Abstract
Activation of the mitogen-activated protein (MAP) kinase pathway has been associated with both cell proliferation and differentiation. Constitutively activated forms of Mek (MAP kinase/Erk kinase) and Erk (MAP kinase) have been previously shown capable of inducing differentiation or proliferation in nonhematopoietic cells. To specifically examine the role of Erk activation in megakaryocytic growth and development, we activated the MAP kinase pathway by the transfection of constitutively activated Mek or Erk cDNA into a human megakaryoblastic cell line, CMK, by electroporation. The CMK transfectant clones that expressed constitutively activated Mek or Erk showed morphologic changes of differentiation. Transfected cells also showed expression of mature megakaryocytic cell surface markers. The MAP kinase pathway was also activated by treatment of the hematopoietic cells with a cytokine that activates Erk. The treatment of CMK cells with stem cell factor (SCF ) caused MAP kinase activation and induced differentiation by the expression of mature megakaryocytic cell surface markers. The effects of the SCF treatment were inhibited by pretreatment with a specific inhibitor of the MAP kinase pathway, PD98059. In this report, we conclude that activation of the MAP kinase pathway was both necessary and sufficient to induce differentiation in this megakaryoblastic cell line.
SIGNALS TRANSMITTED through receptor tyrosine kinases (RTKs) regulate cell proliferation and differentiation.1 One downstream event following the interaction of RTKs with certain growth factors, such as epidermal growth factor (EGF ), nerve growth factor (NGF ), and stem cell factor (SCF ), is the activation of a class of intracellular protein serine/threonine kinases termed mitogen-activated protein (MAP) kinases, also known as extracellular signal-regulated kinases (Erks).2-4 Activation of Erk involves the phosphorylation of two crucial tyrosine and threonine amino acids, and one enzyme responsible for Erk phosphorylation is Mek (MAP kinase/Erk kinase).5-7 Mek is activated by c-Raf through phosphorylation of specific serine amino acids.8-10 This cascade of phosphorylation from c-Raf to Erk can lead to translocation of Erk to the nucleus and result in the phosphorylation of certain transcription factors such as Elk-1.11 12 Erk activation may be a crucial step that links an extracellular stimulus to transcriptional activation and signals a cell to differentiate or proliferate.
Activation of the MAP kinase pathway has been associated with both cell proliferation and differentiation in nonhematopoietic cells, and it appears that the duration of Erk activation can determine cell fate.4 For example, NGF treatment of PC12 cells, a pheochromocytoma cell line, induces sustained Erk activation associated with neurite outgrowth and cessation of cell division. In contrast, the treatment of PC12 cells with EGF induces transient Erk activation and cell proliferation.4 The MAP kinase pathway can be activated in cells indirectly by treatment with cytokines or directly through the expression of activated kinases. Constitutively activated Mek mutants have been previously described that phosphorylate Erk at a rate greater than 100 times that of WT Mek.13-15 One mutation, DE Mek, encodes two acidic amino acid substitutions [Asp218 Glu222] for crucial serine residues.15 In addition, an Erk mutation thought to lead to constitutive activation was identified by a mutagenesis screen in Drosophila.16 This Erk mutation, D334N, restored a normal phenotype in the receptor tyrosine kinase deficient sevenless Drosophila. The mechanism of activation is unknown in this Erk mutant.
Direct evidence linking sustained Erk activation to PC12 differentiation has been reported. Microinjection of DE Mek cDNA into PC12 cells induces differentiation without the addition of growth factors.13,14 Also, microinjection of the D334N Erk RNA induces mesoderm differentiation in Xenopus.17 The mechanism by which sustained Erk activation leads to differentiation in PC12 cells, whereas transient Erk activation causes cell proliferation, is unclear. However, the cellular response to sustained or transient Erk activation differs in other cell lines. For example, sustained activation of the Erk pathway induces proliferation in NIH 3T3 cells. Transfection of constitutively activated DE Mek cDNA into NIH 3T3 cells induced morphologic transformation of the transfected cells. These Mek transformed cells were also capable of anchorage-independent growth in soft agar and formed tumors when injected into nude mice.13 14 Thus, activation of the MAP kinase pathway is sufficient to induce cellular changes in many cell types. However, the effects on differentiation or proliferation are cell type-specific.
The role of the MAP kinase pathway in hematopoietic cells in regard to cell fate is not well-defined. Erk activation has been linked to hematopoietic cell proliferation, differentiation, and cell survival. Several hematopoietic growth factors signal through RTKs and activate the MAP kinase pathway. For instance, the interaction of SCF with c-kit plays a crucial role in the development of multiple hematopoietic lineages,18-20 and c-kit activation leads to rapid activation of Erk21,22 when stimulated by SCF. Another growth factor, granulocyte-colony stimulating factor (G-CSF ), stimulates a proliferative response that is linked to rapid activation of Erk.23 Previous studies have also shown that activation of the MAP kinase pathway is essential in thymocyte differentiation.24 25 Taken together, this evidence suggests that Erk activation could play a crucial role in determining cellular fate in certain hematopoietic cells, but the role of this pathway in determining differentiation versus proliferation may vary between cell lineages.
To directly examine the role of the MAP kinase pathway in megakaryocytic development, we used a human megakaryoblastic cell line, CMK, which was established from a patient with acute megakaryoblastic leukemia.26 Previous studies have shown that CMK cells differentiate morphologically when stimulated with phorbol, 12-myristate, 13-acetate (PMA).26,27 PMA has also been shown to be a strong activator of Erk in T-cell lines.28,29 We introduced cDNAs expressing either Mek13-15 or Erk16 17 that encode a constitutively active protein into CMK cells. Expression of either constitutively activated Mek or Erk in these transfected cells was associated with morphologic differentiation and the expression of mature megakaryocytic cell surface antigens. The differentiation of these cells was associated with in vitro evidence of increased Erk activity. The MAP kinase pathway was also activated in CMK cells by the treatment with SCF. Treatment of CMK cells with SCF induced megakaryocytic differentiation, measured by the expression of mature megakaryocytic cell surface markers. The activation of MAP kinase was significantly inhibited by pretreating CMK cells with an inhibitor of Mek, PD98059. The treatment with PD98059 also inhibited the differentiation effects of SCF. From these studies, we conclude that activation of the MAP kinase pathway was necessary and sufficient to induce megakaryocytic differentiation of CMK cells.
MATERIALS AND METHODS
Cell culture. CMK cells26 (a gift from T. Sato, Chiba, Japan) and their subclones were cultured in RPMI (GIBCO-BRL, Gaithersburg, MD) medium with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and 1% penicillin and streptomycin (P/S) (GIBCO-BRL). Cells were grown at 37°C in a 10% CO2 incubator, subcultured every 3 to 4 days and split at a 1:10 dilution. Cells were treated with varying concentrations of PMA (Calbiochem, San Diego, CA) from 10 ng/mL to 1 μg/mL for 30 minutes to 72 hours. Cells were treated with the Mek inhibitor PD98059 (New England Biolabs, Beverly, MA) to a final concentration of 100 μmol/L during the indicated time course experiments. Cytospins were made using 5 × 104 cells per slide.
Generation of cells expressing mutant Mek and Erk. Plasmids expressing Mek mutants were constructed as follows: Apa I/BamHI fragments of wild-type (WT) Mek and constitutively activated DE Mek mutant were excised from pcDNA/Amp15 (a gift of R.L. Erikson, Cambridge, MA) and inserted into pcDNA3 (Invitrogen, San Diego, CA). We generated a constitutively activated D330N Erk mutant16,17 by subcloning WT Erk (a gift of R.L. Erikson) into pGEX-3X (Pharmacia Biotech, Piscataway, NJ ) and creating a site-specific mutant using the Sculptor in vitro mutagenesis system according to the manufactures instructions (Amersham, Arlington Heights, IL). D330N Erk was generated by using the primer CTACGATCCGACAAATGAGCCAGTGGCC, which encodes a substitution of wild type aspartate330 for asparagine330. The Not I fragment of pGEX-3X:WT Erk and pGEX-3X:D330N Erk were subsequently subcloned into pcDNA3. An adapter with the sequence of GATCCTCTAGACCACCATGGGGG containing an Xba I site, an ATG site, and a sequence for improved translation was added to pcDNA3 at the BamHI/EcoRI restriction sites.30
The plasmids were introduced into CMK cells by electroporation of 5 to 20 μg of linearized plasmid DNA, 100 μg of salmon sperm carrier DNA, and CMK cells at a concentration of 1 to 2 × 107 cells in 0.6 mL in Hanks' buffered salt solution (HBSS) using a Gene Pulser (Bio-Rad Laboratories, Richmond, CA). Cells were electroporated at 220 mV, 960 μF capacitance, and immediately resuspended in RPMI/10% FBS plus 1% P/S. The CMK cells were grown overnight and viable mononuclear cells were isolated using Ficoll-Paque (Pharmacia Biotech), washed once in media, and then resuspended in RPMI/10% FBS/1% P/S containing a final concentration of 1.0 mg/mL G418 (GIBCO-BRL). Cells were plated in 96-well plates with 200 μL per well at a concentration of 5 × 104 cells/mL. Viable cells were identified in five to 10 of the wells per plate within 2 to 3 weeks, subcultured, and maintained in 0.5 mg/mL G418. CMK clones were grown to confluence and frozen in 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen. Aliquots of cells were maintained in culture and used for flow cytometry, cytospins, or were washed in RPMI, and flash frozen in liquid nitrogen and stored at −80°C for further analysis.
Flow cytometric analysis. Stable transfected clones were washed in phosphate buffered saline (PBS) (Mediatech, Hearndon, VA) plus 5% FBS, centrifuged, and resuspended in 0.5 mL of PBS/5% FBS with a 1:5,000 dilution fluorescein isothiocyanate (FITC)-conjugated, anti-CD41 (Gen Trak, Plymouth, PA) or anti-CD61 (Becton Dickinson, Lincoln Park, NJ) for 30 minutes at 4°C. Stained cells were washed with PBS/5% FBS, and resuspended in a final concentration of 0.5 μg/mL of propidium iodine (Calbiochem) for 15 minutes. The stained cells were analyzed by FACScan (Becton Dickinson). Parental CMK cells were analyzed in an identical fashion before and after induction with PMA.
Assays of Erk activity. CMK cells were starved of FBS in RPMI for 2 hours. Approximately 8 × 106 cells were flash frozen, and the cell pellets were Dounce-homogenized in a Kontes 1-mL dounce in 200 to 500 μL of lysis buffer A[10 mmol/L K2HPO4 , pH 7.1/1 mmol/L EDTA/5 mmol/L EGTA/10 mmol/L MgCl2 /50 mmol/L β-glycerophosphate/1 mmol/L Na3VO4 /1% Triton X-100/2 mmol/L dithiothreitol/1 mmol/L phenylmethane-sulfonyl fluoride/leupeptin (10 μg/mL)/pepstatin A (1 μg/mL)], and incubated on ice for 30 minutes. The cytosolic protein fraction was clarified by ultracentrifugation at 30,000g for 30 minutes at 4°C. Total protein concentration of the supernatants was determined by the bicinchoninic acid (BCA) method (Pierce, Rockford, IL). Erk activity was measured using a myelin basic protein (MBP) assay which consisted of 10 μL of cell lysate with 5 μg of MBP substrate incubated with 20 mmol/L Tris (pH 7.5), 10 mmol/L MgCl2 , 20 μg of bovine serum albumin (BSA), 500 μmol/L unlabeled adenosine triphosphate (ATP), and 5 μCi of [γ-32P] ATP in a total reaction volume of 30 μL. Each reaction was incubated at 30°C for 15 minutes and stopped by the addition of 7.5 μL of 5× sample buffer and boiled for 2 minutes. The kinase reaction mixtures were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 12.5% gel, stained in 0.2% Coomassie Brilliant Blue solution, and dried on Whatman 3-mm paper (Whatman, Maldstone, England). After autoradiography on Fuji film (Fuji Photo Film Co, Tokyo, Japan), the radiolabeled phosphate incorporated into substrate was measured by liquid scintillation counting of the MBP gel bands. The cpm value was then used to calculate Erk activity in pmol phosphate incorporated into MBP/minute/μg protein in the cell lysate.
Western analysis. Cell extracts prepared as described above were resolved on 10% SDS-PAGE gels and transferred to a polyvinylidene difluoride blotting membrane (Millipore, Bedford, MA) using the Bio-Rad Western transfer system. Membranes were blocked in Tris-buffered saline with 0.1% (vol/vol) Tween-20 (Pierce), 5% dry instant milk, and probed with either Phospho-MAPK antibody (New England Biolabs) at a 1:1,000 dilution or polyclonal Erk antibody at 1:10,000 for 45 minutes at room temperature. Detection of labeled protein utilized horseradish peroxidase-coupled secondary antibody and the enhanced chemiluminescence system ECL (Amersham).
RESULTS
Erk activation in CMK cells. PMA and SCF have been shown to activate the MAP kinase pathway in several hematopoietic cell lines.29 We treated CMK cells with PMA or SCF to activate Erk. The treatment of CMK cells with 50 ng/mL PMA caused significant activation of Erk within 5 minutes, which was sustained through 120 minutes (Fig 1A). Similarly, treatment of CMK cells with 100 ng/mL SCF induced Erk activation at 5 minutes which was sustained to 60 minutes (Fig 1B). To examine whether the PMA- or SCF-induced activation of the MAP kinase pathway could be inhibited, we pretreated CMK cells with a specific Mek inhibitor, PD98059. Pretreatment of CMK cells with PD98059 did not block Erk activation in the PMA treated cells (Fig 1A). In contrast, pretreatment of cells with PD98059 did block activation of Erk when cells were stimulated with SCF (Fig 1B). To further confirm Erk activation in SCF-treated CMK cells, we performed Western blot analysis on the cell lysates. The SCF-treated cells had phosphorylated Erk detected by the Phospho-MAPK antibody at 5 and 15 minutes, which decreased by 60 minutes. In contrast, Erk phosphorylation mediated by SCF was almost completely inhibited by the pretreatment of cells with the Mek inhibitor (Fig 1C, lanes 6 to 9 v 15 to 18). In contrast, PMA-treated cells had phosphorylated Erk detected at all time points in the presence or absence of the Mek inhibitor (Fig 1C, lanes 2 to 5 and 11 to 14) The total amount of Erk in each of the samples, detectable by Western blotting with an Erk antibody, was equivalent and unchanged over the 2-hour stimulation (data not shown).
(A) Erk activity in PMA-treated CMK cells. CMK cells were serum-starved for 2 hours (•) or starved and then treated with 100 μmol/L of the Mek inhibitor, PD98059 for 1 hour (○). The cells were then stimulated with 50 ng/mL of PMA for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in all stimulated time points (P < .001 all PMA treated time points, P < .01 all PMA+PD98059-treated time points, compared with baseline by analysis of variance [ANOVA], Student-Newman-Keuls Multiple Comparisons Test). (B) CMK cells were serum starved for 2 hours (▪) or starved and then treated with 100 μmol/L of the Mek inhibitor PD98059 for 1 hour (□). The cells were then stimulated with 100 ng/mL SCF for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in samples stimulated for 5 or 15 minutes in the abscence of Mek inhibitor (* P < .05, ** P < .001 compared with baseline by ANOVA, Student-Newman-Keuls Multiple Comparisons Test). (C) Western blot analysis of above cell lysates using an anti-Phospho–MAPK antibody as described in Materials and Methods. Conditions are as indicated on the figure.
(A) Erk activity in PMA-treated CMK cells. CMK cells were serum-starved for 2 hours (•) or starved and then treated with 100 μmol/L of the Mek inhibitor, PD98059 for 1 hour (○). The cells were then stimulated with 50 ng/mL of PMA for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in all stimulated time points (P < .001 all PMA treated time points, P < .01 all PMA+PD98059-treated time points, compared with baseline by analysis of variance [ANOVA], Student-Newman-Keuls Multiple Comparisons Test). (B) CMK cells were serum starved for 2 hours (▪) or starved and then treated with 100 μmol/L of the Mek inhibitor PD98059 for 1 hour (□). The cells were then stimulated with 100 ng/mL SCF for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in samples stimulated for 5 or 15 minutes in the abscence of Mek inhibitor (* P < .05, ** P < .001 compared with baseline by ANOVA, Student-Newman-Keuls Multiple Comparisons Test). (C) Western blot analysis of above cell lysates using an anti-Phospho–MAPK antibody as described in Materials and Methods. Conditions are as indicated on the figure.
Erk activity of CMK stable transfectants. (A) Western blot analysis of individual clones of transfected CMK cells using an anti-Phospho–MAPK antibody. (B) Erk activity (mean ± standard error of mean [SEM]) of lysates from three to nine separate CMK clones transfected with vector, WT Mek, DE Mek, or D330N Erk as indicated. The mean Erk activity in DE Mek and D330N Erk transfected cells was significantly greater than that found in vector and WT Mek transfected cells (* P < .05, ** P < .01 by ANOVA).
Erk activity of CMK stable transfectants. (A) Western blot analysis of individual clones of transfected CMK cells using an anti-Phospho–MAPK antibody. (B) Erk activity (mean ± standard error of mean [SEM]) of lysates from three to nine separate CMK clones transfected with vector, WT Mek, DE Mek, or D330N Erk as indicated. The mean Erk activity in DE Mek and D330N Erk transfected cells was significantly greater than that found in vector and WT Mek transfected cells (* P < .05, ** P < .01 by ANOVA).
Expression of constitutively activated Mek and Erk in transfected CMK cells. Isolated clones of CMK cells transfected with WT Mek and constitutively activated DE Mek and D330N Erk were analyzed first for expression and activity of recombinant protein. The expression of transfected Mek or Erk protein determined by Western blotting was not significantly increased over the endogenous proteins (data not shown). However, cell lysates isolated from several DE Mek clones showed increased Erk phosphorylation by Western blotting using a Phospho-MAPK antibody (Fig 2A). This antibody will recognize only the phosphorylated and activated form of Erk. In comparison, Western analysis showed no significant phosphorylated Erk in the cells transfected with pcDNA3 vector and WT Mek (Fig 2A). Expression of WT Mek protein did not lead to increased Erk phosphorylation, presumably because only activated Mek can phosphorylate Erk. However, a small amount of Erk phosphorylation was seen in two of the D330N Erk clones (Fig 2A).
To further analyze the effect of the transfected Mek and Erk cDNAs on Erk activity, we measured the incorporation of phosphate into the substrate MBP. Erk activity in whole cell lysates of isolated clones transfected with pcDNA3 vector and WT Mek was not significantly different. Untransfected CMK cells had similar Erk activity (data not shown). However, clones transfected with DE Mek and D330N Erk showed significantly increased Erk activity compared with vector and WT Mek transfected cells. Figure 2B shows the mean Erk activity of MBP assays in three to seven separate clones of each group of transfectants.
Expression of constitutively activated DE Mek and D330N Erk induces morphologic changes in CMK cells. The parental CMK cells were treated with 1 μg/mL of PMA for 72 hours and examined by cytospin to confirm morphologic changes associated with PMA treatment. As previously demonstrated, CMK cells treated with PMA showed a differentiated morphology compared with untreated cells26 27 (Fig 3A and B). Exposure to 10 ng/mL of PMA for as little as 30 minutes was sufficient to induce differentiation in CMK cells at 24 hours (data not shown). However, the pretreatment of the cells with PD98059, a Mek inhibitor, before treatment with PMA was unable to block differentiation (data not shown), which was consistent with its inability to block activation of Erk.
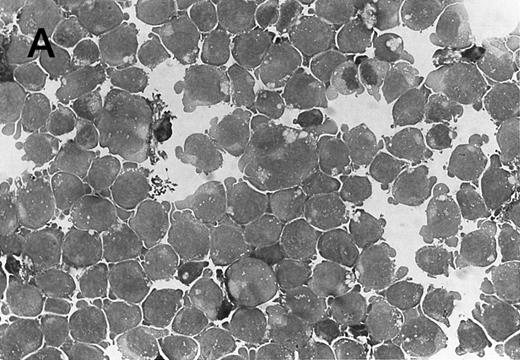
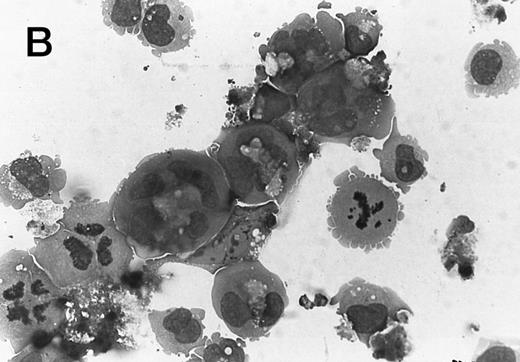
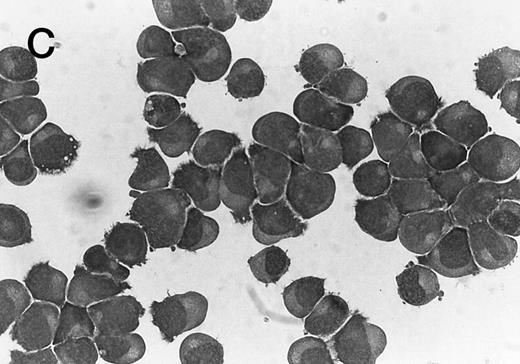
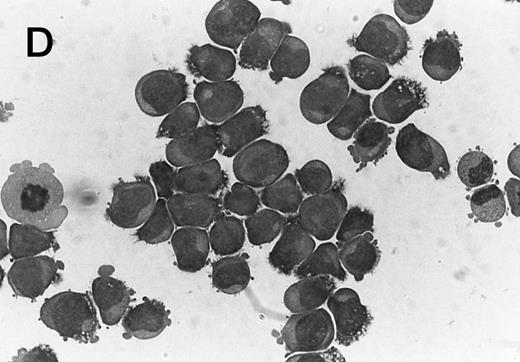
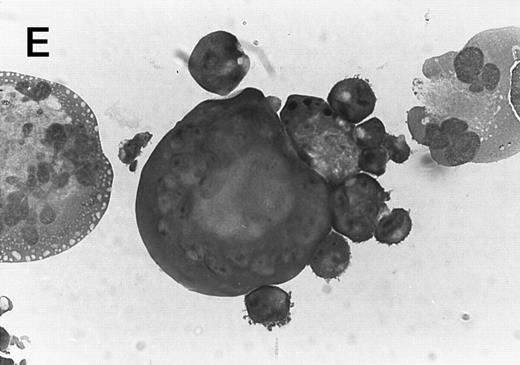
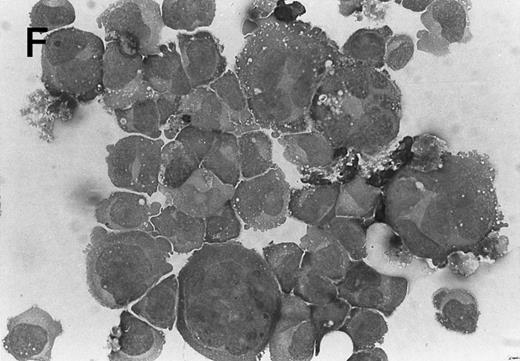
Morphology of CMK cell cytospins, Wright stained, and photographed at 400×. (A) Parental CMK cells showing an immature phenotype. The majority of cells have a single noncondensed nucleus with a small amount of cytoplasm. (B) Parental CMK cells treated with PMA. The cells show signs of differentiation including increased cell size, increased amount of cytoplasm, increased vacuolation of the cytoplasm, and an increased nuclear density with areas of multinucleation. (C) Vector control transfected CMK clone showing an immature phenotype. (D) WT Mek transfected CMK clone showing an immature phenotype. (E) DE Mek transfected clone showing a differentiated phenotype. (F ) D330N Erk transfected clone showing a differentiated phenotype.
Morphology of CMK cell cytospins, Wright stained, and photographed at 400×. (A) Parental CMK cells showing an immature phenotype. The majority of cells have a single noncondensed nucleus with a small amount of cytoplasm. (B) Parental CMK cells treated with PMA. The cells show signs of differentiation including increased cell size, increased amount of cytoplasm, increased vacuolation of the cytoplasm, and an increased nuclear density with areas of multinucleation. (C) Vector control transfected CMK clone showing an immature phenotype. (D) WT Mek transfected CMK clone showing an immature phenotype. (E) DE Mek transfected clone showing a differentiated phenotype. (F ) D330N Erk transfected clone showing a differentiated phenotype.
To examine the effect of specific activation of the MAP kinase pathway in CMK cells, we analyzed CMK transfectants that expressed constitutively activated Mek or Erk for megakaryocytic differentiation. The CMK cells transfected with pcDNA3 vector or WT Mek showed no changes in morphology (Fig 3C and D). In contrast, stable transfectants expressing activated mutants of Mek or Erk showed differentiation in the absence of PMA (Fig 3E and F ).
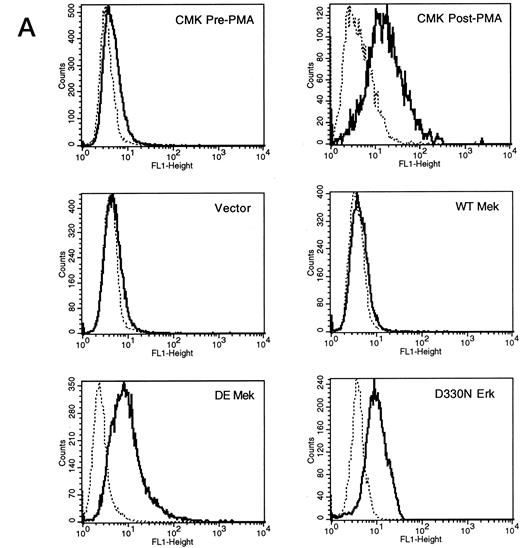
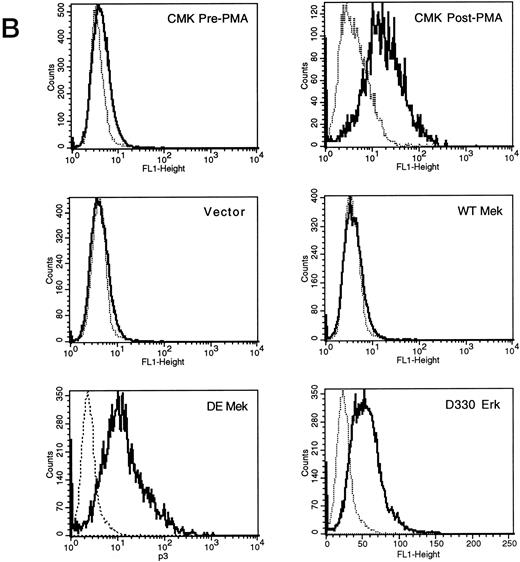
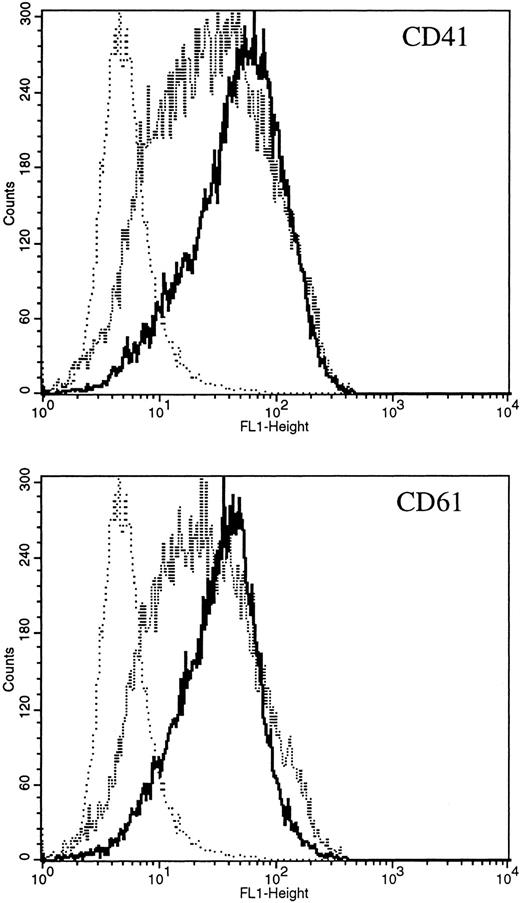
Expression of constitutively activated DE Mek and D330N Erk induce expression of megakaryocytic specific cell surface markers. As an independent measure of differentiation, we analyzed CMK cells for expression of megakaryocytic cell surface markers associated with differentiation. Glycoprotein GPIIb/IIIa, recognized by CD41 and CD61 antibodies, is one specific cell surface marker that is expressed during the differentiation of megakaryoblasts into platelets. Using parental CMK cells, we confirmed that unstimulated CMK cells expressed low levels of CD41 and CD61, and that CD41 and CD61 expression was significantly increased after phorbol ester treatment (Fig 4). The CMK cells transfected with pcDNA3 vector or WT Mek showed levels of CD41 and CD61 expression (Fig 4A and B, respectively) similar to the parental CMK cells. In contrast, clones transfected with constitutively activated Mek and Erk showed increased expression of both CD41 and CD61 (Fig 4A and B, respectively).
FACScan analysis of parental CMK cells treated with PMA or transfected with vector, WT Mek, DE Mek or D330N Erk. CMK cells were analyzed by FACScan as described. Cells were gated to exclude nonviable cells using propidium iodine staining. (⋅⋅⋅⋅⋅⋅) Represents the isotype control, (– – –) denotes the staining with markers of megakaryocytic differentiation, CD41 or CD61. (A) CD41; (B) CD61.
FACScan analysis of parental CMK cells treated with PMA or transfected with vector, WT Mek, DE Mek or D330N Erk. CMK cells were analyzed by FACScan as described. Cells were gated to exclude nonviable cells using propidium iodine staining. (⋅⋅⋅⋅⋅⋅) Represents the isotype control, (– – –) denotes the staining with markers of megakaryocytic differentiation, CD41 or CD61. (A) CD41; (B) CD61.
Activation of Erk is necessary to induce differentiation in CMK cells. The MAP kinase pathway is strongly activated by PMA treatment or through transfection with constitutively activated Mek or Erk proteins. We have shown that strong activation by PMA was sufficient to induce differentiation in CMK cells, but that activation could not be inhibited. We next used SCF to determine whether a hematopoietic growth factor known to stimulate the MAP kinase pathway would induce differentiation. Treatment of CMK cells with SCF induced Erk activation that was inhibited by pretreatment with PD98059 (Fig 1A). To determine whether SCF stimulation was sufficient to induce differentiation in CMK cells, we treated cells with 100 ng/mL SCF for 5 days and examined the cells for expression of CD41 and CD61. Treatment with SCF induced differentiation of CMK cells, as measured by increased expression of CD41 and CD61. This differentiation was significantly inhibited by pretreating cells with PD98059 for 1 hour and maintaining the inhibitor in culture for 5 days (Fig 5).
FACScan analysis of parental CMK cells treated with SCF with and without pretreatment with the Mek inhibitor, PD98059. CMK cells were analyzed by FACScan as described. Cells were gated to exclude nonviable cells using propidium iodine staining. (⋅⋅⋅⋅⋅) Represents the isotype control, (- - - - - - -) denotes the CMK cells treated with SCF and PD98059, (– – –) denotes the CMK cells treated with SCF alone. Staining with markers of megakaryocytic differentiation, CD41, or CD61 as indicated. The Mek inhibitor significantly decreased expression of both CD41 and CD61 (P < .001 comparing SCF with and without PD98059, by the Kolmogorov-Smirnov two sample test for both CD41 and CD61).
FACScan analysis of parental CMK cells treated with SCF with and without pretreatment with the Mek inhibitor, PD98059. CMK cells were analyzed by FACScan as described. Cells were gated to exclude nonviable cells using propidium iodine staining. (⋅⋅⋅⋅⋅) Represents the isotype control, (- - - - - - -) denotes the CMK cells treated with SCF and PD98059, (– – –) denotes the CMK cells treated with SCF alone. Staining with markers of megakaryocytic differentiation, CD41, or CD61 as indicated. The Mek inhibitor significantly decreased expression of both CD41 and CD61 (P < .001 comparing SCF with and without PD98059, by the Kolmogorov-Smirnov two sample test for both CD41 and CD61).
DISCUSSION
The MAP kinase signal transduction pathway involving Mek and Erk plays a crucial role in proliferation and differentiation in several cell lineages. Direct evidence on the need for activation of the MAP kinase pathway in hematopoietic cells has been shown in thymocytes, where Erk activity is required for thymocyte differentiation.24,25,31 Indirect evidence suggests that the MAP kinase pathway may be important in the development of megakaryocytes. Several cytokines are required for growth and differentiation of megakaryocytic cells, including SCF, thrombopoietin, and interleukin (IL)-11. All of these cytokines induce the phosphorylation and activation of Erk.4,32-34 We found that CMK cells were very sensitive to PMA treatment and differentiated in response to PMA treatment for 30 minutes. The PMA stimulation of CMK cells resulted in increased Erk activity, which persisted up to 120 minutes. However, the MAP kinase pathway, as measured by Erk activation, was not significantly blocked by the pretreatment of CMK cells with the Mek inhibitor, PD98059. PD98059 has been previously shown incapable of blocking strong activators of the MAP kinase pathway.35 The MAP kinase pathway was also activated through the cytokine receptor, c-kit, by the treatment of CMK cells with SCF. Although the level of Erk activation was comparable, the duration of activity induced by SCF was shorter than that induced by PMA. The kinetics of Erk activation by PMA suggests alternate pathways not involving Mek may exist to augment or induce Erk activation, bypassing the activity of Mek. The SCF-induced Erk activation was also correlated with induction of differentiation in CMK cells. The pretreatment of cells with the Mek inhibitor PD98059 was able to block Erk activation by SCF and was sufficient to significantly block SCF-induced differentiation as determined by expression of the megakaryocytic markers CD41 and CD61.
Transfection of activated MAP Kinase pathway mutants increased the basal MAP Kinase activity in the transfected cells. The mechanism leading to differentiation induced by expression of the D330N Erk mutant has not been previously reported. The original D330N Erk mutation was isolated by a genetic screen in Drosophila.16 The presence of increased Erk activity and Erk phosphorylation in D330N Erk-transfected cells in our study is the first data suggesting possible mechanisms of action. The phosphorylation of Erk detected by the Phospho-MAPK antibody suggests that the amino acid substitution at D330N induces a conformational change that affects the mechanisms regulating the phosphorylation-dephosphorylation of the critical threonine and tyrosine residues at positions 183 and 185. The D330N Erk mutation could either expose those crucial sites for phosphorylation or prevent their dephosphorylation. Alternately, the possibility of an autocrine feedback mechanism for increasing phosphorylation and activation of the endogenous Erk cannot be ruled out because the Phosho-MAPK antibody would recognize either the endogenous or transfected D330N Erk. Regardless, dissecting out the signaling pathway activated beyond Erk, which is important for megakaryocytic differentiation, will be facilitated by the study of these CMK transfectants.
The direct role of activation of the MAP kinase pathway in megakaryocytopoiesis has not been previously delineated. We bypassed the cytokine receptor-induced activation of the MAP kinase pathway by introducing constitutively activated Mek or Erk cDNA into CMK cells. The transfected CMK cells showed that constitutive activation of Mek or Erk was sufficient for megakaryocytic differentiation. Combined with the Mek inhibitor data through SCF signaling, we conclude Erk activation is both involved in differentiation and sufficient to induce differentiation in this megakaryocytic cell line.
NOTE ADDED IN PROOF
Since submission of our report, Whalen et al published their observations of a similar effect of an activated MAP kinase pathway mutant, concerning megakaryocyte differentiation in K562 cells (Mol Cell Biol 17:1947, 1997).
ACKNOWLEDGMENT
We thank R.L. Erikson for the gifts of Mek and DE Mek mutant and D.A. Williams for critical review of the manuscript.
Supported in part by research grants from the Riley Memorial Association (to T.A.V.) and Riley Cancer Research for Children (to A.S.M.).
Address reprint requests to Terry A. Vik, MD, Wells Center for Pediatric Research, Room 2600, Riley Hospital for Children, 702 Barnhill Dr, Indianapolis, IN 46202.

![Fig. 1. (A) Erk activity in PMA-treated CMK cells. CMK cells were serum-starved for 2 hours (•) or starved and then treated with 100 μmol/L of the Mek inhibitor, PD98059 for 1 hour (○). The cells were then stimulated with 50 ng/mL of PMA for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in all stimulated time points (P < .001 all PMA treated time points, P < .01 all PMA+PD98059-treated time points, compared with baseline by analysis of variance [ANOVA], Student-Newman-Keuls Multiple Comparisons Test). (B) CMK cells were serum starved for 2 hours (▪) or starved and then treated with 100 μmol/L of the Mek inhibitor PD98059 for 1 hour (□). The cells were then stimulated with 100 ng/mL SCF for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in samples stimulated for 5 or 15 minutes in the abscence of Mek inhibitor (* P < .05, ** P < .001 compared with baseline by ANOVA, Student-Newman-Keuls Multiple Comparisons Test). (C) Western blot analysis of above cell lysates using an anti-Phospho–MAPK antibody as described in Materials and Methods. Conditions are as indicated on the figure.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3462/4/m_bl_0020f1a.jpeg?Expires=1769277980&Signature=Y3Md6Jpicp2OkisGiXVUGFYGm24RcYj95h22C~Qa~OuzvyG4HoxhiJVqqgxLI8B4VzubfeFd~WQpphX~nn7s~WhL4mSyQfsfrfQ8quTuBhsfBb6eI4EyDtEylIAE3C6nS89idQ64ubspNDd~6w2cIka2jKvKxFP58jYWCX8xK0Ng2VboqpFo0z1nAyXzkidPK2sjxylVVKyeYgUrZkXmJUJuQX8vNKl2z2QfP0B-ui9Lvh9qJb1WZMoSa19QOkaLJ9le~DWq5I8em9M~GG-dZXVBIijcVWSDtLoZLp49mHXrBRtS3nUuAKxAXrxt2rZSfApFiGL4b2v~ZzEesSgToQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Erk activity in PMA-treated CMK cells. CMK cells were serum-starved for 2 hours (•) or starved and then treated with 100 μmol/L of the Mek inhibitor, PD98059 for 1 hour (○). The cells were then stimulated with 50 ng/mL of PMA for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in all stimulated time points (P < .001 all PMA treated time points, P < .01 all PMA+PD98059-treated time points, compared with baseline by analysis of variance [ANOVA], Student-Newman-Keuls Multiple Comparisons Test). (B) CMK cells were serum starved for 2 hours (▪) or starved and then treated with 100 μmol/L of the Mek inhibitor PD98059 for 1 hour (□). The cells were then stimulated with 100 ng/mL SCF for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in samples stimulated for 5 or 15 minutes in the abscence of Mek inhibitor (* P < .05, ** P < .001 compared with baseline by ANOVA, Student-Newman-Keuls Multiple Comparisons Test). (C) Western blot analysis of above cell lysates using an anti-Phospho–MAPK antibody as described in Materials and Methods. Conditions are as indicated on the figure.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3462/4/m_bl_0020f1b.jpeg?Expires=1769277980&Signature=HRagcRZiQYpWTuPX9-48zQUQhyATGV1q91KCOzPDFq~QRwd98eOBwYaKZUuo7vx8PWJJ0RBuiiwnVa2qXwrEXUIuGsod5GVtJLD-r-eGqLc6HzimOsefZnOBeIcnpMxj9ViLegGEu7T2GO5YblWxts26iVUyqjaYZ2MMOnqeEjG76lnaHUpYGKtvpKqsl2TC0JxnoG38fja4YDbq0j3gNNM3futu8kBmMgROQWAVTrp4F-TSLUQVopoT99G69xJRmQ2qSKvQLIyde235lbWZzGU1hSualFoOGDDPcQHsTQk~JTwqjJz3swLMt~XS9x4xanyvBvq-p88lhocGvz3tkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Erk activity in PMA-treated CMK cells. CMK cells were serum-starved for 2 hours (•) or starved and then treated with 100 μmol/L of the Mek inhibitor, PD98059 for 1 hour (○). The cells were then stimulated with 50 ng/mL of PMA for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in all stimulated time points (P < .001 all PMA treated time points, P < .01 all PMA+PD98059-treated time points, compared with baseline by analysis of variance [ANOVA], Student-Newman-Keuls Multiple Comparisons Test). (B) CMK cells were serum starved for 2 hours (▪) or starved and then treated with 100 μmol/L of the Mek inhibitor PD98059 for 1 hour (□). The cells were then stimulated with 100 ng/mL SCF for various times as indicated. Activity was plotted as relative activation compared with baseline control. Significant increased Erk activity was seen in samples stimulated for 5 or 15 minutes in the abscence of Mek inhibitor (* P < .05, ** P < .001 compared with baseline by ANOVA, Student-Newman-Keuls Multiple Comparisons Test). (C) Western blot analysis of above cell lysates using an anti-Phospho–MAPK antibody as described in Materials and Methods. Conditions are as indicated on the figure.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3462/4/m_bl_0020f1c.jpeg?Expires=1769277980&Signature=YAFjTSTZOYDlXTFtOQkkc-lniMjMchyah0nnOcI6nEBhnyVFaMxwi5OHuOdJGuEjlIoZR6sW-QZ4lWs0vvszP75muzuW5mcuKmH6b7jSEGxlNgZlMdMjNkEy705yCQLf97Mb9In9ZXZGSsXgFwn7vombpgFJQ~14GBP~Gzv1XvP2PqGZ0srQvo44oC6~g0~qF4hvL~-qHQxQi2b9s7p9vta0yq6lgJ0nbNCuzBTDUYpD0Jq8pprIzF40jsoS1D7pj7MgqP1onplAdce5hCQUUL-zg-6hhi5l3PYpdeL8TI40g4YD0Ctbub4OQBtg855KBzzr0-tvFx2hxX8b3pDldw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Erk activity of CMK stable transfectants. (A) Western blot analysis of individual clones of transfected CMK cells using an anti-Phospho–MAPK antibody. (B) Erk activity (mean ± standard error of mean [SEM]) of lysates from three to nine separate CMK clones transfected with vector, WT Mek, DE Mek, or D330N Erk as indicated. The mean Erk activity in DE Mek and D330N Erk transfected cells was significantly greater than that found in vector and WT Mek transfected cells (* P < .05, ** P < .01 by ANOVA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3462/4/m_bl_0020f2a.jpeg?Expires=1769277980&Signature=NvguP5kTeKQw5g4ZKEDfTwp5Y~2naV3KU-tVYAD1vbnM9~UTpQY8PI1y5rwhnk~BbGSvUywBzkphZxtiQAnFgPAbiqsVefcXs-oCME8zLZIU4qxUPn~lLL1FnqOm4HIkRtsEi98OCvhLyO-hA5Fuc7wksv5Rx31QiD9l2NkvM-o1i01QnF2wZCjZVdp~C5Naxf20ovCJpTkzIyi4uTaXEWPR9iTkHE5vEWEbJnZQBEP3ZTjePgGCweVQCAvFLmaaQFCJ0a51jp5Jy2Oz-Yeq4THsvTDkvYk2At0uYkGAHhWqmU0IO0E0c2etnX~AdRE6dbe5LuJg5owfzyiRTeB6-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Erk activity of CMK stable transfectants. (A) Western blot analysis of individual clones of transfected CMK cells using an anti-Phospho–MAPK antibody. (B) Erk activity (mean ± standard error of mean [SEM]) of lysates from three to nine separate CMK clones transfected with vector, WT Mek, DE Mek, or D330N Erk as indicated. The mean Erk activity in DE Mek and D330N Erk transfected cells was significantly greater than that found in vector and WT Mek transfected cells (* P < .05, ** P < .01 by ANOVA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3462/4/m_bl_0020f2b.jpeg?Expires=1769277980&Signature=c3fiCsX~RAPxRKRTMWxMgjIiKuiW4Qvr1YQun8imRA3TNKKpwL-fy3KIbbdYF2TS13DXK1jfeDjraEMlfGTYaak7GKJd~lCVhnKb1xIOWrj07rBdqGKYJ4VEVvVTAHqMGXWpfQrPD2P6V1SZjZdzNkSPufO3jQwG7rDX1nVA5kL5dZfoUavzBe2INH69TI1v6SoT1ryTgJiHLo~4-2EkzSwgkrxUYYpmEKgMRyndowyFYwJoosTAVALM6bXVOhOpTepNGI4MT-9PNPTlaYvDJqNnMqO~X8vJ-P1h52kdYQB2jIpzTAO6kxZKRGSj0l1DOuAkvDlKHq9ro0X~BYlAug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal