) also labeled with anti-TFPI (large gold). The anti-TFPI also labels the cell surface. Bar = 0.2 μm. (C) Cell labeled with anti-LAMP (small gold), which is found in late endosomes and lysosomes. The anti-TFPI (large gold, arrowheads) is present in LAMP 1-positive structures. Bar = 0.2 μm.
Colocalization of anti-TFPI antibodies with endosomes and lysosome marker proteins on cryosections of HUVEC. (A) Cell labeled with anti-mannose-6-phosphate receptor (MPR, small gold), considered to be a marker for late endosomes. The anti-TFPI (large gold, arrowheads) labeling is present in MPR-positive structures. Bar = 0.2 μm. (B) Smaller, MPR-positive vesicles (small gold) also labeled with anti-TFPI (large gold). The anti-TFPI also labels the cell surface. Bar = 0.2 μm. (C) Cell labeled with anti-LAMP (small gold), which is found in late endosomes and lysosomes. The anti-TFPI (large gold, arrowheads) is present in LAMP 1-positive structures. Bar = 0.2 μm.
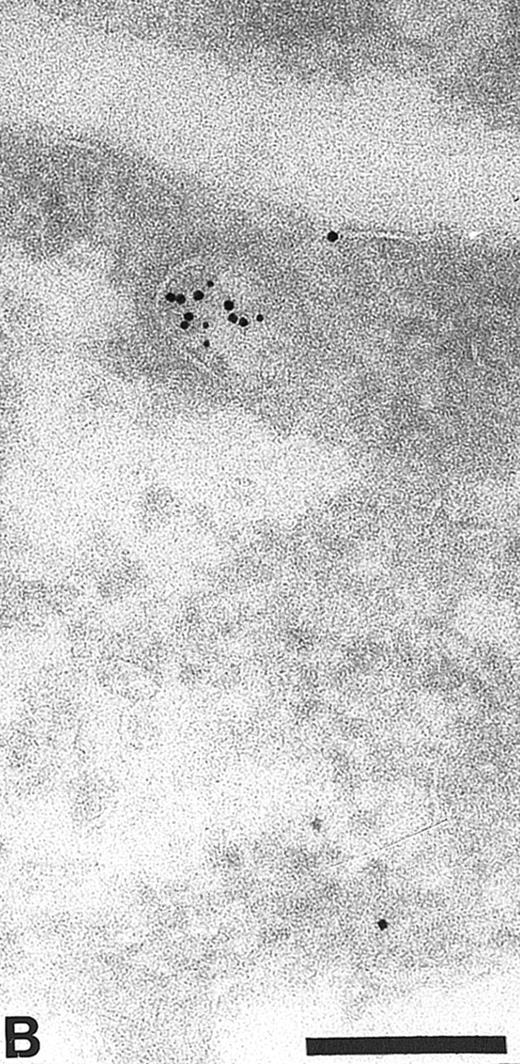
Finally, to show the presence of TFPI in early endosomes, a compartment which has the ability to recycle back to the plasma membrane, the TFPI was colocalized with antibodies to the human transferrin receptor (HTfR). Using both polyclonal and monoclonal antibodies to the human TfR we have shown a colocalization with TFPI in membranous organelles on sections of endothelial cells (Fig 5 and Table 1). This demostrated the possibility that TFPI could be recycled back to the cell surface.
Cryosections of HUVEC labeled with antibodies to human transferrin receptor (HTfR, small gold) and anti-TFPI (large gold). Transferrin receptor recycles from the cell surface via early endosomes. Small vesicles (arrows) label with anti-HTfR and anti-TFPI. Bar = 0.2 μm.
Cryosections of HUVEC labeled with antibodies to human transferrin receptor (HTfR, small gold) and anti-TFPI (large gold). Transferrin receptor recycles from the cell surface via early endosomes. Small vesicles (arrows) label with anti-HTfR and anti-TFPI. Bar = 0.2 μm.
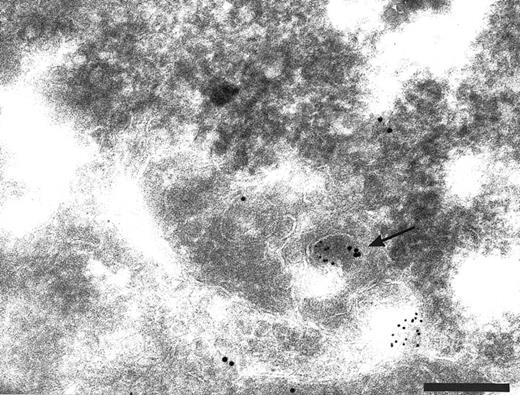
To investigate whether endogenous membrane-bound TFPI in HUVEC was being internalized, newly internalized surface biotin was colocalized with TFPI using antibodies on cryosections through biotinylated cells that had been incubated for 30 minutes at 37°C. Biotin-specific antibodies bound to sites within labeled cells that also contained anti-TFPI label. These structures included small vesicles close to the cell surface (Fig 6A) and in larger vesicles (Fig 6B) similar to those that accumulated DAMP and labeled with anti-LAMP 1 antibodies. Although it is possible that the internalized biotin is being transported to endocytic structures involved in TFPI storage, it is more likely that the TFPI is being co-internalized with the surface biotin.
Cryosections of HUVEC incubated with biotin before endocytosis and fixation. The cell surface labeled with anti-TFPI (large gold) and anti-biotin (small gold). Colocalization of anti-TFPI and anti-biotin was seen in small vesicles close to the cell surface (A, arrow) and in larger vesicles (panel B). Bars = 0.2 μm.
Cryosections of HUVEC incubated with biotin before endocytosis and fixation. The cell surface labeled with anti-TFPI (large gold) and anti-biotin (small gold). Colocalization of anti-TFPI and anti-biotin was seen in small vesicles close to the cell surface (A, arrow) and in larger vesicles (panel B). Bars = 0.2 μm.
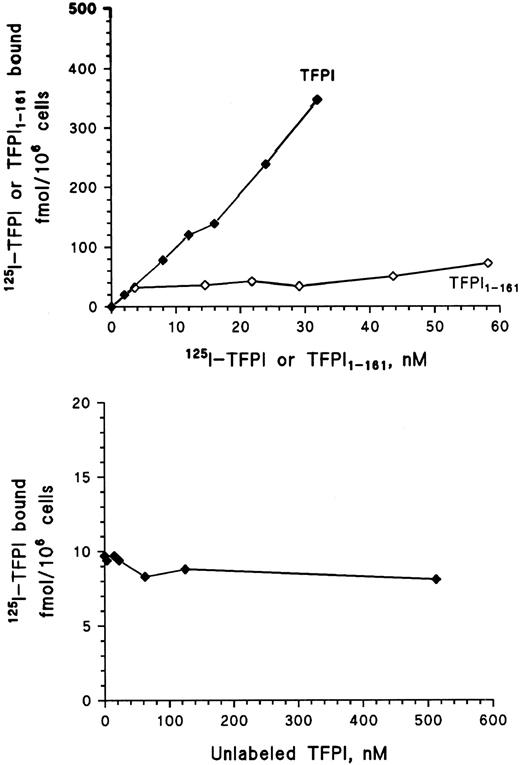
Binding of 125I-TFPI and 125I-TFPI161 to HUVEC. To investigate whether TFPI was able to associate with endothelial cells, binding studies with 125I-TFPI were performed at 4°C to avoid possible ligand uptake and degradation. The binding of 125I-TFPI increased linearly to HUVEC over the concentration range of 0.5 to 32 nmol/L without saturation (Fig 7, upper panel). To examine whether the carboxy terminus of TFPI was required for the interaction with HUVEC, a recombinant C-terminally truncated TFPI, lacking the third Kunitz domain and basic carboxy terminus, was iodinated (125I-TFPI161 ) and binding studies were performed at 4°C. The binding of 125I-TFPI161 to HUVEC increased over the concentration range, but accounted for less than 10% of full-length TFPI binding (Fig 7, upper panel). This observation indicates that the carboxy terminus is necessary for the interaction between TFPI and the cell surface in HUVEC. The presented data represent the means of triplicate determinations from at least two independent experiments.
Line graph showing the binding of increasing amounts of iodinated recombinant full-length TFPI (TFPI) and C-terminal truncated TFPI (TFPI1-161 ) to HUVEC after incubation for 2 hours at 4°C (upper panel). Addition of increasing amounts of unlabeled TFPI simultanously with 0.5 nmol/L 125I-TFPI was conducted to see if unlabeled TFPI would inhibit an interaction between 125I-TFPI and a specific receptor (lower panel). Each symbol represents the mean of triplicate determinations.
Line graph showing the binding of increasing amounts of iodinated recombinant full-length TFPI (TFPI) and C-terminal truncated TFPI (TFPI1-161 ) to HUVEC after incubation for 2 hours at 4°C (upper panel). Addition of increasing amounts of unlabeled TFPI simultanously with 0.5 nmol/L 125I-TFPI was conducted to see if unlabeled TFPI would inhibit an interaction between 125I-TFPI and a specific receptor (lower panel). Each symbol represents the mean of triplicate determinations.
Addition of increasing amounts of unlabeled TFPI before or simultanously with 125I-TFPI was conducted to see if unlabeled TFPI would be able to inhibit an interaction between 125I-TFPI and a possible specific membrane receptor. Unlabeled TFPI over the concentration range of 0 to 500 nmol/L did not influence the binding of 0.5 nmol/L 125I-TFPI (Fig 7, lower panel), indicating that the interaction between TFPI and the endothelial membrane did not involve a specific receptor. The binding between TFPI with its positively charged C-terminus and the endothelial surface may be electrostatic in nature through binding to negatively charged sulfate groups in glycosaminoglycans (GAG). Thus, to examine whether heparin would inhibit the association between 125I-TFPI and the endothelial surface, increasing amounts of heparin (0 to 10 IU/mL) were added and incubated for 2 hours during a binding assay of 0.5 nmol/L 125I-TFPI. Heparin inhibited the binding of 125I-TFPI in a dose-dependent manner, accounting for 60% inhibition at the highest heparin concentration (data not shown).
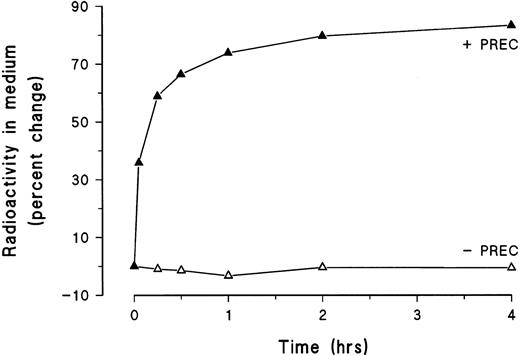
To assess possible degradation of exogenously added and bound TFPI, 125I-TFPI was incubated with HUVEC for 2 hours at 4°C to allow surface binding. After removal of unbound ligand, possible ligand uptake and degradation was initiated by incubating the cells at 37°C. The overlying medium was collected at selected time intervals and subjected to trichloroacetic acid precipitation, and the endothelial monolayers were immediately lysed with lysis buffer. As seen in Fig 8, approximately 60% of the 125I-TFPI dissociated from the cell surface within 15 minutes and continued to accumulate in the overlying medium for 4 hours. The rapid dissociation of TFPI from the cell surface is consistent with its low-affinity binding to HUVEC. During the 4-hour degradation assay, exogenously added and membrane-associated 125I-TFPI was not degraded (Fig 8).
Line graph showing the percent dissociation (precipitable with trichloroacetic acid, + PREC) and degradation (ligand fragments soluble in trichloroacetic acid, − PREC) of cell-surface–bound 125I-TFPI into the medium at selected time intervals for 4 hours at 37°C. Each symbol represents the mean of triplicate determinations.
Line graph showing the percent dissociation (precipitable with trichloroacetic acid, + PREC) and degradation (ligand fragments soluble in trichloroacetic acid, − PREC) of cell-surface–bound 125I-TFPI into the medium at selected time intervals for 4 hours at 37°C. Each symbol represents the mean of triplicate determinations.
DISCUSSION
In this study we used immunocytochemical and biochemical methods to investigate the association of TFPI with endothelial cells. TFPI was localized to the cell surface of endothelial cells and to various intracellular organelles. HUVEC have previously been reported to synthesize16 and constitutively secrete TFPI into conditioned medium,41,42 and the presence of TFPI in the Golgi apparatus supports other observations that endothelial cells are the principle sites of TFPI synthesis.16 It has been hypothesized that exocytosed, newly synthesized TFPI molecules remain anchored to the cell surface where they act as a vessel-wall-bound anticoagulant,43 and we have shown herein specific labeling of TFPI at the outer leaflet of the endothelial cell membrane. The importance of endothelial-bound TFPI has recently been documented by Grabowski et al,44 who reported a several-fold enhancement of TF-induced activation of factor X in a flow model, after neutralization of TFPI on the endothelial cell surface.
In the present study, exogenous, radiolabeled, recombinant, full-length TFPI (rTFPI) was bound to the endothelium in a dose-dependent manner within a concentration range of 0 to 32 nmol/L. Lack of saturation of the binding curve, together with no competitive inhibition with up to a thousand-fold molar excess of unlabeled TFPI, indicates that no specific receptors are involved in binding of exogenous TFPI to the endothelial cell surface. The present observation that only trace amounts of C-terminal truncated TFPI (TFPI1-161 ) bind to endothelial cells suggests that the interaction between TFPI and the endothelial cell surface is electrostatic in nature, and confirms previous studies in hepatoma cells22 indicating that the C-terminus of TFPI is required for binding to the cell surface. These findings are supported by other circumstantial evidence suggesting that TFPI is bound to glycosaminoglycans on the endothelial cell surface.15,19 45
In mammalian cells, the pathway of endocytosis involves the initial internalization of receptor-ligand complexes in clathrin-coated vesicles and their subsequent fusion with early endosomes, a population of tubules and vesicles in the peripheral cytoplasm. Because of slightly acidic pH in early endosomes, ligands dissociate from their receptors and are transported to late endosomes and finally to lysosomes, where they encounter progressively more acidic and hydrolase-rich environments.46 Lysosomal enzymes are delivered to lysosomes from the Golgi complex in transport vesicles that contain the MPR, a receptor that binds to mannose residues on newly formed enzymes and recycles after enzyme delivery.47 Free receptors, such as the transferrin receptor,48 escape transport to late compartments and are returned from early endosomes back to the cell surface via a distinct population of recycling vesicles.
It is possible to differentiate between early and late compartments within the endocytic pathway using antibodies to specific marker proteins. The early endosomes and recycling compartments contain transferrin receptor and the late endosomal compartments are enriched in lysosomal glycoproteins (LAMPs). The presence of MPR is generally considered to be a characteristic of late endosomes.46
It has previously been reported that the endothelium is involved in the endocytosis and degradation of membrane-associated substances such as thrombomodulin25 and other circulatory components of the coagulation system.23 24 By using antibodies to markers of endocytic compartments as well as markers that identify low luminal pH, we have shown the presence of TFPI throughout the endocytic pathway of these cells. The TFPI was found in compartments that accumulated DAMP as well as compartments that labeled with antibodies to human transferrin receptor, mannose phosphate receptor, and LAMP 1. In the micrographs we showed examples of the labeling patterns observed with the specific antibodies we used, but these results are better represented as stereological estimates. For this reason we also presented the patterns as numerical estimates, obtained by systematic random sampling of the total population, to give a better idea of how the antibodies labeled the sections.
We have clearly shown specific labeling of TFPI within early endosomes as well as late endocytic compartments. Because these organelles are components of the endocytic pathway, these observations led us to speculate that endothelial cells are involved with endocytosis of TFPI. However, it is not known whether uptake of both circulatory and membrane-anchored TFPI is occurring. In our study, exogenously added 125I-TFPI was bound to the cell surface, but not degraded by HUVEC. These findings differ from the clearance mechanism of TFPI seen in hepatoma cells, where the LPR-mediated clearance of TFPI appears to involve a two-step process.22 As with endothelial cells, the initial binding of TFPI to the hepatoma cell surface could be inhibited by pretreatment with heparin and was dependent on an intact C-terminal domain.22
The reasons for the apparent discrepancy between the appearance of TFPI within the endocytic compartment and the absence of degradation of exogenously added 125I-TFPI is not known. One possible explanation could be that endocytosis and degradation of TFPI in endothelial cells is restricted to de novo exocytosed and membrane-anchored TFPI. Recently, Sevinsky et al49 provided evidence for TFPI-specific association with glycosyl phosphatidylinositol (GPI)-anchored binding sites on external membranes. Thus, it is possible that recycling and degradation of TFPI may be restricted to GPI-anchored binding sites of membrane TFPI, whereas GAG-bound TFPI may serve as reservoir for the circulatory pool of TFPI which could be released by heparins. This suggestion is supported by the observation of endogenous TFPI in endocytic compartments, by colocalization of biotin and TFPI labeling during endocytosis, and by the colocalization of TFPI within organelles containing transferrin receptor, indicating the possibility of TFPI recycling. Furthermore, exogenously added TFPI which requires an intact C-terminus for proper association with the cell surface, probably by GAG interaction, was not degraded by the endothelial cells.
It may also be speculated that a ligand is needed to promote endocytosis of surface-bound TFPI. This hypothesis is supported by the recent findings by Ho et al,50 who showed that receptor-mediated endocytosis of coagulation factor Xa required cell-surface bound TFPI, and demonstrated a parallel internalization and degradation of factor Xa and TFPI in hepatoma cells. It was suggested that endothelial cell surface TFPI may be actively involved in the clearance of factor Xa from the circulation. Similarly, TFPI has been shown to be responsible for the downregulation of TF-dependent proteolytic function (TF-VIIa complex) in stimulated endothelial cells by triggering translocation of the inhibited complex into cavelae.49 Thus, in addition to its direct function as a endothelial-bound anticoagulant, surface-bound TFPI may also have an indirect anticoagulant impact by regulating the clearance of factor Xa, a plasma serine protease catalyzing the conversion of prothrombin to thrombin and subsequently leads to the generation of the fibrin clot and by downregulation of TF-VIIa proteolytic activity.
ACKNOWLEDGMENT
We owe a special thanks to Dr Mirella Ezban at Novo Nordic (Gentofte, Denmark) for providing us with anti-TFPI antibodies and recombinant TFPI, without which this study could not have been initiated. We thank Profs I. Mellman (Yale University, New Haven, CT) and B. Hoflack (EMBL, Heidelberg, Germany) for their generous gifts of anti-dinitrophenol and anti-MPR antibodies, respectively. The LAMP 1 was obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, under contract N01-HD-6-2915 from the NICHD.
Supported in part by a grant from the Norwegian Council on Cardiovascular Diseases.
Address reprint requests to John-Bjarne Hansen, MD, Department of Medicine, Institute of Clinical Medicine, University of Tromsø N-9037 Tromsø, Norway.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal