Abstract
The MpL ligand (ML) is a potent stimulus for thrombocytopoiesis. To create an in vivo model of ML deficiency, we injected dogs with a recombinant human ML (rhML) to determine whether cross-reacting antibodies would develop and cause thrombocytopenia. RhML was administered subcutaneously for 8 weeks to three normal dogs (mean platelets, 197 ± 5.5 × 103/μL). Within 5 days their platelet counts were twice baseline and greater than 4 times baseline by day 21. Then, uniformly, chronic thrombocytopenia developed. At 1 week after terminating rhML, mean platelets were 0.5 times baseline and at 2 months 0.25 times baseline. Early in treatment, marrow biopsies showed increased megakaryocyte number and ploidy, which decreased as platelets declined. Paralleling these changes, high titer anti-rhML antibodies developed. Autologous 51Cr-labeled platelet recovery and survival measurements indicated that the thrombocytopenia was principally due to decreased production. Infusion of plasma from the thrombocytopenic dogs into two normal dogs and one dog previously made thrombocytopenic with rhML caused platelet counts to fall gradually. These studies show that dogs with anti-rhML antibodies develop thrombocytopenia, presumably because the cross-reacting antibodies neutralize endogenous canine ML. The results strongly suggest that ML plays an essential role in maintaining normal platelet levels.
IN 1994, SEVERAL research groups identified the ligand for the cytokine receptor c-Mpl and subsequently showed that it is a potent stimulus to platelet production.1-5 Bone marrow CD34+ cells express c-Mpl and selectively differentiate to megakaryocytes on exposure to the c-Mpl ligand (ML).2,6,7 Mice with embryonal stem cell disruption of the c-Mpl or ML genes are thrombocytopenic,8,9 and mice overexpressing c-Mpl ligand have markedly increased marrow megakaryocytes.10 Accumulating evidence also indicates that there is a reciprocal relationship between plasma levels of ML and platelet counts.11
Across mammalian species there are close homologies for the hematopoietic growth factors, but the amino acid sequences are species specific. For this reason human growth factors administered to animals may lead to development of cross-reacting antibodies that neutralize the biologic activities of the native molecule. This is the proposed mechanism of the anemia in dogs administered recombinant human erythropoietin12 and the neutropenia in dogs after administration of recombinant human granulocyte colony-stimulating factor (G-CSF ).13 These experiments also showed the critical role of these specific growth factors in maintaining normal blood levels of erythrocytes and neutrophils. To extend these studies and investigate the role of ML in maintaining normal platelet counts, we administered recombinant human ML (rhML) to dogs and observed the acute and chronic effects on platelet counts.
MATERIALS AND METHODS
Dogs
Healthy, mixed-breed, female dogs weighing 19 to 20 kg were housed in facilities accredited by the American Association for Laboratory Animal Care and studied under a protocol approved by the Animal Care Committee, University of Washington. The dogs were examined at least once per day for general health, temperature, and any evidence of bleeding or other problems.
Growth Factors
A truncated form of the human ML, including the erythropoietin-like domain, was produced in Escherichia coli, purified to homogeneity and derivitized with polyethylene glycol. This molecule was formulated in aqueous buffer, sterilized by filtration, and provided as a gift by Amgen (Thousand Oaks, CA). Before injection, the material was diluted to 100 μg/mL with phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) and injected subcutaneously.
Recombinant human interleukin-6 (rhIL-6) (0.2 mg/mL) for investigational use expressed and purified from E coli was also supplied by Amgen and administered subcutaneously at concentrations of 200 μg/mL.
Blood and Bone Marrow Examination
Complete blood counts (CBCs) were performed daily using a Coulter Counter (model T-540, Hialeah, FL) and differential counts on Wright's stained smears were performed manually. All counts were performed just before injection of growth factors. Bone marrow aspirates and biopsies from the iliac crest were performed on anesthetized animals and prepared by standard methods.
Autologous Chromium-51 (Cr51) Platelet Kinetics
Autologous platelet kinetic studies were performed as previously described.14 Acid citrate dextrose (ACD) anticoagulated venous blood (30 mL, 1:10 ACD to whole blood) was removed and the platelets separated by centrifugation and labeled by incubating with 300 μCi 51Cr. The labeled platelets were then reinfused and 3 mL blood samples were drawn serially for 5 days to determine blood platelet specific activity. Platelet survival was calculated from the specific activity data using the least squares method and recovery determined by extrapolation to time 0.15 By this method, normal mean (± standard error of mean [SEM]) recovery is 55% ± 1% and mean platelet survival is 5.4 ± 0.1 days.
Plasma Collection
On days 49 through 56 after initiation of rhML, approximately 25 mL/d of ACD plasma was collected from each dog and frozen at −80°C for later infusion studies.
Antibody Assays
Anti-ML assays. Serum samples were analyzed for specific neutralization of ML using a modification of a previously described assay.3 Murine 32D cells transfected with the human Mpl receptor were suspended in Minimum Essential Media (GIBCO, Grand Island, NY) supplemented with 10% Fetal Clone II (Hyclone, Colorado Springs, CO) plus 250 pg/mL rhML (Amgen, Thousand Oaks, CA) and plated at 10,000 cells/well (200 μL total volume/well) in 96-well tissue culture plates (Becton Dickinson Labware, Lincoln Park, NJ). The standard curve for anti-ML antibody activity was generated using serial dilution of a polyclonal rabbit anti-rhML diluted in normal serum. Cultures were incubated for 48 hours at 37°C in humidified air with 10% CO2 .
The plates were analyzed using the standard colorimetric method, MTS (Cell Titer 96; Promega, Madison, WI). The growth was measured by optical density (OD) at 490 nm absorbance with a Spectra Max 250 plate reader (Molecular Devices, Sunnyvale, CA) and SoftMax Pro software (Molecular Devices). The OD at which 50% of maximum growth was reached was defined as the inhibitory concentration 50% (IC50 ). Readings of samples that fell below the IC50 were considered inhibitory. The titer was determined by the lowest dilution of a sample resulting in an OD value higher than the IC50 . Maximum growth was determined from cells cultured without the addition of serum. Specificity of inhibition was demonstrated by the inability of the antibody to inhibit growth stimulated with murine recombinant IL-3. Results were the mean of quadruplicate samples and expressed as the reciprocal of the lowest dilution resulting in an OD greater than the IC50 for the positive control.
To confirm that the inhibitor activity was due to an antibody, the IgG fraction was purified with a Hi-Trap Protein A affinity column (Pharmacia Biotech, Uppsala, Sweden). Serum was diluted twofold in equilibration buffer (50 mmol/L Tris, 150 mmol/L NaCI, pH 8.0), filtered through a 22-μm filter, loaded onto the column, and incubated for 1 hour at room temperature. IgG was eluted by 100 mmol/L glycine (pH 3.0) into tubes containing 1 mol/L Tris HCI (pH 9.5).
IgG, specifically binding to rhML, was detected by a solid phase radioimmunoassay (RIA). A truncated, recombinant form of ML was absorbed to Immulon II Removawells (Dynatech, Chantilly, VA) by incubating at room temperature for 2 hours. Duplicate test sera, purified IgG, as well as negative and positive controls, were diluted in 1% BSA in 1× PBS, added to previously coated wells and incubated for 2 hours. Iodinated protein A (125I protein A) (Dupont, Wilmington, DE) was then added to all samples and incubated for 1 hour. All wells were decanted between steps and washed with 1× wash solution (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Radioactivity was measured using gamma spectroscopy-LKB Wallac Gamma Master (Turku, Finland).
Anti–IL-6 Antibody Assay
A commercial immunoassay kit for IL-6 (R&D Systems, Minneapolis, MN) with a sensitivity range for measurement of IL-6 of 2 to 200 pg/mL was used to detect antibodies to IL-6. The presence of antibody was detected by addition of the study dog's serum to the standard assay. Normal canine serum was used as a control.
Study Protocol
Serial CBCs, bone marrow aspirate, and biopsy were performed pretreatment and rhML was administered daily (6.25 μg/kg for 2 weeks, 12.5 μg/kg for 2 weeks, 25 μg/kg for 4 weeks). The dogs were then observed with serial blood cell counts for 6 months or until platelet counts were greater than 100 × 103/μL. In one dog at 3 weeks after the rhML injections were completed, daily injections of rhIL-6 were administered at 25 μg/kg/d for 28 days.
Statistics
All values are presented as mean ± SEM unless otherwise noted. The Student's t-test (two-tailed) was used for all statistical comparisons.
RESULTS
Administration of rhML caused no local or systemic reactions in any of the dogs.
Blood Cell Counts
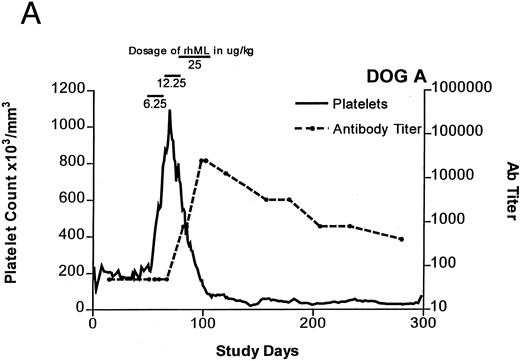
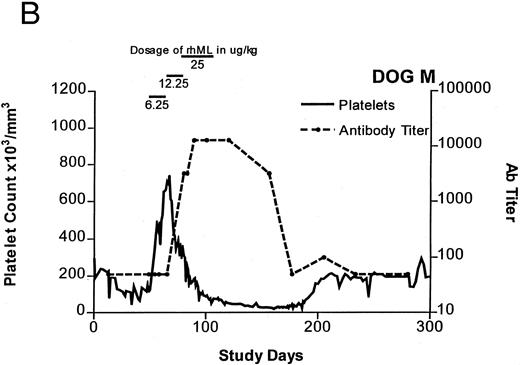
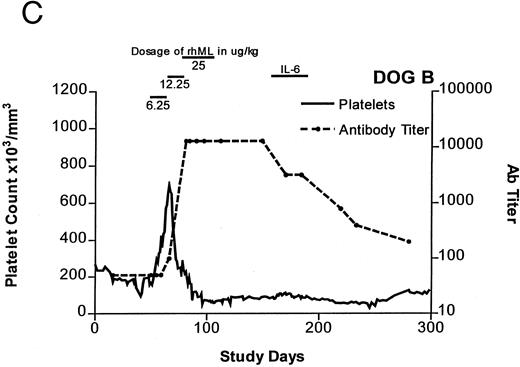
Treatment-induced thrombocytosis in all three animals. Platelet counts rose from 171 ± 54 × 103/μL to a peak of 841 ± 219 × 103/μL by day 19 (P < .05) (see Fig 1A-C and Table 1). In all three dogs, while therapy with rhML continued, platelet counts fell despite increasing dose. Platelet nadirs occurred at approximately 2 to 3 months after initiation of rhML treatment. The counts then gradually recovered, taking more than 3 months from the nadir to recover to a level greater than 100 × 103/μL. Hematocrit levels gradually rose over the whole study period in these young dogs, but not while on rhML treatment, probably due to blood loss with the platelet kinetic studies and other blood tests. The rhML treatment caused a significant increase in neutrophil counts in two of the three dogs (Table 1), but these counts returned to baseline levels when rhML was discontinued.
Platelet counts and anti-rhML antibody titers for dogs repeatedly administered daily rhML. The schedule for dosing is shown at the top of the panel. Dog B was also administered recombinant human IL-6 for the days shown. Antibody (Ab) titers are expressed as the lowest titer of serum having an inhibitory concentration more than the IC50 for a standard antibody.
Platelet counts and anti-rhML antibody titers for dogs repeatedly administered daily rhML. The schedule for dosing is shown at the top of the panel. Dog B was also administered recombinant human IL-6 for the days shown. Antibody (Ab) titers are expressed as the lowest titer of serum having an inhibitory concentration more than the IC50 for a standard antibody.
Blood Cell Counts With rhML Treatment
| . | ||||
|---|---|---|---|---|
| . | Before Weeks 1-7 . | On Weeks . | After Weeks 17-45 . | Recovery After Week 45 . |
| . | . | 8-16 . | . | . |
| Dog A | ||||
| Platelets | 197.4 ± 5.5 | 493.5 ± 40.7* | 46.2 ± 1.8* | 118.9 ± 3.9* |
| Hematocrit | 41.8 ± .9 | 41.9 ± .6 | 47.5 ± 0.5* | 52.4 ± 0.6* |
| WBC | 10.3 ± .2 | 13.7 ± 4* | 9.0 ± 0.1* | 7.4 ± 0.4* |
| ANC | 4904.9 ± 203.0 | 7020.8 ± 293.7* | 4809.8 ± 109.8 | 4152.4 ± 288.9 |
| Before Weeks 1-7 | On Weeks | After Weeks | Recovery After Week 42 | |
| 8-16 | 17-42 |
| . | ||||
|---|---|---|---|---|
| . | Before Weeks 1-7 . | On Weeks . | After Weeks 17-45 . | Recovery After Week 45 . |
| . | . | 8-16 . | . | . |
| Dog A | ||||
| Platelets | 197.4 ± 5.5 | 493.5 ± 40.7* | 46.2 ± 1.8* | 118.9 ± 3.9* |
| Hematocrit | 41.8 ± .9 | 41.9 ± .6 | 47.5 ± 0.5* | 52.4 ± 0.6* |
| WBC | 10.3 ± .2 | 13.7 ± 4* | 9.0 ± 0.1* | 7.4 ± 0.4* |
| ANC | 4904.9 ± 203.0 | 7020.8 ± 293.7* | 4809.8 ± 109.8 | 4152.4 ± 288.9 |
| Before Weeks 1-7 | On Weeks | After Weeks | Recovery After Week 42 | |
| 8-16 | 17-42 |
| Dog B | ||||
| Platelets | 187.2 ± 8.0 | 244.0 ± 25.1 | 78.7 ± 1.9* | 116.6 ± 2.8* |
| Hematocrit | 44.2 ± 1.0 | 39.6 ± 0.5* | 45.3 ± 0.4 | 48.0 ± 0.6* |
| WBC | 10.1 ± .3 | 10.8 ± 0.4 | 8.3 ± 0.2* | 8.1 ± 0.4* |
| ANC | 5641.6 ± 376.6 | 6131.8 ± 295.7 | 4374.0 ± 122.6* | 4317.1 ± 223.4 |
| Before Weeks 1-7 | On Weeks | After Weeks | Recovery After Week 29 | |
| 8-16 | 17-29 |
| Dog B | ||||
| Platelets | 187.2 ± 8.0 | 244.0 ± 25.1 | 78.7 ± 1.9* | 116.6 ± 2.8* |
| Hematocrit | 44.2 ± 1.0 | 39.6 ± 0.5* | 45.3 ± 0.4 | 48.0 ± 0.6* |
| WBC | 10.1 ± .3 | 10.8 ± 0.4 | 8.3 ± 0.2* | 8.1 ± 0.4* |
| ANC | 5641.6 ± 376.6 | 6131.8 ± 295.7 | 4374.0 ± 122.6* | 4317.1 ± 223.4 |
| Before Weeks 1-7 | On Weeks | After Weeks | Recovery After Week 29 | |
| 8-16 | 17-29 |
| Dog M | ||||
| Platelets | 134.8 ± 11.2 | 330.2 ± 25.9* | 42.4 ± 2.5* | 189.7 ± 5.5* |
| Hematocrit | 41.0 ± 0.8 | 40.3 ± 0.5 | 45.1 ± 0.3* | 47.2 ± 0.9* |
| WBC | 11.1 ± 0.3 | 13.7 ± 0.3* | 10.3 ± 0.2* | 13.3 ± 0.5* |
| ANC | 5548.4 ± 251.8 | 7162.8 ± 239.7* | 5496.0 ± 170.8 | 8343.5 ± 581.7 |
| Dog M | ||||
| Platelets | 134.8 ± 11.2 | 330.2 ± 25.9* | 42.4 ± 2.5* | 189.7 ± 5.5* |
| Hematocrit | 41.0 ± 0.8 | 40.3 ± 0.5 | 45.1 ± 0.3* | 47.2 ± 0.9* |
| WBC | 11.1 ± 0.3 | 13.7 ± 0.3* | 10.3 ± 0.2* | 13.3 ± 0.5* |
| ANC | 5548.4 ± 251.8 | 7162.8 ± 239.7* | 5496.0 ± 170.8 | 8343.5 ± 581.7 |
Abbreviations: WBC, white blood cell; ANC, absolute neutrophil count.
Mean ± SEM values significantly different from baseline, P < .05.
Bone Marrow Examinations
Compared with the pretreatment bone marrows, all three dogs showed only an increase in marrow megakaryocytes at 1 week on treatment, as reported in other studies.9 16-19 Subsequent biopsies at 5, 6, 10, and 16 weeks after initiation of rhML showed only decreased marrow megakaryocytes with pycnotic nuclei. Marrows performed after platelet counts had returned to normal were similar to baseline.
Platelet Kinetic Studies
Platelet kinetic studies were performed at 2, 3, and 5 weeks of therapy, timed to correspond to increasing, peak, and declining platelet counts. These studies showed normal percent recovery, but statistically significant decreases in platelet survival for the studies on weeks 2 and 5 (Table 2).
Platelet Kinetics
| Dogs . | No. . | Platelets . | Recovery (%) . | Half-Life (days) . |
|---|---|---|---|---|
| . | . | (× 103/μL) . | . | . |
| Normal dogs | 41 | 290 ± 20* | 55 ± 1 | 5.4 ± 0.1 |
| Dogs on rhML: | ||||
| 2 weeks | 3 | 680 ± 120† | 57 ± 16 | 3.6 ± 0.2† |
| 3 weeks | 3 | 830 ± 230† | 54 ± 9 | 4.8 ± 0.5 |
| 5 weeks | 3 | 260 ± 150 | 51 ± 7 | 4.4 ± 1.1† |
| Dogs . | No. . | Platelets . | Recovery (%) . | Half-Life (days) . |
|---|---|---|---|---|
| . | . | (× 103/μL) . | . | . |
| Normal dogs | 41 | 290 ± 20* | 55 ± 1 | 5.4 ± 0.1 |
| Dogs on rhML: | ||||
| 2 weeks | 3 | 680 ± 120† | 57 ± 16 | 3.6 ± 0.2† |
| 3 weeks | 3 | 830 ± 230† | 54 ± 9 | 4.8 ± 0.5 |
| 5 weeks | 3 | 260 ± 150 | 51 ± 7 | 4.4 ± 1.1† |
Mean ± SEM.
Values significantly different from normal, P < .05.
Anti-rhML Antibodies
Before treatment, the sera for all three dogs were negative for anti-ML antibodies. With treatment, antibodies neutralizing ML developed, with increases over baseline by about 3 weeks and peaking approximately 2 to 3 months after initiation of treatment (see Fig 1A-C). Subsequently, the antibody titers declined gradually as the dogs' platelet counts returned to normal. Titrations of individual serum samples showed that for the period corresponding to the nadir of platelet counts and the peak of antibody activity, the serum samples were completely inhibitory of recombinant human ML at all dilutions up to 1:800. Using IgG purified from these samples, identical results were obtained.
Recombinant hIL-6 Therapy
One dog, while thrombocytopenic, was administered rhIL-6 at a dose of 25 μg/kg subcutaneously for 4 weeks (see Fig 1C). During this period, there was a small, but statistically significant, increase in the platelet count. Platelet counts averaged 85.0 ± 1.75 × 103/μL for the 3 weeks before this treatment. They were 95.9 ± 1.9 × 103 μL (P < .05) on rhIL-6 and 76.5 ± 2.85 × 103/μL for the 4 weeks after IL-6 treatment (P < .05 for comparison of before and on rh IL-6). Assays for serum antibodies to rhIL-6 performed at the end of IL-6 therapy showed complete inhibition of the rhIL-6 standard at the highest concentration tested (50 pg/mL) indicating that an immunizing dose of the human protein had been administered.
Plasma Infusions
Two dogs with normal platelet counts, were infused with 120 mL of plasma from dogs made thrombocytopenic by administration of rhML. In both of these dogs, platelet counts decreased gradually over the next 3 to 4 weeks, reaching a nadir of approximately 0.5 times baseline (Fig 2). In a third dog, one of the dogs treated with rhML, reinfusion of the dog's own plasma caused thrombocytopenia, with a drop in the platelet count also occurring gradually over a 5-week period (Fig 2).
Platelet counts after infusion of plasma containing anti-rhML antibodies in three dogs, two normal dogs (dogs C and D) and one dog made thrombocytopenic 10 months previously with repeated administration of rhML (dog A). (□) Represents dog A, infused with dog A's plasma; (○) represents dog C, infused with dog B's plasma; (⋄) represents dog D, infused with dog M's plasma.
Platelet counts after infusion of plasma containing anti-rhML antibodies in three dogs, two normal dogs (dogs C and D) and one dog made thrombocytopenic 10 months previously with repeated administration of rhML (dog A). (□) Represents dog A, infused with dog A's plasma; (○) represents dog C, infused with dog B's plasma; (⋄) represents dog D, infused with dog M's plasma.
DISCUSSION
This study shows that rhML is a potent stimulus for increasing platelet production in dogs, results consistent with reports in other species. In this study, the evidence for increased platelet production includes the increase in platelet counts and the increase in marrow megakaryocytes. Gradually the dogs, however, failed to maintain the rhML-induced thrombocytosis. Beginning about 2 and a half weeks after starting the rhML injections, they gradually developed moderately severe thrombocytopenia. This occurred concomitant with the development of an IgG antibody response to rhML. Both a modest decrease in platelet survival and marked decrease in marrow megakaryocytes developed, suggesting that the dominant effect of the antibody was to reduce platelet production. Although we have not shown that the antibodies produced in these dogs are reacting with endogenous canine ML, we believe this is the likely mechanism because of known differences in the amino acid sequence for human and canine ML.19 Cross-reacting and neutralizing antibodies to homologous proteins administered repeatedly have been demonstrated for insulin and other hormones in addition to the hematopoietic growth factors, erythropoietin and G-CSF.12 13
It is noteworthy in this study that rhML induced a significant increase in the absolute neutrophil count in two of the three dogs, but no other hematologic effects. The basis for the increase in blood neutrophils is not known, but may relate to the effects of ML to synergize with other endogenous factors to increase proliferation of early hematopoietic cells.20 Alternatively, it may be related to an antigen-antibody response to the injections of rhML. Because ML and erythropoietin have similarities of their amino acid sequence and because erythroid precursors respond to ML,21 we thought that chronic anemia might possibly develop in these animals, but this did not occur.
The results of this study are very similar to those with embryonal cell disruption or “gene knockout” experiments in mice with disruption of either the receptor for ML or ML itself.8,9 In this canine model and in the “knockout” mice, there was moderately severe thrombocytopenia, but continuation of some platelet production. In one of our dogs, we attempted to determine if treatment with recombinant human IL-6 would lead to a more severe thrombocytopenia by induction of anti-rhIL-6 antibodies, assuming that IL-6 is critical for maintaining the basal level of platelet production in the rhML-treated animals.22,23 Although platelet counts increased and then decreased and anti–IL-6 activity was induced, the magnitude of these changes was relatively small. Other cytokines, such as IL-1, IL-3, or IL-11 are also candidates for a role in maintaining platelet production in states of ML deficiency. Although we have not directly examined the effects of IL-11 administration in dogs made thrombocytopenic by repeated administration of rhML, some relevant information is available. Normal dogs given recombinant human IL-11 develop thrombocytosis. With 2 weeks of treatment and longer follow-up, they do not develop thrombocytopenia.24 Serum samples from these animals, however, show that treatment induces anti–rhIL-11 activity by immunoassay (Dale and Nash, unpublished observations).
The results of this study point out the critical role of ML in maintaining normal platelet levels. They also serve to emphasize that species-specific hematopoietic growth factors are required for long-term studies of the physiologic or pharmacologic effects of these cytokines. In this regard, it is also important to maintain surveillance for the potential antigenicity of each new growth factor introduced into clinical practice, even minor differences from the natural hormones may result in blunted effects due to antibody formation.
ACKNOWLEDGMENT
The authors gratefully acknowledge the technical assistance of Linda Weber, Elin Rodger, Richard Person, Nathan Bittner, Michael Chan, Craig Abrams, and Niket Shrivastava and the assistance of Phyllis Child in preparing this manuscript.
Supported by Grant No. RO1-18951 from the National Institutes of Health, Bethesda, MD, and a grant from Amgen, Thousand Oaks, CA.
Presented in part at the 37th Annual Meeting of the American Society of Hematology, Seattle, WA, December 1-5, 1995 (Blood 86:368a, 1995 [suppl 1, abstr]).
Address reprint requests to David C. Dale, MD, Department of Medicine, Box 356422, University of Washington, 1959 NE Pacific, Seattle, WA 98195-6422.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal