Abstract
Highly purified CD34++CD38−Lin− hematopoietic progenitors isolated from human fetal liver were infected with the murine retroviral vector, MFG nls-LacZ, which encodes a modified version of the Escherichia coli β-galactosidase gene. Progenitors that were cocultured with the packaging cell line could reconstitute human bone marrow or thymus implanted in SCID-hu mice. Expression of the β-galactosidase gene was observed in primitive and committed clonogenic progenitors, mature myeloid, B-lineage cells, and T-lineage cells for up to 4 months after injection into SCID-hu mice. Furthermore, hematopoietic reconstitution by genetically modified progenitor cells could be achieved by the injection of the cells generated from as few as 500 CD34++CD38−Lin− cells, suggesting efficient retroviral gene transfer into fetal liver progenitors.
GENETIC MANIPULATION of hematopoietic stem cells will be beneficial in a broad range of clinical settings. Stem cells can give rise to all the lineages of blood cells, and therefore gene introduction into stem cells would result in the expression of the transgene in their mature progenies for long periods of time. Defective murine retroviral vectors are widely used for gene transduction of various cell types.1 Amphotropic retroviruses are able to infect human cells and to integrate genes into the host DNA, thus allowing the transmission of the newly introduced genetic information to daughter cells. Efficient retroviral gene integration, however, requires target cells to be in cycle,2 whereas most, if not all, of hematopoietic stem cells are known to be in a quiescent state.3-5 Although the use of cytokines has been shown to improve the efficacy of gene transduction of hematopoietic progenitors,6 it is unclear whether cytokines control the entry of quiescent stem cells into cell cycle.7,8 It is also controversial whether in vitro manipulation of stem cells results in a profound alteration of stem cell properties, especially their ability to mediate long-term reconstitution of myeloablated individuals. Some studies have shown that murine9 and human10 progenitors cultured in the presence of combinations of cytokines maintain their reconstitution potential, whereas others showed the loss of reconstitution potential following mock retroviral infection11 or exposure to cytokines.12
Studies in nonhuman primates,13 in patients,14-18 and in immunodeficient mouse models with human cells19-22 have shown low levels of hematopoietic reconstitution by genetically modified progenitors. All of these studies were conducted either with unfractionated mononuclear cells or with CD34+ cells isolated from adult bone marrow (BM), mobilized peripheral blood (PB), or cord blood (CB). In this study we explored the possibility of purified CD34++CD38−Lin− cells derived from human fetal liver as targets for retroviral gene transduction. The progenitors with CD34++CD38−Lin− phenotype isolated from adult BM,8,23,24 CB,25 and fetal liver26 were shown to be highly enriched for the primitive progenitor activity, and therefore will be an appropriate target for stem cell–based gene therapy. However, it is likely that they are less susceptible to retroviral gene transduction, as only a small proportion of this fraction of cells are in cycle.8,25 Indeed, the retroviral gene transduction into CD34+CD38− cells has been shown to be inefficient as long as cells isolated from CB27 or PB28 were used. In contrast, primitive progenitors isolated from fetal liver were shown to go into cell cycle more efficiently while maintaining the primitive phenotype compared with those derived from adult BM or even from CB.29 The difficulty of retroviral infection of quiescent stem cells, therefore, may be overcome by using fetal liver–derived progenitor cells.
In addition, we investigated the effects of different cytokine combinations on retroviral gene transduction and on the maintenance of reconstitution potential, including Flk2/Flt3 ligand (FL).30,31 FL has been shown to be a potent cofactor that stimulates the proliferation of primitive progenitors.32-34 Furthermore, it was recently reported that FL supported the maintenance of multilineage, long-term hematopoietic reconstitution activity during the culture for retroviral gene transduction.35
The hematopoietic reconstitution potential of ex vivo–manipulated progenitors was evaluated using SCID-hu mice, which provide a dynamic model of human in vivo hematopoiesis.36-38 We showed that a population of human fetal liver cells highly enriched for stem cell activity can be efficiently transduced with a retroviral vector while it maintains the hematopoietic reconstitution activity.
MATERIALS AND METHODS
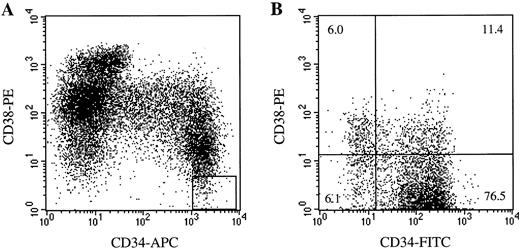
Isolation of fetal liver progenitors. Human fetal tissues were obtained from Advanced Bioscience Resources (Alameda, CA), in compliance with regulations issued by the state and federal government, and were used with the approval of the committee for the Protection of Human Subjects at DNAX Research Institute. Fetal liver mononuclear cells were prepared for fluorescence-activated cell sorting (FACS) as described.26 Cells expressing high levels of CD34 but lacking for CD38 and the cocktail of lineage markers (CD3, CD8, CD10, CD14, CD16, CD19, CD20, and glycophorin A) were isolated (Fig 1A).
The phenotype of freshly isolated and cultured CD34++CD38−Lin− cells. (A) The electronic gate on the Lin− cell population used to define the CD34++CD38−Lin− cells is shown. (B) The phenotype of CD34++CD38−Lin− cells cultured for 2 days in the presence of IL-3, IL-6, GM-CSF, KL, and FL, and additional 3 days on the packaging cells in the presence of same cytokine combination is shown. Most of the cells retain the CD34+CD38− phenotype.
The phenotype of freshly isolated and cultured CD34++CD38−Lin− cells. (A) The electronic gate on the Lin− cell population used to define the CD34++CD38−Lin− cells is shown. (B) The phenotype of CD34++CD38−Lin− cells cultured for 2 days in the presence of IL-3, IL-6, GM-CSF, KL, and FL, and additional 3 days on the packaging cells in the presence of same cytokine combination is shown. Most of the cells retain the CD34+CD38− phenotype.
Retroviral infection of progenitor cells. Isolated progenitor cells were incubated for 2 days in RPMI 1640 (JRH Biosciences, Lenexa, KS), supplemented with 10% fetal bovine serum (FBS; BioWhitakker Inc, Walkersville, MD), 1% L-glutamine, 1% penicillin-streptomycin (GIBCO BRL, Grand Island, NY), and the combination of cytokines described below. Cells were then transferred onto mitomycin (Sigma Chemical Co, St Louis, MO) pretreated A7.21, a Psi-Crip–derived producing cell line, kindly provided by L. Cohen (Somatix Therapy Co). This cell line produces the MFG-S-nls LacZ retroviral vector encoding a modified version of the Escherichia coli β-galactosidase gene (nls-LacZ).39,40 Three days after coculture cells were collected by vigorous flushing. Adherent packaging cells were removed by plating cells twice for 2 hours. Cytokine combinations used in these experiments were: condition (C) 1; recombinant human (rh) interleukin-3 (IL-3; R & D Systems, Inc, Minneapolis, MN), rhIL-6 (produced in DNAX by Dr R. Kastelein), rh granulocyte-macrophage colony-stimulating factor (GM-CSF; Schering-Plough Co, Kenilworth, NJ; 10 ng/mL each), and rh c-kit ligand (KL; R & D Systems, Inc; 100 ng/mL), C2; C1 combination plus recombinant mouse (rm) FL (100 U/mL) produced at DNAX,32 C3; rhIL-6, rhKL, and rmFL.
SCID-hu mouse reconstitution assays. C.B-17 scid/scid (SCID) mice transplanted with either fetal thymus and liver or fetal BM (SCID-hu mice) were prepared as described.36 37 Mice were selected so that human grafts and fetal liver cells were mismatched for commonly distributed major histocompatibility complex (MHC) Class I alloantigens. Mice received 250 cGy whole body γ-irradiation, immediately followed by the injection of genetically modified fetal liver cells directly into grafts. The numbers of injected cells correspond to the numbers of CD34++CD38−Lin− cells at the initiation of the culture.
Clonogenic progenitor assays. Methylcellulose colony assays were performed with Methocult 4230 (Stem Cell Technology Inc, Vancouver, Canada) supplemented with rhIL-3, rhIL-6, rh granulocyte (G)-CSF (Amgen, Thousand Oaks, CA; 10 ng/mL each), rhKL (100 ng/mL), and rh erythropoietin (Epo; Amgen; 2 U/mL). Colonies formed in methylcellulose culture (CFC-MC) were enumerated on day 14. The sum of colony-forming unit granulocyte macrophage (CFU-GM), CFU-G, CFU-M, and burst-forming unit-erythroid (BFU-E) were shown as CFU-MC. A double-layered agarose culture supplemented with rhIL-3, rhGM-CSF, rhKL (20 ng/mL each) was used for the detection of high-proliferative potential (HPP)-colony-forming cells (CFC) and low-proliferative potential (LPP)-CFC, as described.26 Colonies were enumerated on day 21. HPP-CFC were defined as progenitors giving rise to colonies greater than or equal to 0.5 mm in diameter. Culture conditions used for CFC assays were confirmed to induce proliferation of human, but not murine, progenitors.
Flow cytometry. Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MoAbs) recognizing the MHC Class I alloantigen (HLA-A2, -A3, and -B7; obtained from American Type Culture Collection, Rockville, MD) were used to determine the contribution of fetal liver–derived cells in hematopoietic reconstitution. Cells were stained with a FITC-conjugated anti-HLA antibody in combination with phycoerythrin (PE)-conjugated CD19, CD33, CD34, CD3, CD4, CD8, CD45 (Becton Dickinson Immunocytometry Systems, San Jose, CA), or DX17 (recognizes monomorphic epitope of MHC Class I antigen; a gift from Dr L. Lanier at DNAX) and analyzed by FACScan in the presence of 1 μg/mL propidium iodide. In some experiments, recovered BM cells were stained with FITC-CD19, PE-CD33, and PE-CD14 and were sorted into B cells and myeloid cells by FACS Vantage. Thymocytes were stained with FITC-CD8 and PE-CD4 and were sorted into CD4+CD8+, CD4+CD8−, and CD4−CD8+ populations.
Detection of the nls-LacZ gene. Expression of the introduced nls-LacZ gene was examined by a standard X-gal staining39 for E coli β-galactosidase activity, on colonies formed in both methylcellulose and agarose assays, and on cytospin slides prepared with 2.5 × 104 suspension cells recovered from BM or thymus grafts. Fixed cells were incubated with X-gal reaction solution at 37°C for 2 hours for cytospin preparations and for 12 hours for colonies. Cytospin-prepared cells were then lightly stained with hematoxylin. The reaction of X-gal staining was highly specific because none of the cells or colonies derived from the human grafts that were not injected with gene transduced cells showed positive reaction. Furthermore, investigation of individual colonies generated by transduced progenitor cells before the injection into SCID-hu mice exhibited completely matched results by X-gal staining and by DNA-PCR analysis.
Genomic polymerase chain reaction (PCR) was conducted using the following primers: primer 5′LacZ6: 5′ CTTCTCTAGGCGCCCCCATAT 3′, localized in the retroviral gag sequence, and primer 3′ LacZ7: 5′ CGTTGTAAAACGACGGCCAGT 3′, localized in the LacZ sequence. The expected size of the amplified fragment was 470 bp. Templates were amplified for 35 cycles with 1 minute at 94°C, 1 minute at 58°C, and 2 minutes at 72°C, followed by a last step of 7 minutes at 72°C. Specificity of amplification was checked by Southern blot analysis with an internal primer LacZs2: 5′ CTAGAACCTCGCTGGAAAGG 3′. Under this condition, a single A7.21 cell that contains more than 2 copies of the nls-LacZ gene in 1,000 negative cells can be reproducibly detected.
RESULTS
CD34++CD38−Lin− fetal liver cells can be gene transduced in vitro. CD34++CD38−Lin− cells were isolated by cell sorting (Fig 1A) from 14 fetal livers of gestational ages ranging from 16 to 24 weeks and were cultured in the presence of three different combinations of cytokines (C1: IL-3 + IL-6 + GM-CSF + KL; C2: IL-3 + IL-6 + GM-CSF + KL + FL; C3: IL-6 + KL + FL) for a total of 5 days. The expansion of cells and clonogenic progenitors are shown in Fig 2A. Although cell recovery from C3 cultures appeared to be lower than those obtained from C1 and C2 cultures, similar levels of expansion of clonogenic cells were achieved in all conditions. On average, 20- to 40-fold expansion was found in CFC-MC, 30- to 60-fold in LPP-CFC, and 10-fold in HPP-CFC. In addition, the effects of cytokine combinations on the efficiency of retroviral gene transduction were investigated (Fig 2B). Expression of the transduced β-galactosidase gene was observed in 5% to 10% of clonogenic cells of all types, including HPP-CFC. Statistical analysis, using a Wilcoxon test for paired samples, showed no significant difference among the results observed with different cytokine combinations, either for expansion or infection efficiency. All fetal liver samples shown in this figure were subsequently injected into SCID-hu mice.
Effects of cytokine combinations on ex vivo expansion and gene transduction. (A) Fold expansions in cellularity, CFC in methylcellulose cultures (CFC-MC), LPP-CFC, and HPP-CFC induced under various cytokine combinations are illustrated. (B) The percentages of transduced nls-LacZ gene expressing colonies are summarized. (□) C1: IL-3 + IL-6 + GM-CSF + KL; (▧) C2: IL-3 + IL-6 + GM-CSF + KL + FL; (▪) C3: IL-6 + KL + FL. Numbers of fetal liver samples analyzed are shown in parentheses.
Effects of cytokine combinations on ex vivo expansion and gene transduction. (A) Fold expansions in cellularity, CFC in methylcellulose cultures (CFC-MC), LPP-CFC, and HPP-CFC induced under various cytokine combinations are illustrated. (B) The percentages of transduced nls-LacZ gene expressing colonies are summarized. (□) C1: IL-3 + IL-6 + GM-CSF + KL; (▧) C2: IL-3 + IL-6 + GM-CSF + KL + FL; (▪) C3: IL-6 + KL + FL. Numbers of fetal liver samples analyzed are shown in parentheses.
The expression of the CD34 and CD38 antigens on cultured CD34++CD38−Lin− cells were analyzed in parallel to the reconstitution studies. Results showed that 67.7 ± 4.1, 74.3 ± 1.2, and 68.7 ± 3.4% of the cells cultured under C1, C2, and C3, respectively, expressed CD34 yet negative or low to CD38 antigen (Fig 1B). There were no statistically significant differences among the cultures with different cytokine combinations. Thus, these results indicate that, on average, 70% of the injected cells still maintained CD34+CD38− phenotype after gene transduction procedure.
Ex vivo–manipulated CD34++CD38−Lin− cells maintain their hematopoietic reconstitution potential. Hematopoietic reconstitution by ex vivo–manipulated CD34++ CD38−Lin− fetal liver cells was assessed 4 to 17 weeks after injection into human BM in SCID-hu mice37 (Table 1). Injected cell numbers are indicated as the number of CD34++CD38−Lin− cells at initiation of the culture (day 0) to normalize the variable levels in cell expansion induced by different cytokine combinations. The actual number of injected cells ranged from 2.8 × 104 to 5.2 × 105. The contribution of fetal liver–derived cells to hematopoiesis was evaluated by flow cytometry, based on the expression of MHC Class I alloantigens (Fig 3A). Forty-two out of 43 BM samples, from 11 independent experiments, showed varying degrees of chimerism between fetal liver donor–derived and BM donor–derived cells. Percentages of fetal liver donor–derived cells tended to increase after 7 weeks following injection. All of the 33 BM samples, which were subjected to the further analysis, contained fetal liver–derived CD19+ B cells, CD33+ myeloid cells, and CD34+ progenitor cells for as long as 17 weeks after injection. It was shown that the CD33+ and CD34+ compartments were more rapidly reconstituted relative to the CD19+ compartment.
BM Reconstitution Potential of Ex Vivo–Manipulated CD34++CD38−Lin− Fetal Liver Cells
| Experiment No. . | Weeks . | Cytokine* . | Injected Cells† . | Reconstitution (%)‡ . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | . | Total . | CD19+ . | CD33+ . | CD34+ . |
| 1 | 4 | C1 | 8,400 | 54 | 27 | 78 | 60 |
| C1 | 8,400 | 55 | 33 | 65 | 64 | ||
| C2 | 6,400 | 26 | 9 | 53 | 38 | ||
| C2 | 6,400 | 29 | 9 | 57 | 33 | ||
| C2 | 6,400 | 47 | 25 | 61 | 50 | ||
| 2 | 4 | C2 | 13,800 | 48 | 36 | 56 | 53 |
| C1 | 13,800 | 35 | 10 | ND | ND | ||
| 7 | C2 | 13,800 | 100 | 89 | 71 | 73 | |
| 3 | 4 | C1 | 7,500 | 43 | 24 | 71 | 58 |
| C2 | 5,000 | 29 | 17 | 47 | 13 | ||
| C2 | 5,000 | 50 | 57 | 65 | 54 | ||
| 4 | 8 | C1 | 5,400 | 66 | 65 | 66 | 71 |
| C1 | 5,400 | 26 | 18 | 56 | 39 | ||
| C1 | 600 | 42 | 22 | 38 | 23 | ||
| C1 | 600 | 75 | 79 | 50 | 65 | ||
| C2 | 5,400 | 77 | 59 | 83 | ND | ||
| 5 | 8 | C1 | 7,200 | 75 | 65 | 82 | 65 |
| C1 | 7,200 | 93 | 90 | 93 | 91 | ||
| C1 | 1,500 | 94 | 96 | 90 | 86 | ||
| C1 | 1,500 | 10 | 4 | 37 | 21 | ||
| C1 | 750 | 98 | 98 | 96 | 91 | ||
| C1 | 750 | 91 | 99 | 98 | 95 | ||
| C2 | 7,200 | 98 | 97 | 99 | 99 | ||
| C2 | 7,200 | 91 | 86 | 97 | 96 | ||
| C2 | 1,500 | 87 | 86 | 87 | 88 | ||
| C2 | 750 | 86 | 73 | 97 | 98 | ||
| 6 | 8 | C2 | 24,000 | 98 | 99 | 97 | 95 |
| C3 | 2,700 | 94 | 95 | 94 | 96 | ||
| C3 | 24,000 | 97 | 98 | 97 | 95 | ||
| 7 | 9 | C2 | 17,600 | 92 | 65 | 92 | 65 |
| C2 | 2,000 | 74 | 90 | 93 | 91 | ||
| C3 | 17,600 | 51 | 40 | 27 | 31 | ||
| 8 | 10 | C1 | 4,500 | 90 | ND | ND | ND |
| C1 | 500 | 74 | ND | ND | ND | ||
| C2 | 4,500 | 100 | ND | ND | ND | ||
| C2 | 500 | 89 | ND | ND | ND | ||
| 9 | 11 | C2 | 6,300 | 76 | ND | ND | ND |
| 11 | 14 | C2 | 10,600 | 96 | 99 | 92 | 95 |
| 12 | 17 | C2 | 4,300 | 99 | 99 | ND | ND |
| C2 | 4,300 | 97 | 98 | ND | ND | ||
| C3 | 13,100 | 95 | 96 | 95 | 91 | ||
| C3 | 4,300 | 95 | 97 | ND | ND | ||
| Experiment No. . | Weeks . | Cytokine* . | Injected Cells† . | Reconstitution (%)‡ . | |||
|---|---|---|---|---|---|---|---|
| . | . | . | . | Total . | CD19+ . | CD33+ . | CD34+ . |
| 1 | 4 | C1 | 8,400 | 54 | 27 | 78 | 60 |
| C1 | 8,400 | 55 | 33 | 65 | 64 | ||
| C2 | 6,400 | 26 | 9 | 53 | 38 | ||
| C2 | 6,400 | 29 | 9 | 57 | 33 | ||
| C2 | 6,400 | 47 | 25 | 61 | 50 | ||
| 2 | 4 | C2 | 13,800 | 48 | 36 | 56 | 53 |
| C1 | 13,800 | 35 | 10 | ND | ND | ||
| 7 | C2 | 13,800 | 100 | 89 | 71 | 73 | |
| 3 | 4 | C1 | 7,500 | 43 | 24 | 71 | 58 |
| C2 | 5,000 | 29 | 17 | 47 | 13 | ||
| C2 | 5,000 | 50 | 57 | 65 | 54 | ||
| 4 | 8 | C1 | 5,400 | 66 | 65 | 66 | 71 |
| C1 | 5,400 | 26 | 18 | 56 | 39 | ||
| C1 | 600 | 42 | 22 | 38 | 23 | ||
| C1 | 600 | 75 | 79 | 50 | 65 | ||
| C2 | 5,400 | 77 | 59 | 83 | ND | ||
| 5 | 8 | C1 | 7,200 | 75 | 65 | 82 | 65 |
| C1 | 7,200 | 93 | 90 | 93 | 91 | ||
| C1 | 1,500 | 94 | 96 | 90 | 86 | ||
| C1 | 1,500 | 10 | 4 | 37 | 21 | ||
| C1 | 750 | 98 | 98 | 96 | 91 | ||
| C1 | 750 | 91 | 99 | 98 | 95 | ||
| C2 | 7,200 | 98 | 97 | 99 | 99 | ||
| C2 | 7,200 | 91 | 86 | 97 | 96 | ||
| C2 | 1,500 | 87 | 86 | 87 | 88 | ||
| C2 | 750 | 86 | 73 | 97 | 98 | ||
| 6 | 8 | C2 | 24,000 | 98 | 99 | 97 | 95 |
| C3 | 2,700 | 94 | 95 | 94 | 96 | ||
| C3 | 24,000 | 97 | 98 | 97 | 95 | ||
| 7 | 9 | C2 | 17,600 | 92 | 65 | 92 | 65 |
| C2 | 2,000 | 74 | 90 | 93 | 91 | ||
| C3 | 17,600 | 51 | 40 | 27 | 31 | ||
| 8 | 10 | C1 | 4,500 | 90 | ND | ND | ND |
| C1 | 500 | 74 | ND | ND | ND | ||
| C2 | 4,500 | 100 | ND | ND | ND | ||
| C2 | 500 | 89 | ND | ND | ND | ||
| 9 | 11 | C2 | 6,300 | 76 | ND | ND | ND |
| 11 | 14 | C2 | 10,600 | 96 | 99 | 92 | 95 |
| 12 | 17 | C2 | 4,300 | 99 | 99 | ND | ND |
| C2 | 4,300 | 97 | 98 | ND | ND | ||
| C3 | 13,100 | 95 | 96 | 95 | 91 | ||
| C3 | 4,300 | 95 | 97 | ND | ND | ||
Results of 42 BM samples that were reconstituted by injected fetal liver cells are shown.
Abbreviation: ND, not determined.
C1: IL-3 + IL-6 + GM-CSF + KL; C2: IL-3 + IL-6 + GM-CSF + KL + FL; C3: IL-6 + KL + FL.
Number of injected cells are indicated as the number of CD34++CD38−Lin− cells at the initiation of culture.
Contribution of fetal liver–derived cells was assessed based on the HLA alloantigen expression.
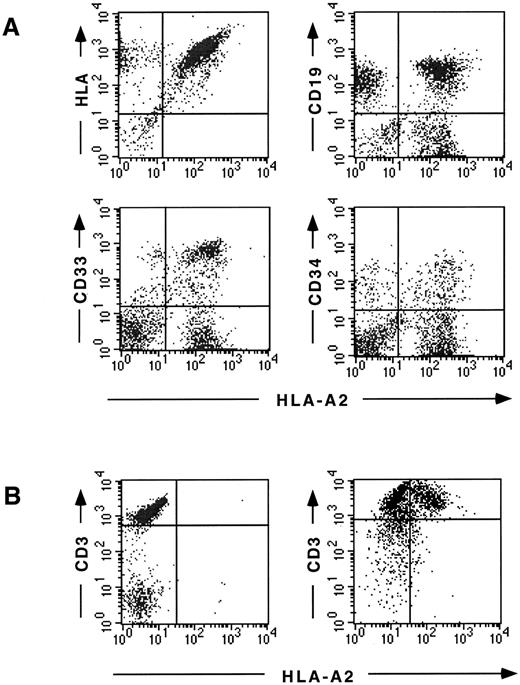
Flow cytometric detection of fetal liver donor–derived cells after reconstitution in SCID-hu mice. (A) BM cells recovered 8 weeks after injection. HLA-A2 positive, fetal liver donor–derived cells are identified in the HLA expressing human cell population (top left), CD19+ B cells (top right), CD33+ myeloid cells (bottom left), and CD34+ progenitor cells (bottom right). A smaller population of HLA-A2 negative, bone donor–derived cells is found in each population. (B) Thymocytes recovered 12 weeks after injection. HLA-A2 positive, fetal liver donor–derived cells can be clearly detected in the CD3high population (upper right quadrant) in the reconstituted thymus (right) but not in the uninjected thymus (left).
Flow cytometric detection of fetal liver donor–derived cells after reconstitution in SCID-hu mice. (A) BM cells recovered 8 weeks after injection. HLA-A2 positive, fetal liver donor–derived cells are identified in the HLA expressing human cell population (top left), CD19+ B cells (top right), CD33+ myeloid cells (bottom left), and CD34+ progenitor cells (bottom right). A smaller population of HLA-A2 negative, bone donor–derived cells is found in each population. (B) Thymocytes recovered 12 weeks after injection. HLA-A2 positive, fetal liver donor–derived cells can be clearly detected in the CD3high population (upper right quadrant) in the reconstituted thymus (right) but not in the uninjected thymus (left).
To evaluate the reconstitution efficacy of cultured progenitor cells, different doses of cells were injected in some experiments (Experiments 4 to 8). Although we used cell doses that corresponded to as low as 500 to 750 CD34++ CD38−Lin− cells, no clear dose effects were observed in the levels of contribution by fetal liver–derived cells in hematopoietic reconstitution (Table 1). In vivo expansion of injected fetal liver cells was evaluated. BM samples injected with high doses of cells (>1 × 105 total cells that were generated by >1,500 CD34++CD38−Lin− cells) and those with low doses of cells (<5 × 104 total cells that were generally generated by 500 to 750 CD34++CD38−Lin− cells) were analyzed separately (Table 2). Despite the large standard errors primarily caused by the variations in the size of bone grafts, these analyses provided valuable information in understanding the kinetics of BM reconstitution in this model. Shortly after injection (4 weeks), yields of fetal liver–derived cells were less than the numbers injected. This reduction in cellularity, however, was followed by increases in the fetal liver–derived cells after 7 weeks. At that time, the contribution of fetal liver–derived cells in the CD19+, CD33+, or CD34+ compartments increased, indicating that the injected progenitors actively proliferated and differentiated during 4 to 7 weeks.
In Vivo Expansion of Ex Vivo–Manipulated CD34++CD38−Lin− Fetal Liver Cells in the Human BM
| Weeks . | . | High Dose* . | Low Dose* . | ||
|---|---|---|---|---|---|
| . | . | C1† . | C2† . | C1† . | C2† . |
| 4 | Cell yield (×104) | 17.8 ± 3.2 (n = 4) | 34.6 ± 11.2 (n = 6) | ND | ND |
| (12.4-26.6) | (2.2-68.8) | ||||
| Fold expansion | 0.5 ± 0.1 (0.4-0.7) | 0.9 ± 0.3 (0.1-1.7) | ND | ND | |
| 7-11 | Cell yield (×104) | 64.5 ± 23.8 (n = 7) | 120.8 ± 23.6 (n = 9) | 54.3 ± 21.0 (n = 5) | 145.0 ± 50.3 (n = 3) |
| (5.0-181.4) | (4.1-225.3) | (12.6 ± 132.3) | (60.3-234.5) | ||
| Fold expansion | 2.1 ± 0.7 (0.5-4.5) | 5.0 ± 1.0 (0.1-8.7) | 14.1 ± 5.6 (2.5-33.1) | 57.5 ± 30.8 (15.1-117.3) | |
| Weeks . | . | High Dose* . | Low Dose* . | ||
|---|---|---|---|---|---|
| . | . | C1† . | C2† . | C1† . | C2† . |
| 4 | Cell yield (×104) | 17.8 ± 3.2 (n = 4) | 34.6 ± 11.2 (n = 6) | ND | ND |
| (12.4-26.6) | (2.2-68.8) | ||||
| Fold expansion | 0.5 ± 0.1 (0.4-0.7) | 0.9 ± 0.3 (0.1-1.7) | ND | ND | |
| 7-11 | Cell yield (×104) | 64.5 ± 23.8 (n = 7) | 120.8 ± 23.6 (n = 9) | 54.3 ± 21.0 (n = 5) | 145.0 ± 50.3 (n = 3) |
| (5.0-181.4) | (4.1-225.3) | (12.6 ± 132.3) | (60.3-234.5) | ||
| Fold expansion | 2.1 ± 0.7 (0.5-4.5) | 5.0 ± 1.0 (0.1-8.7) | 14.1 ± 5.6 (2.5-33.1) | 57.5 ± 30.8 (15.1-117.3) | |
The mean ± standard error of mean and the ranges are shown.
Abbreviation: ND, not determined.
High dose: >1 × 105 total cells (ranges: 1 - 6 × 105) that were generated from >1,500 CD34++CD38−Lin− cells; low dose: <5 × 104 cells (ranges: 1 - 5 × 104) that were generated by 500-750 CD34++CD38−Lin− cells, except for one sample received cells generated by 2,000 CD34++CD38−Lin− cells.
C1: IL-3 + IL-6 + GM-CSF + KL; C2: IL-3 + IL-6 + GM-CSF + KL + FL.
Although cells cultured under C2 exhibited consistently higher levels of in vivo cell expansion, the differences between C1 and C2 were not statistically significant. More interestingly, there were no significant differences in cell yields between BMs injected with high and low doses of cells. Yields of fetal liver–derived cells from BM injected with high doses of cells cultured either in C1 or C2 were 96.1 ± 17.8 × 104 (n = 16), whereas those injected with low doses were 88.3 ± 26.5 × 104 (n = 8). As a consequence, it was shown that higher levels of expansion were achieved when low number of cells were injected. Injections of cells generated by 500 to 750 CD34++CD38−Lin− cells resulted in 2.5- to 117.3-fold expansion, whereas those by more than 1,500 cells resulted in only 0.4- to 8.7-fold expansion. These results indicated that cells generated from 500 to 750 CD34++CD38−Lin− fetal liver cells during 5-days culture were sufficient to fully reconstitute the human hematopoietic activity in the bone grafts.
The potential to reconstitute the T-cell compartment was investigated using SCID-hu mice transplanted with human fetal thymus along with fetal liver under the kidney capsule.36 The level of reconstitution is indicated as the percentage of fetal liver–derived cells in the CD3high population, which expresses high levels of MHC Class I antigens (Fig 3B). All thymic grafts injected with cultured CD34++CD38−Lin− cells resulted in reconstitution by fetal liver–derived cells 6 to 16 weeks postinjection (Table 3). As illustrated by a series of experiments, the levels of reconstitution increased as a function of time.
T-Cell Reconstitution Potential of Ex Vivo–Manipulated CD34++CD38−Lin− Fetal Liver Cells
| Experiment No. . | Weeks . | Cytokine3-150 . | Injected Cells3-151 . | Reconstitution in CD3high Cells (%)3-152 . | Expression of . |
|---|---|---|---|---|---|
| . | . | . | . | . | β-Galactosidaseρ . |
| 1 | 6 | C1 | 8,400 | ND | ++ |
| C2 | 6,400 | 25.3 | ++ | ||
| 3 | 6 | C1 | 7,500 | 8.5 | +++ |
| C2 | 5,000 | 15.2 | +++ | ||
| 9 | 11 | C1 | 6,300 | 41.3 | ++ |
| C2 | 6,300 | 32.9 | ++ | ||
| 10 | 12 | C1 | 4,400 | 68.0 | + |
| C2 | 4,400 | 33.5 | + | ||
| 13 | 16 | C2 | 3,600 | 97.9 | ++ |
| C3 | 3,600 | ND | − |
| Experiment No. . | Weeks . | Cytokine3-150 . | Injected Cells3-151 . | Reconstitution in CD3high Cells (%)3-152 . | Expression of . |
|---|---|---|---|---|---|
| . | . | . | . | . | β-Galactosidaseρ . |
| 1 | 6 | C1 | 8,400 | ND | ++ |
| C2 | 6,400 | 25.3 | ++ | ||
| 3 | 6 | C1 | 7,500 | 8.5 | +++ |
| C2 | 5,000 | 15.2 | +++ | ||
| 9 | 11 | C1 | 6,300 | 41.3 | ++ |
| C2 | 6,300 | 32.9 | ++ | ||
| 10 | 12 | C1 | 4,400 | 68.0 | + |
| C2 | 4,400 | 33.5 | + | ||
| 13 | 16 | C2 | 3,600 | 97.9 | ++ |
| C3 | 3,600 | ND | − |
Abbreviation: ND, not determined.
See footnote in Table 1.
See footnote in Table 1.
Contribution of fetal liver–derived cells was assessed based on the HLA alloantigen expression in the CD3high population.
ρ Expression of the nls-LacZ gene was determined by X-gal staining of cytospin prepared cells. The levels of expression were scored as: −, no β-gal+ cells in the slide; +, 1 - 10 β-gal+ cells/slide; ++, 11 - 50 β-gal+ cells/slide; +++, >50 β-gal+ cells/slide.
Genetically modified fetal liver progenitor cells can reconstitute lymphoid, myeloid, and progenitor cell compartments. Expression of transduced nls-LacZ gene in recovered BM cells was investigated by the standard X-gal staining (Table 4). The results revealed that 28 out of 41 (68%) BM samples contained β-galactosidase positive (β-gal+) cells as analyzed in cytospin preparations. Of those, 3 samples contained greater than 50 β-gal+ cells, 10 contained 11 to 50 β-gal+ cells, and 15 contained 1 to 10 β-gal+ cells per slide that were prepared with 2.5 × 104 cells. Although percentages of fetal liver–derived cells increased after 7 weeks of incubation, the frequencies of β-gal+ cells decreased as a function of time, with less than 10 β-gal+ cells detected in 6 out of 16 BM samples analyzed, and no β-gal+ cells found in 10 BM samples after 9 weeks. Cells with lymphoid morphology and cells with the features of varying stages of myeloid differentiation were observed to express the nls-LacZ gene (Fig 4A). The X-gal reaction product was localized in the nuclear and perinuclear space, a characteristic feature of the transduced E coli β-galactosidase gene caused by association with the nuclear localization signal sequence of SV40.39
Expression of the nls-LacZ Gene in the Reconstituted BM Cells
| Experiment No. . | Weeks . | Cytokine4-150 . | Injected Cells4-151 . | % Reconstitution‡ . | β-Galactosidase Expression . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Cytospinρ . | HPP-CFC (%) . | LPP-CFC (%) . | CFC-MC (%)¶ . |
| 1 | 4 | C1 | 8,400 | 54 | +++ | 0/6 | 6/153 (3.9) | 15/209 (7.2) |
| C1 | 8,400 | 55 | +++ | 3/24 (12.5) | 17/226 (7.5) | 30/246 (12.2) | ||
| C2 | 6,400 | 26 | ++ | 0/23 | 5/121 (4.1) | 23/260 (8.9) | ||
| C2 | 6,400 | 29 | ++ | 3/13 (23.1) | 13/130 (10) | 32/263 (12.2) | ||
| C2 | 6,400 | 47 | ++ | 1/10 (10) | 3/81 (3.7) | 9/177 (5) | ||
| 2 | 4 | C1 | 13,800 | 35 | − | ND | ND | 0/341 |
| C2 | 13,800 | 48 | − | 0/15 | 1/223 (0.5) | 1/146 (0.7) | ||
| 7 | C2 | 13,800 | 99 | + | 0/55 | 19/491 (3.9) | 12/244 (4.9) | |
| 3 | 4 | C1 | 7,500 | 43 | ++ | 11/55 (20) | 66/361 (18.3) | 42/215 (19.5) |
| C2 | 5,000 | 29 | ++ | 4/45 (8.9) | 22/215 (10.2) | 24/241 (10) | ||
| C2 | 5,000 | 50 | +++ | 13/94 (13.8) | 59/337 (17.5) | 34/309 (11) | ||
| 4 | 8 | C1 | 5,400 | 66 | + | 0/22 | 4/182 (2.2) | 1/153 (0.7) |
| C1 | 5,400 | 26 | ++ | 1/15 (6.7) | 22/217 (10.1) | 12/211 (5.7) | ||
| C1 | 600 | 42 | + | 1/32 (3.1) | 3/151 (2.0) | 4/22 (1.8) | ||
| C1 | 600 | 75 | + | 0/31 | 0/110 | 0/147 | ||
| C2 | 5,400 | 77 | + | 0 | 0/39 | 0/20 | ||
| 5 | 8 | C1 | 7,200 | 75 | + | 6/28 (21.4) | 30/260 (11.5) | 6/302 (2) |
| C1 | 7,200 | 93 | + | 2/26 (7.7) | 24/409 (5.9) | 9/383 (2.4) | ||
| C1 | 1,500 | 94 | ++ | 2/39 (5.1) | 23/271 (8.5) | 9/242 (3.7) | ||
| C1 | 750 | 98 | + | 6/40 (15) | 28/281 (10) | 21/308 (6.8) | ||
| C1 | 750 | 91 | ++ | 0/36 | 9/275 (3.3) | 3/261 (1.2) | ||
| C2 | 7,200 | 98 | + | 3/41 (7.3) | 48/332 (14.5) | 12/278 (4.3) | ||
| C2 | 7,200 | 91 | ++ | 0/54 | 31/363 (8.5) | 12/303 (4) | ||
| C2 | 1,500 | 87 | ++ | 1/42 (2.4) | 23/363 (6.4) | 22/298 (7.4) | ||
| C2 | 750 | 86 | − | 0/38 | 35/464 (7.5) | 5/283 (1.8) | ||
| 7 | 9 | C2 | 17,600 | 92 | − | 5/112 (4.5) | 17/377 (4.5) | 6/181 (3.3) |
| C2 | 2,000 | 74 | − | 0/90 | 3/489 (0.6) | 1/156 (0.6) | ||
| C3 | 17,600 | 51 | − | 0/27 | 2/500 (0.4) | 0/199 | ||
| 8 | 10 | C1 | 4,500 | 90 | − | ND | ND | 3/64 (4.7) |
| C1 | 500 | 74 | + | 0/4 | 1/62 (1.6) | 1/47 (2) | ||
| C2 | 4,500 | 99 | + | 0/9 | 3/119 (2.5) | 2/60 (3.3) | ||
| C2 | 500 | 89 | + | 0/3 | 0/79 | 0/48 | ||
| 9 | 11 | C2 | 6,300 | 76 | − | ND | ND | 6/90 (6.7) |
| 10 | 14 | C1 | 4,400 | ND | ND | ND | ND | 1/16 (6.3) |
| C1 | 4,400 | ND | + | 1/51 (2) | 2/302 (0.7) | 4/245 (1.6) | ||
| C2 | 4,400 | ND | + | 0/32 | 10/430 (2.3) | 1/211 (2.8) | ||
| C2 | 4,400 | ND | + | 0/18 | 3/290 (1) | 1/73 (1.4) | ||
| 11 | 14 | C2 | 10,600 | 96 | − | 0/6 | 2/105 (1.9) | 0/81 |
| 12 | 17 | C2 | 4,300 | 99 | − | ND | ND | 2/114 (1.8) |
| C2 | 4,300 | 97 | − | 0/21 | 0/126 | 0/127 | ||
| C3 | 13,100 | 95 | − | 0/18 | 1/230 (0.4) | 3/225 (1.3) | ||
| C3 | 4,300 | 95 | − | ND | ND | 0/78 | ||
| Experiment No. . | Weeks . | Cytokine4-150 . | Injected Cells4-151 . | % Reconstitution‡ . | β-Galactosidase Expression . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Cytospinρ . | HPP-CFC (%) . | LPP-CFC (%) . | CFC-MC (%)¶ . |
| 1 | 4 | C1 | 8,400 | 54 | +++ | 0/6 | 6/153 (3.9) | 15/209 (7.2) |
| C1 | 8,400 | 55 | +++ | 3/24 (12.5) | 17/226 (7.5) | 30/246 (12.2) | ||
| C2 | 6,400 | 26 | ++ | 0/23 | 5/121 (4.1) | 23/260 (8.9) | ||
| C2 | 6,400 | 29 | ++ | 3/13 (23.1) | 13/130 (10) | 32/263 (12.2) | ||
| C2 | 6,400 | 47 | ++ | 1/10 (10) | 3/81 (3.7) | 9/177 (5) | ||
| 2 | 4 | C1 | 13,800 | 35 | − | ND | ND | 0/341 |
| C2 | 13,800 | 48 | − | 0/15 | 1/223 (0.5) | 1/146 (0.7) | ||
| 7 | C2 | 13,800 | 99 | + | 0/55 | 19/491 (3.9) | 12/244 (4.9) | |
| 3 | 4 | C1 | 7,500 | 43 | ++ | 11/55 (20) | 66/361 (18.3) | 42/215 (19.5) |
| C2 | 5,000 | 29 | ++ | 4/45 (8.9) | 22/215 (10.2) | 24/241 (10) | ||
| C2 | 5,000 | 50 | +++ | 13/94 (13.8) | 59/337 (17.5) | 34/309 (11) | ||
| 4 | 8 | C1 | 5,400 | 66 | + | 0/22 | 4/182 (2.2) | 1/153 (0.7) |
| C1 | 5,400 | 26 | ++ | 1/15 (6.7) | 22/217 (10.1) | 12/211 (5.7) | ||
| C1 | 600 | 42 | + | 1/32 (3.1) | 3/151 (2.0) | 4/22 (1.8) | ||
| C1 | 600 | 75 | + | 0/31 | 0/110 | 0/147 | ||
| C2 | 5,400 | 77 | + | 0 | 0/39 | 0/20 | ||
| 5 | 8 | C1 | 7,200 | 75 | + | 6/28 (21.4) | 30/260 (11.5) | 6/302 (2) |
| C1 | 7,200 | 93 | + | 2/26 (7.7) | 24/409 (5.9) | 9/383 (2.4) | ||
| C1 | 1,500 | 94 | ++ | 2/39 (5.1) | 23/271 (8.5) | 9/242 (3.7) | ||
| C1 | 750 | 98 | + | 6/40 (15) | 28/281 (10) | 21/308 (6.8) | ||
| C1 | 750 | 91 | ++ | 0/36 | 9/275 (3.3) | 3/261 (1.2) | ||
| C2 | 7,200 | 98 | + | 3/41 (7.3) | 48/332 (14.5) | 12/278 (4.3) | ||
| C2 | 7,200 | 91 | ++ | 0/54 | 31/363 (8.5) | 12/303 (4) | ||
| C2 | 1,500 | 87 | ++ | 1/42 (2.4) | 23/363 (6.4) | 22/298 (7.4) | ||
| C2 | 750 | 86 | − | 0/38 | 35/464 (7.5) | 5/283 (1.8) | ||
| 7 | 9 | C2 | 17,600 | 92 | − | 5/112 (4.5) | 17/377 (4.5) | 6/181 (3.3) |
| C2 | 2,000 | 74 | − | 0/90 | 3/489 (0.6) | 1/156 (0.6) | ||
| C3 | 17,600 | 51 | − | 0/27 | 2/500 (0.4) | 0/199 | ||
| 8 | 10 | C1 | 4,500 | 90 | − | ND | ND | 3/64 (4.7) |
| C1 | 500 | 74 | + | 0/4 | 1/62 (1.6) | 1/47 (2) | ||
| C2 | 4,500 | 99 | + | 0/9 | 3/119 (2.5) | 2/60 (3.3) | ||
| C2 | 500 | 89 | + | 0/3 | 0/79 | 0/48 | ||
| 9 | 11 | C2 | 6,300 | 76 | − | ND | ND | 6/90 (6.7) |
| 10 | 14 | C1 | 4,400 | ND | ND | ND | ND | 1/16 (6.3) |
| C1 | 4,400 | ND | + | 1/51 (2) | 2/302 (0.7) | 4/245 (1.6) | ||
| C2 | 4,400 | ND | + | 0/32 | 10/430 (2.3) | 1/211 (2.8) | ||
| C2 | 4,400 | ND | + | 0/18 | 3/290 (1) | 1/73 (1.4) | ||
| 11 | 14 | C2 | 10,600 | 96 | − | 0/6 | 2/105 (1.9) | 0/81 |
| 12 | 17 | C2 | 4,300 | 99 | − | ND | ND | 2/114 (1.8) |
| C2 | 4,300 | 97 | − | 0/21 | 0/126 | 0/127 | ||
| C3 | 13,100 | 95 | − | 0/18 | 1/230 (0.4) | 3/225 (1.3) | ||
| C3 | 4,300 | 95 | − | ND | ND | 0/78 | ||
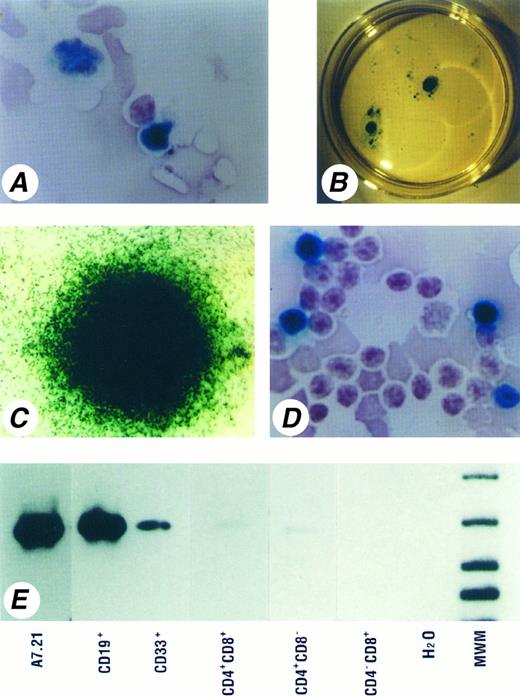
Integration and expression of the nls-LacZ gene in multilineage cells. (A) The nls-LacZ gene expressing BM cells recovered 4 weeks after injection. Cells with lymphoid (lower right) and myeloid (upper left) morphology are β-gal+. Note the nuclear localization of the β-gal signal. (Original magnification, × 200). (B) Macroscopic view of β-gal+ colonies derived from BM cells recovered 4 weeks after injection. The nls-LacZ expressing HPP-CFC and LPP-CFC are evident in a 60-mm culture dish. (C) Microscopic view of one of the nls-LacZ–expressing HPP-CFC shown in (B). (Original magnification, × 5). (D) The nls-LacZ–expressing thymocytes. Approximately 10% of the cells express nls-LacZ in this sample obtained 8 weeks after injection. (Original magnification, × 200). (E) Southern blot of genomic PCR products from FACS-sorted cell populations. Both CD19+ B cells and CD33+ myeloid cells isolated from a BM 14 weeks after injection (Experiment 11, C2 shown in Table 5) showed strong bands of 470 bp size. In thymocytes recovered 11 weeks after injection, faint bands can be detected in the CD4+CD8+ and CD4+CD8− subsets, but not in the CD4−CD8+ subset. Reanalysis of sorted cells showed a purity of 99.8% for CD19+ cells, 99.5% for CD33+ cells, 99.0% for CD4+CD8+ cells, 99.4% for CD4+CD8− cells, and 98.9% for CD4−CD8+ cells.
Integration and expression of the nls-LacZ gene in multilineage cells. (A) The nls-LacZ gene expressing BM cells recovered 4 weeks after injection. Cells with lymphoid (lower right) and myeloid (upper left) morphology are β-gal+. Note the nuclear localization of the β-gal signal. (Original magnification, × 200). (B) Macroscopic view of β-gal+ colonies derived from BM cells recovered 4 weeks after injection. The nls-LacZ expressing HPP-CFC and LPP-CFC are evident in a 60-mm culture dish. (C) Microscopic view of one of the nls-LacZ–expressing HPP-CFC shown in (B). (Original magnification, × 5). (D) The nls-LacZ–expressing thymocytes. Approximately 10% of the cells express nls-LacZ in this sample obtained 8 weeks after injection. (Original magnification, × 200). (E) Southern blot of genomic PCR products from FACS-sorted cell populations. Both CD19+ B cells and CD33+ myeloid cells isolated from a BM 14 weeks after injection (Experiment 11, C2 shown in Table 5) showed strong bands of 470 bp size. In thymocytes recovered 11 weeks after injection, faint bands can be detected in the CD4+CD8+ and CD4+CD8− subsets, but not in the CD4−CD8+ subset. Reanalysis of sorted cells showed a purity of 99.8% for CD19+ cells, 99.5% for CD33+ cells, 99.0% for CD4+CD8+ cells, 99.4% for CD4+CD8− cells, and 98.9% for CD4−CD8+ cells.
Most marrow samples (36 out of 42; 86%) contained substantial numbers of β-gal+ clonogenic cells. A significant proportion (2.0% to 23.1%) of HPP-CFC expressed nls-LacZ (Fig 4B and C) for as long as 14 weeks after injection. Although fewer β-gal+ HPP-CFC were detected at the later time points (after 10 weeks), it should be noted that the numbers of HPP-CFC obtained in these experiments were low and therefore did not allow the reliable detection of β-gal+ HPP-CFC at a frequency of less than 3%. LacZ expression was also observed in 0.4% to 18.3% and 0.6% to 19.5% of LPP-CFC and CFC-MC, respectively. The number of CFC-MC represents the sum of all types of colonies formed in methylcellulose culture, including CFU-GM, CFU-G, CFU-M, and BFU-E. Expression of the nls-LacZ gene was observed in all types of colonies. Although there was a tendency that the percentages of β-gal+ clonogenic cells decreased after 8 weeks, β-gal+ clonogenic cells could be consistently recovered up to 17 weeks after injection.
X-gal staining performed on cytospin preparations of thymocytes revealed that 9 out of 10 grafts contained β-gal+ lymphoid cells (Fig 4D), although the levels of reconstitution by nls-LacZ–expressing cells varied between experiments (Table 3). Occasionally cells with macrophage-like morphology were observed to be positive for nuclear β-gal expression among thymocytes (data not shown).
Reconstitution of B-lymphoid as well as myeloid compartments by genetically modified progenitor cells was further confirmed using FACS-sorted cell populations. CD19+ B cells and CD33+ myeloid cells purified from reconstituted BM samples showed integration of the nls-LacZ gene as demonstrated by genomic PCR analysis (Fig 4E) and/or expression of the nls-LacZ gene as detected by X-gal staining (Table 5). One sorting experiment with thymocytes showed the reconstitution by nls-LacZ–expressing cells from CD4+CD8+ double-positive through CD4+ or CD8+ single-positive stages of thymocytes. These results were further confirmed by the detection of the transgene with genomic PCR in the CD4+CD8+ and the CD4+CD8− subsets. The fact that the transgene and its expression could not always be detected by both PCR and cytochemistry is likely because of the low number of sorted cells available for both assays.
Integration and Expression of the nls-LacZ Gene in the FACS-Sorted Cell Populations
| Experiment No. . | Tissue . | Weeks . | Cytokine5-150 . | Injected Cells5-151 . | Genomic PCR nls-LacZ/nls-LacZ Expressionρ . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | CD19+ . | . | . | ||
| . | . | . | . | . | . | CD33+ . | . | . | |
| . | . | . | . | . | . | . | . | . | . |
| 14 | BM | 10 | C2 | 4,500 | +/+ | ||||
| −/+ | |||||||||
| C2 | 500 | +/− | |||||||
| −/+ | |||||||||
| 15 | BM | 11 | C2 | 6,300 | +/+ | ||||
| ND/+ | |||||||||
| 16 | BM | 14 | C1 | 8,100 | −/ND | ||||
| −/ND | |||||||||
| C2 | 8,100 | +/+ | |||||||
| +/ND | |||||||||
| C2 | 8,100 | +/+ | |||||||
| −/ND | |||||||||
| CD4+ CD8+ | CD4+ CD8− | CD4− CD8+ | |||||||
| Experiment No. . | Tissue . | Weeks . | Cytokine5-150 . | Injected Cells5-151 . | Genomic PCR nls-LacZ/nls-LacZ Expressionρ . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | CD19+ . | . | . | ||
| . | . | . | . | . | . | CD33+ . | . | . | |
| . | . | . | . | . | . | . | . | . | . |
| 14 | BM | 10 | C2 | 4,500 | +/+ | ||||
| −/+ | |||||||||
| C2 | 500 | +/− | |||||||
| −/+ | |||||||||
| 15 | BM | 11 | C2 | 6,300 | +/+ | ||||
| ND/+ | |||||||||
| 16 | BM | 14 | C1 | 8,100 | −/ND | ||||
| −/ND | |||||||||
| C2 | 8,100 | +/+ | |||||||
| +/ND | |||||||||
| C2 | 8,100 | +/+ | |||||||
| −/ND | |||||||||
| CD4+ CD8+ | CD4+ CD8− | CD4− CD8+ | |||||||
| 9 | Thymus | 11 | C1 | 6,300 | +/+ | +/+ | −/+ |
| C2 | 6,300 | +/− | +/+ | −/+ |
| 9 | Thymus | 11 | C1 | 6,300 | +/+ | +/+ | −/+ |
| C2 | 6,300 | +/− | +/+ | −/+ |
In summary, we showed that ex vivo–manipulated CD34++CD38−Lin− cells isolated from fetal liver efficiently reconstituted primitive progenitors, committed progenitors of myeloerythroid lineages, and mature myeloid cells, B cells, and T cells with substantial contribution from gene transduced cells. Furthermore, reconstitution could be achieved by cells generated from as few as 500 to 750 CD34++CD38−Lin− cells.
DISCUSSION
In the present study, we introduced a foreign gene into purified CD34++CD38−Lin− cells derived from human fetal liver. The CD34++CD38−Lin− cells isolated from various sources were shown to be highly enriched for stem cell activity8,23-26 and represent only approximately 1% of total CD34+ cells in the fetal liver.26 By a standard transduction protocol, efficient gene transduction was achieved as evidenced by expression of the transduced nls-LacZ gene in 5% to 10% of clonogenic progenitors including HPP-CFC, LPP-CFC, and CFC-MC. It was shown that the level of expression of amphotropic retroviral receptors in the CD34+CD38− population is significantly lower than the CD34+CD38+ population.41 The frequency of cycling cells is also lower in the former population.8,25 Taking these biological features of the CD34+CD38− cells into consideration, the level of gene transduction achieved in this study is quite efficient, because it is only slightly lower than those reported for the CD34+ population.14,16,18 Qualitative differences in the primitive progenitor cells derived from ontogenically different sources have been documented. Primitive progenitors of fetal liver origin were shown to possess an approximately 10-fold higher proliferative potential as compared with those isolated from CB after a 7-day culture.29 Cloning efficiencies of singly sorted CD34+CD38− cells isolated from fetal liver were fourfold higher than those from CB.42 It was also shown that most fetal liver progenitors undergo multiple cell divisions during 7-day culture while maintaining the CD34 expression.29 In the present study, we also observed that fetal liver CD34++CD38−Lin− cells achieved on average 50-fold cell expansion during 5-day culture, whereas 70% of the progeny maintained CD34+CD38− phenotype. The vigorous proliferation of fetal liver progenitors may contribute, at least in part, to the high levels of gene transduction achieved in this study.
After in vivo reconstitution, transgene expression was observed in multiple lineages, including T cells, B cells, myeloid cells, and clonogenic progenitor cells, for over 4 months. Results of cell dose experiments showed that the cells generated from only 500 to 750 CD34++CD38−Lin− cells were sufficient to reconstitute full spectrum of hematopoiesis with a substantial contribution from genetically modified cells. Interestingly, β-gal+ HPP-CFC were recovered from reconstituted BM up to 14 weeks after injection, suggesting the possibility of gene transduction into the progenitors that can give rise to HPP-CFC in vivo. Because injected cells contained progenitors that have differentiated during ex vivo manipulation, and the numbers of progenitors injected in most of the experiments described were more than sufficient to reconstitute the bone grafts, it is possible that multiple primitive and committed progenitors that were gene transduced contributed to the reconstitution of multiple lineages. Further studies, such as the determination of proviral integration sites,20 43 will be necessary to investigate the multilineage potential of progenitors as well as the numbers of clones contributed for the reconstitution.
The injection of residual mitomycin-treated packaging cells might also cause infection of multilineage human hematopoietic cells in animals. However, this possibility is extremely low because injection of genetically modified CD34+ peripheral blood cells, using the same protocol, resulted in a very low frequency of β-gal+ cells (Humeau et al, manuscript in preparation). Furthermore, no evidence of persistence of the packaging cells was reported in previous clinical studies performed with cocultured cells.17
A recent study in which reconstitution potential of genetically-modified human progenitor cells was evaluated in nonobese diabetic mice with severe combined immunodeficiency disease (NOD/SCID mice) showed that only a very few genetically marked clonogenic progenitor cells could be recovered from the reconstituted mice at 6 weeks after reconstitution.22 Thus, it was concluded that pluripotent human progenitor cells that were capable of repopulating NOD/SCID mice were rarely transduced with retroviruses. In contrast, our results showed that gene transduction into primitive progenitors that can give rise to clonogenic progenitor cells in vivo for up to 4 months could be achieved. Several factors may account for the different outcome observed in these two studies. First, the source of progenitor cells used for the experiments may significantly affect the reconstitution potential as well as gene transduction efficiency. Although the CB progenitors were reported to be more efficiently gene transduced than those in adult BM or PB,44 retroviral gene transduction into CD34+CD38− fraction from CB was still not efficient.27 A significantly higher proliferative potential of fetal liver progenitors that were used in our study as compared with that of progenitors from CB that were used in the NOD/SCID study is likely to contribute to the observed differences. Secondly, we used highly purified (>97%) CD34++CD38−Lin− cells for gene transduction, whereas the Lin− fraction was used in the other study as a source of CD34+-enriched cells. A 100-fold enrichment for the primitive progenitor cells increased the multiplicity of infection 100-fold, and thereby may increase the gene transduction efficiency. Finally, animal models used to read out the reconstitution potential of genetically modified cells are different. Both the NOD/SCID model45,46 and the SCID-hu model38,47 48 have been used to investigate the biology of human hematopoietic cells in vivo. Human hematopoietic cells reside in murine hematopoietic organs in the NOD/SCID model, and therefore proliferation and differentiation of human progenitor cells largely depend on the molecules provided by murine environment. In contrast, human hematopoietic progenitor cells proliferate, differentiate, and function in the hematopoietic microenvironment of human origin in the SCID-hu model. Thus, the biological differences of the animal models used in each study may significantly affect the outcome.
The difference in the frequencies of nls-LacZ expression observed in clonogenic progenitors (1% to 10%) and in mature hematopoietic cells (1 in 1,000 to 10,000), especially at later time points of reconstitution (>8 weeks), is intriguing. A very similar finding was observed in the study in which an adenosine deaminase (ADA) gene and a neomycin resistance gene were retrovirally transduced into CD34+ cells isolated from the autologous CB of ADA deficient patients.18 In this clinical study, vector-containing cells were found at frequencies between 1/3,000 and 1/100,000 in PB and in BM, contrasting to the relatively high (4% to 6%) frequency of CFC that contained the vector. Although a host immune response was postulated as a cause of decreased gene expression in another study,49 it cannot explain our results, because SCID mice lack a functional immune system. A more conceivable explanation is that the retroviral long terminal repeat (LTR) is inactivated by hypermethylation, which results in suppression of transgene expression50 in more differentiated cells. Alternatively, transgene expression may be more active in proliferating cells, such as those that are forming colonies in vitro.
An advantage of the nls-LacZ gene used in this study is its easy detection, which enabled us to investigate gene expression in colony assays in situ, as well as in histological analysis. The observed hematopoietic reconstitution mediated by transduced cells suggests that the nls-LacZ gene does not disrupt hematopoietic differentiation. The results are, in general, consistent with those obtained from clinical marking studies with other genetic markers, such as the commonly used neoR gene.14-16 Therefore, the nls-LacZ gene will be a useful genetic marker for the hematopoietic system.
Because FL has been shown to preferentially act on the primitive progenitors32-34 and to support the long-term reconstitution potential during cultures for gene transduction in the absence of stromal cells,35 we investigated the effect of FL on gene transduction efficiency using both in vitro and in vivo assays. There were no significant differences in the level or longevity of reconstitution. Cell yields of BM samples injected with cells cultured in the presence of FL (C2) consistently gave rise to higher levels of in vivo cellular expansion as compared with those cultured in the absence of FL (C1), but the differences were not statistically significant. No significant differences in reconstitution potential were observed in cell dose experiments; however, it should be noted that the lowest number of cells injected was already sufficient to reconstitute the bone grafts for at least 3 to 4 months. The effects of FL, therefore, may appear if cell numbers are further decreased and/or reconstitution is observed for longer periods of time. Alternatively, the effects of FL that could partially replace the supportive roles provided by stromal cells35 might be masked in our study because gene transduction was conducted in the presence of the fibroblastic packaging cells, which may produce FL.
In conclusion, we showed that gene transfer into highly enriched primitive progenitors isolated from fetal liver could be achieved through retroviral infection. Further studies are required to elucidate the conditions that enable efficient gene transduction into primitive progenitors of nonfetal origin. The SCID-hu mouse model provides an ideal tool to optimize conditions for gene transfer. Evaluation of the effects of elements that may influence on the efficacy of gene therapy, such as cytokine combinations and the construction of vectors, on the basis of in vivo reconstitution potential will provide clinically relevant information.
ACKNOWLEDGMENT
We thank N. Carballido-Perrig, S. Antonenko, and C. Maroc for excellent technical assistance; the FACS facility and the Animal facility in DNAX; Dr J.E. de Vries for critical reading of the manuscript; and Drs O. Danos, L. Cohen, and R. Mulligan at Somatix Therapy Co.
Supported by grants from CNAMTS-INSERM (to C.C.), the Comité des Bouches-du-Rhône de la Ligue contre le Cancer (to C.C.), the Ministère de la Recherche et de l'Enseignement Supérieur (to L.H.), GEFLUC (to L.H.), and the Philippe Fondation Inc (France) (to L.H.). DNAX Resrearch Institute is supported by the Schering-Plough Corporation.
Address reprint requests to Reiko Namikawa, MD, PhD, Human Immunolgy Department, DNAX Research Institute, 901 California Ave, Palo Alto, CA 94304.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal