Abstract
We have previously characterized stromal progenitor cells contained in fetal bone marrow by fluorescence-activated cell sorting (FACS) using the differential expression of CD34, CD38, and HLA-DR, and found that a small number were contained within the CD34+ cell fraction. In the present study, the frequency of stromal progenitors in both the CD34+ and CD34− subpopulations from samples of fetal and adult bone marrow was approximately one in 5,000 of the mononuclear cell fraction. Using multiparameter single-cell sorting, one in 20 fetal bone marrow cells with the CD34+, CD38−, HLA-DR−, CDw90+ phenotype were clonogenic stromal progenitors, whereas greater than one in five single cells with the CD34−, CD38−, HLA-DR−, CDw90+ phenotype formed stromal cultures. We found that cultures initiated by hematopoietic and stromal progenitors contained within the CD34+ fraction of bone marrow cells formed mixed hematopoietic/stromal cell cultures that maintained the viability of the hematopoietic progenitor cells for 3 weeks in the absence of added hematopoietic cytokines. We characterized some of the hematopoietic cytokines synthesized by stromal cultures derived from either CD34+ or CD34− bone marrow cells using reverse transcriptase–polymerase chain reaction (RT-PCR) amplification of interleukin-3 (IL-3), stem cell factor (SCF), CD34, Flt3/Flk2 ligand (FL), and thrombopoietin (TPO) mRNA sequences. We found ubiquitous expression of TPO mRNA in greater than 90% of stromal cultures initiated by either CD34+ or CD34− cells, and variable expression of SCF, FL, and CD34 mRNA. In particular, SCF and CD34 mRNA were detected only in stromal cultures initiated by CD34+ bone marrow cells, although the differences between CD34+ and CD34− stromal cells were not statistically significant. IL-3 mRNA was not found in any stromal cultures. An enzyme-linked immunosorbent assay (ELISA) of soluble SCF and TPO present in culture supernatants demonstrated that biologically significant amounts of protein were secreted by some cultured stromal cells: eight of 16 samples of conditioned media from stromal cultures initiated by fetal and adult bone marrow contained more than 32 pg/mL SCF (in the linear range of the ELISA), with a median value of 32 pg/mL (range, 9 to 230), while 13 of 24 samples of conditioned media had more than 16 pg/mL TPO (in the linear range of the ELISA), with a median of 37 pg/mL (range, 16 to 106). Our data indicate that stromal cultures initiated by single bone marrow cells can make FL, SCF, and TPO. Local production of early-acting cytokines and TPO by stromal cells may be relevant to the regulation of hematopoietic stem cell self-renewal and megakaryocytopoiesis in the bone marrow microenvironment.
BONE MARROW provides a microenvironment that supports and regulates the growth, self-renewal, and differentiation of hematopoietic cells.1-7 The hematopoietic microenvironment of bone marrow is composed of a mixed population of endothelial cells, fibroblasts, adventitial reticular cells, macrophages, and adipocytes. They regulate hematopoiesis through direct cell-cell contact and release of growth factors. However, cellular interactions between the hematopoietic stem cell compartment and the microenvironment are not well understood, due in part to the heterogeneity of stromal cells comprising the microenvironment. It has been hypothesized that different subsets of stromal cells exist with positive and negative regulatory effects on hematopoiesis, but the relationship between functional differences in stromal cells and expression of surface markers on stromal cells has not been extensively studied.8-10 Stromal progenitors have been found within the CD34+ fraction of bone marrow, which also contains the hematopoietic progenitor pool.11 Simmons and Torok-Storb,12,13 using fluorescence-activated cell sorting (FACS) and in vitro culture, have suggested that the CD34+, Stro-1+ fraction contains most of the stromal progenitors, whereas other reports, using immunoabsorption columns, have indicated that the majority of stromal progenitors are contained within the CD34− fraction of adult bone marrow cells.14 Thus, the phenotypic identity of the bone marrow stromal progenitor cell remains unclear. A description of the different pathways of stromal cell differentiation in the bone marrow will help to distinguish between these alternatives and to elucidate the different functional properties of these cells.15-17
We began by characterizing the frequency of stromal progenitors in the CD34+ and CD34− fractions of bone marrow, and then compared the patterns of cytokines expressed by cultures initiated by cells with the CD34− or CD34+ phenotype isolated by immunomagnetic bead purification followed by limiting dilution. We then used FACS and single-cell sorting to isolate stromal cell populations defined by the pattern of CD34 and CDw90 expression from fetal bone marrow, and characterized the cytokine profile of cultures derived from single clonogenic adult and fetal bone marrow cells. We present here in a detailed analysis comparing the frequency of clonogenic stromal cells within the CD34+ and CD34− subsets of bone marrow and the functional characteristics of stromal cultures initiated by single clonogenic stromal progenitors.
MATERIALS AND METHODS
Bone marrow sample selection and preparation. Adult bone marrow cells were obtained from 10-mL aliquots of autologous and normal donor bone marrow harvested from patients at the Emory Clinic, after informed consent had been obtained. The mononuclear cell fraction from each bone marrow sample was obtained by Ficoll-Hypaque separation of low-density cells from red blood cells and granulocytes. The mononuclear cell fraction represented 20% of total nucleated cells in the aspirate, so that a typical 10-mL sample of bone marrow yielded about 40 million mononuclear cells. Human fetal bone marrow from 14- to 24-week gestation fetuses was obtained from Advanced Bioscience Resources Inc (Alameda, CA). Single-cell suspensions of fetal bone marrow were prepared by flushing the bone marrow cells out of the humori and femurs using a syringe and a 22-gauge needle into phosphate-buffered saline (PBS) containing 1% (wt/vol) bovine serum albumin (BSA). The low-density mononuclear cell fraction of fetal bone marrow obtained after Ficoll-Hypaque separation consisted of 1 × 106 to 100 × 106 cells from two femurs.
Tissue culture. The media consisted of the Terry Fox long-term myeloid media containing 12.5% (vol/vol) fetal bovine serum and 12.5% (vol/vol) horse serum (TF media). Tissue culture plates (96-well) were kept at 37°C in a 5% (vol/vol) CO2 humidified atmosphere. The growth of stromal cells in each well was determined by visual inspection between 14 and 21 days of culture. Wells were scored as positive for growth of stromal cells when they contained more than 50 adherent fibroblast-like stromal cells. In some cases, cultures initiated by single FACS-sorted bone marrow cells were expanded by a single passage in tissue culture using trypsin/EDTA to detach adherent stromal cells, followed by replating into fresh TF media.
Magnetic cell sorting of human cells. The Mini MACS kit for magnetic labeling and isolation of progenitor cells from bone marrow expressing the human CD34 antigen was purchased from Miltenyi Biotech Inc (Sunnyvale, CA). The bone marrow cell suspension was first stained using an anti-CD34 antibody, followed by a second-step reagent containing immunomagnetic beads that bound to CD34+ cells. CD34+ cells were absorbed to the column matrix in the presence of an external magnetic field. The column was washed, and the retained cells (CD34+ fraction) were eluted by removing the column from the magnetic field. The purity of the CD34+ fraction was routinely 75% to 85% (mean, 80%). CD34+ cells were stained with a second anti-CD34 monoclonal antibody, HPCA-2, that recognizes a different CD34 epitope (HPCA-2; BDIS, San Jose, CA) and analyzed by FACS. The CD34− fraction contained less than 10% of the original content of CD34+ cells when restained with HPCA-2 and analyzed by FACS.
FACS and analysis. Cell suspensions of fetal bone marrow were stained with saturating concentrations of combinations of CD34 (HPCA-2) Allophycocyanine (APC) (BDIS), a combination of CD38 phycoerythrin (PE) (BDIS), and HLA-DR PE (BDIS) and CDw90 fluorescein (PharMingen, San Diego, CA). Cells from each population with the phenotypes (1) CD34+, CD38−, HLA-DR−, CDw90+, (2) CD34+, CD38−, HLA-DR−, CDw90, (3) CD34−, CD38−, HLA-DR−, CDw90+, and (4) CD34−, CD38−, HLA-DR−, CDw90− were sorted into the individual wells of a 96-well flat-bottom tissue culture plate containing cell culture media using a FACS Vantage Cell Sorter (BDIS) equipped with an automatic cell deposition unit (ACDU), a Coherent Enterprise laser tuned at 488 nm (100 mW), and a Coherent Spectrum laser tuned at 647 nm (100 mW). The gate for positively stained cells was set according to a fluorescently isotype-conjugated MoAb, and usually was between the first and second decade of fluorescence intensity. In some cases, such as when the PE-conjugated CD38 antibody was used, the gate for positively stained cells was between the second and third decade of fluorescence intensity (Fig 1).
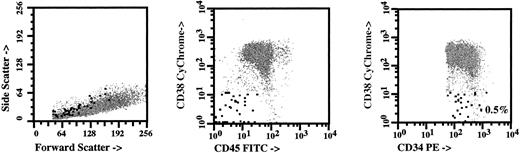
Flow cytometric analysis of CD34+, CD38−, CD45− adult bone marrow cells. Suspensions of low density mononuclear cells from human adult bone marrow were stained with fluorescently labeled monoclonal antibodies. CD34+ cells were collected into a list mode data file and analyzed using the Paint-a-Gate Plus program (BDIS). The axis of each panel is labeled according to the light scatter parameters examined, or the antigen recognized by the monoclonal antibody used, and the corresponding fluorescence conjugate (ie, CD38 Cychrome). The putative stromal cells (CD34+, CD38−, CD45−) were present at frequency of 0.5% and were shown as a large block dots, while CD34+ hematopoietic progenitors are shown as smaller gray dots.
Flow cytometric analysis of CD34+, CD38−, CD45− adult bone marrow cells. Suspensions of low density mononuclear cells from human adult bone marrow were stained with fluorescently labeled monoclonal antibodies. CD34+ cells were collected into a list mode data file and analyzed using the Paint-a-Gate Plus program (BDIS). The axis of each panel is labeled according to the light scatter parameters examined, or the antigen recognized by the monoclonal antibody used, and the corresponding fluorescence conjugate (ie, CD38 Cychrome). The putative stromal cells (CD34+, CD38−, CD45−) were present at frequency of 0.5% and were shown as a large block dots, while CD34+ hematopoietic progenitors are shown as smaller gray dots.
Limiting dilution analysis of clonogenic cells. Serial dilutions of bone marrow cell suspensions were cultured at a range of cell concentrations (10 cells/well to 106 cells/well) in TF media alone. After 18 to 21 days, the cultures were scored for the presence of stromal growth. The frequency of negative wells was plotted against the logarithm of the number of cells plated in each well, and a straight line was fitted against the points for each type of cultured cell. The frequency of clonogenic cells in each population was determined by calculating the intercept on the abscissa at the point at which 1/e (∼37%) of the wells failed to show growth.18
Cocultures of stromal and hematopoietic cells. Stromal cell cultures were initiated with CD34+ and CD34− cells in duplicate. After 2 weeks of culture, the monolayers were washed with media to remove nonadherent cells, and they were then exposed to 20 Gy ionizing radiation from a 137Cs source in a single fraction. CD34+ cells (predominantly hematopoietic progenitors) isolated from an unrelated allogeneic donor by mini MACS and containing 70% CD34+ cells (as assessed by FACS) were added to the irradiated stromal monolayers derived from CD34+ or CD34− bone marrow cells in TF media without additional cytokines.
FACS analysis of nonadherent cells in cocultures of CD34+ hematopoietic cells and stromal monolayer. Following 2 weeks of culture, nonadherent cells from mixed stromal cell/hematopoietic cell cultures were gently aspirated in culture media and collected by centrifugation at 1,300 rpm for 10 minutes. Cell suspensions containing 1 × 105 nonadherent cells were stained with saturating concentrations of combinations of CD19 (a B-cell marker; BDIS), CD13 (a marker of myelomonocytic cells; BDIS), CD33 (a myeloid marker; BDIS), CD34 (a marker for hematopoietic progenitor cells; BDIS), and CD38 (HPCA-2, a marker for differentiated hematopoietic cells; BDIS). List mode files of 20,000 events were collected and analyzed for the presence of hematopoietic progenitors (CD34+) and differentiated hematopoietic cells (CD19+, CD13+, CD33+, and CD38+ subsets).
Methylcellulose cultures. Nonadherent cells from mixed stromal/hematopoietic cultures initiated with CD34+ adult allogenic bone marrow samples were gently aspirated. The nonadherent cells plus media were centrifuged at 1,300 rpm for 10 minutes, resuspended in 1 mL PBS + 5% FBS, and counted. Each sample containing 3 × 105 cells in 300 μL (1 mL media was added to 2.7 mL Methocult GF +H4435 methylcellulose) was vortexed and allowed to settle. Two separate aliquots of 1 mL cells plus media from each sample were plated in gridded NUNC petri dishes at a final concentration of 1 × 105 cells/mL. The cultures were scored for the presence of hematopoietic colonies after 10 to 12 days according to established criteria.19
Plasma clot cultures. Nonadherent cells (3 × 105) from a mixed stromal hematopoietic cell culture initiated by CD34+ cells from adult bone marrow were cultured in 1 mL media containing 10 ng/mL PEG-MGDF and 15% (vol/vol) citrated plasma in a semisolid plasma clot.20 Following 8 days' culture at 37°C, cultures were fixed with methanol:acetone (1:1) and dried overnight. After rehydration, the cells were stained with antiplatelet antibodies IIb, IIIa, and Ib followed by fluorescein-conjugated goat anti-mouse sera (Bio-Design, Kennebunk, ME) and propidium iodide at a concentration of 0.5%.20 A cluster of three or more positive stained cells were scored as one colony-forming unit–megakaryocytes (CFU-MEG).20
RNA extraction. Clonogenic stromal cell cultures derived from single FACS-sorted fetal bone marrow cells or CD34− or CD34+ adult bone marrow cells were allowed to grow to near-confluency in individual wells of a 96-well tissue culture plate. The media and nonadherent cells were removed, and the stromal monolayer was washed twice with PBS. Adherent cells were solubilized by addition of GITC solution (final concentrations, 4 mol/L guanidine isothiocyanate, 0.5% sarcosyl, 25 mmol/L citric acid, pH 7.0, and 0.007% β-mercaptoethanol), and the lysate was sequentially mixed with 0.2 mol/L sodium acetate, pH 4.0, 1 vol phenol, and 1/10 vol 49:1 chloroform:isoamyl alcohol and then cooled on ice for 15 minutes. The aqueous layer was removed and precipitated at −20°C in isopropanol. The RNA was reprecipitated by addition of equal volumes of GITC solution and isopropanol, dried, and resuspended in RNase-free water.21
DNA probes. Synthetic oligonucleotides encoding the cDNA sequence of hematopoietic cytokines were prepared in the Microchemical Facility of the Winship Center at Emory University (Table 3). All oligonucleotides were end-labeled using polynucleotide kinase according to instructions provided by the manufacturer (Promega, Madison, WI).22
Oligonucleotide Primers and Internal Probes
| Fibronectin (305 bp) | Sense: 5′TGTCTATGCTCAGAATCCAAGCGG-3′ |
| Antisense: 5′GCTGGCTCTCCATATCATCGTGC-3′ | |
| Internal probe: 5′TAGGGCGATCAATGTTGGTTACTG-3′ | |
| SCF (502 bp) | Sense: 5′GCCGCTGTTCGTGCAATATGC-3′ |
| Antisense: 5′GGAGTAAAGAGCCTGGGTTCTGGG-3′ | |
| Internal probe: 5′TGTGGAGTGCGTCAAAGA-3′ | |
| CD34 (659 bp) | Sense: 5′CAAGGCAGAAATCAAATGTTCAGGC-3′ |
| Antisense: 5′TCACCTAGCCGAGTCACAATTCG-3′ | |
| Internal probe: 5′TGTGGAGTGCGTCAAAGA-3′ | |
| IL-3 (500 bp) | Sense: 5′TGCTGGACTTCAACAACCTCAATGG-3′ |
| Antisense: 5′CAACCGCACAAGGCCAAATGG-3′ | |
| Internal probe: 5′CACCCACGCGACATCCAA-3′ | |
| TPO (347 bp) | Sense: 5′GTAGGGGTGGGCGTTGGAGCAG-3′ |
| Antisense: 5′AAGTGGCAGCAGGGATTCAGAGC-3′ | |
| Internal probe: 5′CCTAGGAGCCCCGGACATTTC-3′ | |
| FL (517 bp) | Sense: 5′CCCCCGGCCGAAATGACAGTG-3′ |
| Antisense: 5′ATGGGGGTGGCAGGGTTGAGGA-3′ | |
| Internal probe: 5′CACAGCCCCGATCTCCGACTTC-3′ |
| Fibronectin (305 bp) | Sense: 5′TGTCTATGCTCAGAATCCAAGCGG-3′ |
| Antisense: 5′GCTGGCTCTCCATATCATCGTGC-3′ | |
| Internal probe: 5′TAGGGCGATCAATGTTGGTTACTG-3′ | |
| SCF (502 bp) | Sense: 5′GCCGCTGTTCGTGCAATATGC-3′ |
| Antisense: 5′GGAGTAAAGAGCCTGGGTTCTGGG-3′ | |
| Internal probe: 5′TGTGGAGTGCGTCAAAGA-3′ | |
| CD34 (659 bp) | Sense: 5′CAAGGCAGAAATCAAATGTTCAGGC-3′ |
| Antisense: 5′TCACCTAGCCGAGTCACAATTCG-3′ | |
| Internal probe: 5′TGTGGAGTGCGTCAAAGA-3′ | |
| IL-3 (500 bp) | Sense: 5′TGCTGGACTTCAACAACCTCAATGG-3′ |
| Antisense: 5′CAACCGCACAAGGCCAAATGG-3′ | |
| Internal probe: 5′CACCCACGCGACATCCAA-3′ | |
| TPO (347 bp) | Sense: 5′GTAGGGGTGGGCGTTGGAGCAG-3′ |
| Antisense: 5′AAGTGGCAGCAGGGATTCAGAGC-3′ | |
| Internal probe: 5′CCTAGGAGCCCCGGACATTTC-3′ | |
| FL (517 bp) | Sense: 5′CCCCCGGCCGAAATGACAGTG-3′ |
| Antisense: 5′ATGGGGGTGGCAGGGTTGAGGA-3′ | |
| Internal probe: 5′CACAGCCCCGATCTCCGACTTC-3′ |
Primer pairs are shown for the amplification of human cytokine messages by PCR and internal primers used as probes for the hybridization. Sequences are in 5′-3′ orientation.
RT-PCR. cDNA was synthesized from 1.0 g RNA using random primers in a buffer consisting of 20 mmol/L Tris HCl, pH 8.4, 50 mmol/L KCl2 , 1.5 mmol/L MgCl2 , 0.2 mmol/L of each dATP, dCTP, dGTP, and dTTP, 250 ng random hexamers, and 2 U/mL reverse transcriptase (AMV-Reverse transcriptase, GIBCO-BRL, Gaithersburg, MD). After a 50-minute incubation at 42°C, the reaction was terminated by heating to 70°C for 15 minutes, followed by addition of E coli RNase H (2 U/mL) and an additional 20-minute incubation at 37°C. cDNA extraction was performed using the kit for first-strand cDNA synthesis provided by GIBCO-BRL.23 24 The cDNA of actin, fibrinonectin, CD34, or cytokine was amplified using primers specific for the gene of interest. A one-tenth volume of the first-strand cDNA reaction mixture was used for each PCR reaction in RT buffer, containing 0.1 nm of each of the sense and antisense oligonucleotides of interest and 0.025 U/mL Taq polymerase. The reaction mixture was heated to 94°C for 2 minutes to denature the RNA/cDNA hybrid, and then thermocycled 30 times at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes.
Alkaline Southern blot analysis. PCR products were separated by electrophoresis in 1.5% agarose in 1× Tris-borate-EDTA (89 mmol/L Tris-borate and 2 mmol/L EDTA) buffer and visualized using ethidium bromide (0.5 μg/mL). PCR amplification using specific primers yielded bands of the size expected for cDNA generated by the primers used: fibrinonectin (FIB), 305 bp; stem cell factor (SCF), 502 bp; CD34, 659 bp; interleukin-3 (IL-3), 500 bp; thrombopoietin (TPO), 347 bp; Flt3/flk2 ligand (FL), 517 bp. The DNA products in agarose gel were transferred to nylon (N+-Hybond) using 20× SSC (1× SSC is 0.15 mol/L sodium chloride and 0.015 mol/L sodium citrate).25 The identity of PCR products present on the nylon membrane was confirmed by Southern blot analysis using a third 32P-labeled oligonucleotide as a specific hybridization probe containing a cDNA sequence internal primer. The membranes were hybridized with 32P-labeled probe in 6× SSC, 5× Denardt solution (0.1% polyvinylpyrrolidone, 0.1% Ficoll type 400, and 0.1% BSA), 1.0% sodium dodecyl sulfate (SDS), 0.01 mol/L EDTA, and 100 μg/mL denatured sonicated salmon sperm DNA at 65°C for 12 to 18 hours. The membrane was washed in 6× SSC for 15 minutes at 65°C, followed by two washes in 2× SSC for 10 minutes at 65°C. The membranes were exposed to x-ray film (Kodak Biomax Films; Arlington Heights, IL) for 24 hours at −70°C with an intensifying screen.26
TPO ELISA. Polyclonal antibodies raised against recombinant human MPL ligands were used for antigen capture and signal generation. Anti-MPL ligand antibodies and human MPL ligand were generously provided by Janet L. Nichol and Alex C. Hornkohl (Amgen, Thousand Oaks, CA). This assay is designed for analyzing human serum or heparinized plasma. Plates (Costar) were precoated with rabbit anti-rHuMPL ligand antibodies at 2 μg/mL. Culture supernatants were TF media containing 12.5% horse serum and 12.5% fetal bovine serum conditioned by stromal cell cultures initiated by either CD34+ or CD34− cells isolated from fetal and adult bone marrow. Conditioned media samples were diluted to 50% (vol/vol) with dilution buffer (.1% Tween 20, 150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH adjusted to 7.5) and incubated for 18 hours at room temperature. The plates were washed twice with EIA wash buffer (0.05% Tween 20 and 1× TNE). Bound TPO was detected by addition of rabbit anti-rHuMPL ligand and HRPO conjugate (Amgen), followed by addition of TMB peroxidase substrate/peroxidase solution B (1:1) to each well (Kirkegard & Perry Laboratories, Gaithersburg, MD). The reaction was stopped with addition of 100 μL 0.5N H2SO4 , and absorbance was measured at 450 to 650 nm using a Microplate Autoloader (Molecular Device Corp, Sunnyvale, CA). The culture supernatants were analyzed at two half-log dilutions in triplicate, ensuring optical densities within the linear portion of the standard curve. A standard curve using samples containing TPO at concentrations of 8 to 1,024 pg/mL in a 50:50 mixture of dilution buffer and TF media was used to calculate the TPO concentration in experimental samples. The standard curve was linear in the range of TPO concentrations between 8 and 1,024 pg/mL with corresponding OD values of 0.019 and 3.92. Negative controls, which consisted of TF media alone, had an OD of less than 0.01 in this ELISA; positive controls consisted of serum from two reference patients, and had OD values of 0.514 and 2.450, corresponding to TPO concentrations of 110 and 500 pg/mL, respectively.
IL-3 and SCF ELISA. Microtiter plates precoated with a murine monoclonal antibody specific for IL-3 and SCF were purchased from R&D Systems Inc (Minneapolis, MN). Recombinant human IL-3 and SCF standards and samples of conditioned media assayed according to the manufacturers' instructions were pipetted into each well in the amount of 100 μL plus 100 μL assay diluent RD11. After washing three times with Wash Buffer, 200 μL polyclonal antisera specific for either IL-3 or SCF and conjugated to HRPO, were added to the wells and incubated for 2 hours at room temperature. Following a wash to remove any unbound antibody-enzyme reagent, 200 μL stabilized hydrogen peroxide was added to the wells and incubated for 20 minutes at room temperature. The color developed in proportion to the amount of IL-3 or SCF bound in the initial step. Color development was stopped with 50 μL stabilized chromogen, and the OD of each well within 30 minutes was determined using a spectrophotometer set to 450 nm. A standard curve was prepared by plotting OD versus the concentration of IL-3 or SCF in wells containing 8 to 2,048 pg/mL recombinant cytokine supplied by the manufacturer. The concentration of IL-3 and SCF in the unknown samples was determined by comparing the OD of samples against the standard curve. Standard curves for SCF and IL-3 were linear in the range of 32 to 2,000 pg/mL.
RESULTS
Presence of CD34+, CD38−, CD45− cells in adult bone marrow cells detected by FACS analysis. The great majority of clonogenic stromal progenitors (>80%) and hematopoietic stem cells are contained within the mononuclear cell fraction of bone marrow cells.27 We have previously shown that a rare subset of CD34+ mononuclear cells in fetal bone marrow samples contained stromal progenitors.28 We used flow cytometry to analyze the phenotype of CD34+ cells in samples of adult bone marrow, and noted that a population of CD34+, CD38−, CD45− cells were consistently present at a mean frequency of 0.3% ± 0.8% of CD34+ cells and comprised 0.003% of total nucleated cells. The CD34+, CD38−, CD45− phenotype of these cells corresponded to the phenotype of CD34+, CD38−, HLA-DR− stromal cells that we have previously characterized in fetal bone marrow.11 A representative FACS plot of CD34+ cells from adult bone marrow is shown in Fig 1, with the putative stromal cells (CD34+, CD38−, CD45−) shown as large black dots and hematopoietic progenitors shown as smaller gray dots (Fig 1).
Clonogenic stromal cells are most frequent in the CD34−, CDw90+ subset of adult bone marrow isolated by MACS and LDA. We analyzed the frequency of stromal progenitors in bone marrow subsets defined by expression of CD34 and CDw90, isolated by immunomagnetic bead purification (MACS) followed by limiting dilution analysis (LDA) and Poisson analysis (Table 1 and Fig 2). The median frequency of clonogenic stromal cells in 22 samples of unfractionated bone marrow mononuclear cells from adult patients was one in 4,000, with similar frequencies of stromal cells found in the CD34− (1 in 4,500) and CD34+ (1 in 5,000) fractions. The CDw90+, CD34− fraction of adult bone marrow cells had the highest median frequency of stromal progenitors (1 in 500), while the lowest median frequency of stromal progenitor cells was found in the CD34−, CDw90− fraction (1 in 200,000) (Table 1 and Fig 2). We measured the frequency of stromal progenitors in samples of peripheral blood mononuclear cells obtained by apheresis, and found that they were present at a median frequency of less than one in 100,000 (data not shown). Approximately half of the low-density mononucleated cells in bone marrow aspirate are derived from contaminating peripheral blood cells. The frequencies of stromal progenitors that we have calculated are conservative estimates of the true frequency in the bone marrow microenvironment, since contaminant peripheral blood contains very few stromal progenitors.
Poisson Analysis of the Relative Frequency and Absolute Number of CD34+ and CD34− Stromal Progenitors per 1 × 106 Adult Bone Marrow Mononuclear Cells
| . | Bone Marrow . | CD34+ . | CD34− . | CDw90+, . | CDw90−, . |
|---|---|---|---|---|---|
| . | Mononuclear Cells . | . | . | CD34− . | CD34− . |
| Median frequency in BM | 1/4,000 | 1/5,000 | 1/4,500 | 1/500 | 1/200,000 |
| Stromal progenitors per 1 × 106 BM cells | 250 | 2 | 200 | ND | ND |
| n = 22 | n = 22 | n = 22 | n = 5 | n = 5 |
| . | Bone Marrow . | CD34+ . | CD34− . | CDw90+, . | CDw90−, . |
|---|---|---|---|---|---|
| . | Mononuclear Cells . | . | . | CD34− . | CD34− . |
| Median frequency in BM | 1/4,000 | 1/5,000 | 1/4,500 | 1/500 | 1/200,000 |
| Stromal progenitors per 1 × 106 BM cells | 250 | 2 | 200 | ND | ND |
| n = 22 | n = 22 | n = 22 | n = 5 | n = 5 |
CD34+ and CD34− bone marrow subset cells, selected using immunomagnetic beads, were plated at a series of concentrations from 5 × 104 to 1 × 102 cells/well into 8 to 24 wells in a 96-well tissue culture plate containing 200 μL tissue culture medium. Each well was scored for the growth of 50 or more fibroblastic cells after 14 to 21 days of culture at 37°C. The frequency of clonogenic stromal cells was determined as the cell concentration at which 37% of the wells showed no stromal growth. ND, not determined.
Limiting dilution analysis of stromal progenitors in adult bone marrow. Bone marrow cells were plated at 100 to 500,000 cells/well in a 96-well plate with media containing 25% serum. Wells were sorted for the growth of stromal cells after 2 to 3 weeks of culture. The mean frequency (±SEM) of negative wells was plotted against the logarithm of cells plated into each set of wells. The frequency of clonogenic stromal cells was determined from the inverse of the cell concentration at which 37% of the wells lacked the growth of stromal cells.
Limiting dilution analysis of stromal progenitors in adult bone marrow. Bone marrow cells were plated at 100 to 500,000 cells/well in a 96-well plate with media containing 25% serum. Wells were sorted for the growth of stromal cells after 2 to 3 weeks of culture. The mean frequency (±SEM) of negative wells was plotted against the logarithm of cells plated into each set of wells. The frequency of clonogenic stromal cells was determined from the inverse of the cell concentration at which 37% of the wells lacked the growth of stromal cells.
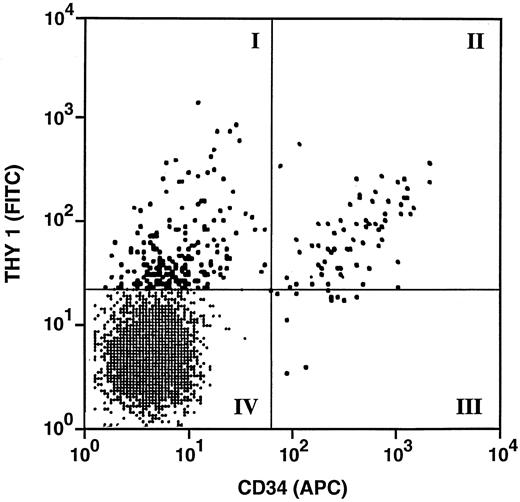
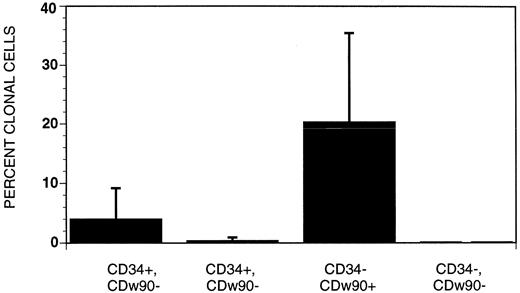
Clonogenic stromal cells are most frequent in the CD34+, CDw90+ and CD34−, CDw90+ fractions of fetal bone marrow isolated by FACS and single-cell sorting. To define the phenotype of stromal progenitors more precisely, four populations of CD38−/HLA-DR− fetal bone marrow cells were sorted based on the pattern of CDw90/Thy-1 and CD34 expression (Fig 3). Of fetal bone marrow cells, 18.9% lacked expression of CD38 and HLA-DR and were within the forward- and side-scatter gate that involved lymphocytes and monocytes (data not shown). The most abundant population consisted of cells that lacked expression of CD34 and CDw90 (lower left quadrant: 94% of CD38−/HLA-DR− bone marrow cells). The next most abundant cells were those that expressed CDw90 without CD34 (upper left quadrant: 3.9% of CD38−/HLA-DR− bone marrow cells), followed by CD34+, CDw90+ cells (upper right quadrant: 1.1% of CD38−/HLA-DR− cells) and very rare CD34+, CDw90− cells (lower right quadrant: 0.4% of CD38−/HLA-DR− cells). Single cells from each of the four quadrants were sorted into individual wells of a 96-well tissue culture plate with media containing 25% (vol/vol) serum. After 18 to 21 days of culture, the cultures were sorted for the presence of stroma growth (Fig 4). The greatest frequency of stromal progenitors was seen in the CDw90+, CD34− population, in which a mean of 20.4% ± 15.1 single sorted cells were clonogenic stromal progenitors. The population with the next highest frequencies of clonogenic stromal progenitors were the CD34+, CDw90+ cells. Stromal progenitors were largely absent in the two CDw90− populations.
FACS sort gates for the isolation of stromal progenitors from the CD38−, HLADR− fraction of fetal bone marrow cells. Four populations were sorted, based on their pattern of CDw90/Thy1 and CD34 expression. Note that there were relatively more CD34−, CDw/Thy-1+ cells compared to CD34+, CDw90/Thy-1 cells. The CD34+ and CD90+ cells are shown as large black dots; the CD34− and CDw90− cells were shown in the lower left quadrant.
FACS sort gates for the isolation of stromal progenitors from the CD38−, HLADR− fraction of fetal bone marrow cells. Four populations were sorted, based on their pattern of CDw90/Thy1 and CD34 expression. Note that there were relatively more CD34−, CDw/Thy-1+ cells compared to CD34+, CDw90/Thy-1 cells. The CD34+ and CD90+ cells are shown as large black dots; the CD34− and CDw90− cells were shown in the lower left quadrant.
Frequency of clonogenic stromal progenitors among different populations of CD38−, HLA-DR–fetal bone marrow cells. Single cells in each of the four quadrants were sorted into individual wells of a 96 multi-well tissue culture plate with media containing 25% vol/vol serum. The greatest frequency of stromal progenitors was seen in the CDw90+ CD34− population, the second most homogeneous population was the CD34+ CDw90+ cells. Stromal progenitors were largely absent in the CDw90− Thy1− populations.
Frequency of clonogenic stromal progenitors among different populations of CD38−, HLA-DR–fetal bone marrow cells. Single cells in each of the four quadrants were sorted into individual wells of a 96 multi-well tissue culture plate with media containing 25% vol/vol serum. The greatest frequency of stromal progenitors was seen in the CDw90+ CD34− population, the second most homogeneous population was the CD34+ CDw90+ cells. Stromal progenitors were largely absent in the CDw90− Thy1− populations.
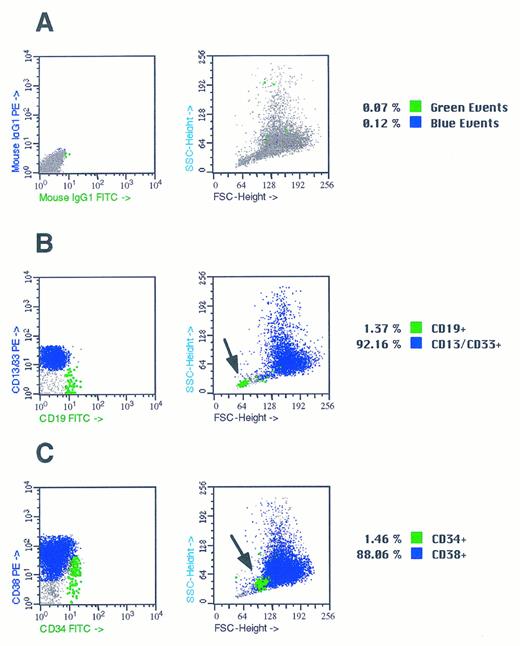
Hematopoietic progenitors survive in stromal cultures initiated by CD34+ and CD34− bone marrow cells in the absence of exogenous cytokines. We noted that bulk cultures of 100 to 10,000 CD34+ bone marrow cells produced a mixture of both stromal and hematopoietic cells after 21 days of culture in TF media containing 25% (vol/vol) serum and no additional cytokines. This observation is consistent with previous studies in which we have demonstrated that separate populations of hematopoietic and stromal progenitors are contained within the CD34+ fraction of bone marrow.11 Morphologic analysis of nonadherent cells present in 3-week cultures of CD34+ bone marrow cells showed round myelomonocytic blasts. FACS analysis of nonadherent cells demonstrated that the majority were committed myelomonocytic cells that expressed CD13 and/or CD33 (Fig 5). A smaller fraction of nonadherent cells expressed the CD34 antigen, and some of these were CD38− (Fig 5). In addition, we consistently noted a population with light-scatter properties of lymphocytes that expressed the B-cell markers CD19 and were present at a frequency of 2% (Fig 5). We then assayed the nonadherent cells in these mixed stromal/hematopoietic cultures for the presence of hematopoietic colony-forming cells in methylcellulose and plasma clot assays. We found significant numbers of erythroid burst-forming units (BFU-E), granulocyte-monocyte colony-forming units (CFU-GM), granulocyte-erythroid-monocyte-macrophage colony-forming units (CFU-GEMM), and colony-forming units–megakaryocyte (CFU-MEG) (Table 2).20 The frequency of CFU-MEG was 2/1 × 105 nonadherent cells in the first experiment and 6 CFU-MEG/1 × 105 nonadherent cells in the second experiment. The frequency of CFU-MEG/1,000 CD34+ cells in nonadherent cells from these cultures (0.15 and 0.48 per 1,000 CD34+ cells) was similar to the frequency of CFU-MEG in fresh aspirates of bone marrow.19 20
Flow cytometric analysis of nonadherent cells in mixed CD34+ hematopoietic and stromal cell cultures. Suspensions of nonadherent cells from 3-week-old mixed stromal cell/hematopoietic cell culture initiated by CD34+ bone marrow cells were analyzed by flow cytometry. Cell suspensions containing 1 × 105 nonadherent cells were stained with fluorescently labeled CD13, CD19, CD33, CD38, and IgG isotope monoclonal antibodies as described in Materials and Methods. (A) FACS plots of nonadherent cells stained with antibodies Isotype control IgG1 monoclonal antibodies conjugated with PE and FITC. Nonspecifically stained cells are shown as large black dots and comprised 0.4% of the events in the data file. (B) FACS plots of nonadherent cells stained with antibodies CD13/33 PE and CD19 FITC. CD13/CD33+ myelomonocytic cells were present at frequency of 80% and CD19 B cells were present at a frequency of 1.4% and are shown as a large green block of dots. The position of the CD19+ cells in plot of forward scatter versus side scatter is shown by the arrow in the right hand panel. (C) FACS plots of nonadherent cells stained with antibodies CD38 PE CD34 FITC. CD38+, CD34− were 88.06%: 5% of the cells were CD34+ and are shown as large black dots in the left panels. The position of the CD34+ cells in the plot of forward scatter versus side scatter is shown by the arrow in the right hand panel.
Flow cytometric analysis of nonadherent cells in mixed CD34+ hematopoietic and stromal cell cultures. Suspensions of nonadherent cells from 3-week-old mixed stromal cell/hematopoietic cell culture initiated by CD34+ bone marrow cells were analyzed by flow cytometry. Cell suspensions containing 1 × 105 nonadherent cells were stained with fluorescently labeled CD13, CD19, CD33, CD38, and IgG isotope monoclonal antibodies as described in Materials and Methods. (A) FACS plots of nonadherent cells stained with antibodies Isotype control IgG1 monoclonal antibodies conjugated with PE and FITC. Nonspecifically stained cells are shown as large black dots and comprised 0.4% of the events in the data file. (B) FACS plots of nonadherent cells stained with antibodies CD13/33 PE and CD19 FITC. CD13/CD33+ myelomonocytic cells were present at frequency of 80% and CD19 B cells were present at a frequency of 1.4% and are shown as a large green block of dots. The position of the CD19+ cells in plot of forward scatter versus side scatter is shown by the arrow in the right hand panel. (C) FACS plots of nonadherent cells stained with antibodies CD38 PE CD34 FITC. CD38+, CD34− were 88.06%: 5% of the cells were CD34+ and are shown as large black dots in the left panels. The position of the CD34+ cells in the plot of forward scatter versus side scatter is shown by the arrow in the right hand panel.
Cultures of CD34+ Stromal and Hematopoietic Bone Marrow Cells Maintain Colony-Forming Cells in the Absence of Exogenous Cytokines
| . | BFU-E . | CFU-E . | CFU-G . | CFU-M . | CFU-GM . | CFU-GEMM . | CFU-MEG . |
|---|---|---|---|---|---|---|---|
| Experiment 1 | 35 | 26 | 50 | 40 | 23 | 2 | 2 |
| Experiment 2 | 17 | 46 | 22 | 44 | 3 | 1 | 6 |
| . | BFU-E . | CFU-E . | CFU-G . | CFU-M . | CFU-GM . | CFU-GEMM . | CFU-MEG . |
|---|---|---|---|---|---|---|---|
| Experiment 1 | 35 | 26 | 50 | 40 | 23 | 2 | 2 |
| Experiment 2 | 17 | 46 | 22 | 44 | 3 | 1 | 6 |
Nonadherent cells were gently aspirated from mixed hematopoietic/stromal cultures that had been initiated with CD34+ cells from adult bone marrow samples. Nonadherent cells plus media were centrifuged at 1,300 rpm for 10 minutes, resuspended in 1 mL PBS + 5% FBS, and counted. For methylcellulose cultures and plasma clot assay, 3 × 105 CD34+ nonadherent cells were plated in triplicate wells. CFC activity in methylcellulose was counted after 2 weeks of culture; the number of CFU-MEG was counted after 8 days of culture.
Stromal cultures initiated by both CD34+ and CD34− stromal progenitors supported the survival and expansion of CD34+ hematopoietic cells. CD34-enriched adult bone marrow cells (5 × 104, containing 3.5 × 104 CD34+ cells) were added to preestablished stromal monolayers that had been initiated by either CD34+ or CD34− stromal progenitors, grown to confluency over 2 to 3 weeks, and then irradiated to 20 Gy. After 2 weeks of coculture on preformed stroma, nonadherent cells were counted, and the fraction expressing CD34, CD19, CD33, and CD38 was assessed by flow cytometry. Control stromal cultures that were irradiated and cultured without addition of nonadherent CD34+ cells showed less than 150 viable nonadherent cells. FACS analysis of nonadherent cells present on CD34− stromal and CD34+ stromal cocultures showed that the majority were hematopoietic cells. Of note, a mean of 18% of nonadherent cells from the coculture of hematopoietic progenitors with CD34− stroma were CD34+, CD38+ and 2.5% were CD34+, CD38− (n = 2). Analysis of nonadherent hematopoietic cells cocultured with stroma generated by CD34+ bone marrow cells showed that a mean of 14% were CD34+, CD38+ and 1.6% were CD34+, CD38− (n = 2). CD19+ B cells were present at a frequency of 1.3% in cultures of hematopoietic cells on either CD34+ or CD34− stroma, with the remainder of nonadherent cells expressing the myelomonocytic markers CD13 or CD33. There was a mean sevenfold expansion of CD34+ hematopoietic cells when hematopoietic progenitors were cocultured on preestablished stroma initiated by CD34+ cells, and a mean 13-fold expansion of CD34+ cells when hematopoietic progenitors were cocultured on preestablished CD34 stroma (n = 2). Thus, stromal cultures initiated by either CD34+ or CD34− bone marrow cells were able to support the survival and expansion of CD34+ hematopoietic progenitor cells.
Cytokine expression profiles of fetal and adult bone marrow stromal cells measured by RT-PCR and Southern blot. We analyzed the presence of mRNA sequences encoding hematopoietic cytokines in stromal cell cultures derived from the single clonogenic CD34+ and CD34− cells of fetal bone marrow and from CD34+ and CD34− cells of adult bone marrow stromal cells. RNA was extracted from 29 primary cultures from fetal bone marrow and 25 primary cultures from adult bone marrow and transcribed into cDNA by addition of random hexamer primers and reverse transcriptase. cDNA sequences were amplified using specific primers and the PCR, followed by Southern blot transfer and hybridization with internal primers specific for the genes of interest21 23 (Table 3). We tested stromal cultures initiated by CD34+ or CD34− stromal progenitor cells for the presence of mRNA encoding SCF, IL-3, TPO, FL, and the CD34 antigen. Fibronectin, a stromal cell–specific marker, and actin were used as controls for the presence of intact mRNA. We found that TPO mRNA was ubiquitously expressed in stromal cultures initiated by either CD34+ (10 of 11) or CD34− cells (23 of 25), and that FL was variably expressed in cultures initiated by either CD34+ (4 of 11) or CD34− (3 of 25) bone marrow cells. CD34 mRNA and SCF mRNA were only found in one of four stromal cultures (CD34) and in two of seven (SCF) initiated by CD34+ bone marrow cells. IL-3 mRNA was not found in any stromal cultures (Table 4). We failed to find statistically significant differences in the pattern of cytokine synthesis between cultures derived from either CD34+ or CD34− stromal progenitor cells or from samples of either fetal or adult bone marrow. A representative Southern blot showing the cytokine pattern in samples of fetal and adult bone marrow is shown in Fig 6.
mRNA Cytokine Expression in Stromal Cultures of Fetal and Adult Bone Marrow
| Phenotype . | Bone . | Fibronectin . | TPO . | FL . | IL-3 . | SCF . | CD34 . |
|---|---|---|---|---|---|---|---|
| . | Marrow . | . | . | . | . | . | . |
| CD34+, THY-1+ | Fetal | 6/7 | 6/7 | 2/7 | 0/4 | 2/2 | 1/2 |
| CD34−, THY-1+ | Fetal | 4/22 | 20/22 | 2/22 | 0/22 | 0/22 | 0/22 |
| CD34+ | Adult | 5/13 | 4/4 | 2/4 | 0/2 | 0/5 | 0/2 |
| CD34− | Adult | 4/12 | 3/3 | 1/3 | 0/2 | 0/4 | 0/2 |
| Unsorted | Adult | 8/13 | 7/8 | 3/8 | ND | ND | ND |
| Phenotype . | Bone . | Fibronectin . | TPO . | FL . | IL-3 . | SCF . | CD34 . |
|---|---|---|---|---|---|---|---|
| . | Marrow . | . | . | . | . | . | . |
| CD34+, THY-1+ | Fetal | 6/7 | 6/7 | 2/7 | 0/4 | 2/2 | 1/2 |
| CD34−, THY-1+ | Fetal | 4/22 | 20/22 | 2/22 | 0/22 | 0/22 | 0/22 |
| CD34+ | Adult | 5/13 | 4/4 | 2/4 | 0/2 | 0/5 | 0/2 |
| CD34− | Adult | 4/12 | 3/3 | 1/3 | 0/2 | 0/4 | 0/2 |
| Unsorted | Adult | 8/13 | 7/8 | 3/8 | ND | ND | ND |
Twenty-nine stromal cell cultures derived from single FACS-sorted fetal bone marrow cells (F) and 25 stromal cultures that had been initiated by single adult bone marrow stromal cells (A) were analyzed for the presence of cytokine mRNA species. The presence of intact RNA in each sample was confirmed by amplifying the fibronectin (stromal cell–specific) and/or actin cDNA sequences, using specific primer sequences. The presence of specific PCR products for each gene of interest (TPO, SCF, IL-3, FL, and CD34) was confirmed by Southern blot hybridization using a 32P-labeled internal probe.
Southern blot analysis of PCR products from stromal cultures initiated by FACS sorted single cells. (A) RNA was extracted from six fetal bone marrow stromal cultures that had been initiated by CD34+ or CD34− FACS sorted single cells. (+) cDNA was synthesized by the addition of random hexamer primers and reverse transcriptase. (−) The same reaction conditions lacking reverse transcriptase were used. The presence of specific PCR products was detected by Southern blot using a Fibronectin, SCF, CD34 32P-labeled specific probes as indicated. (B) RNA was extracted from 4 unselected stromal cultures of adult bone marrow stromal cells. cDNA was synthesized by the addition of random hexamer primers and reverse transcriptase. The presence of specific PCR products was detected by Southern blot using a FL 32P-labeled internal probe as indicated. (C) RNA was extracted from 10 stromal cultures initiated by FACS sorted single cells with the CD34+ or CD34− phenotype. cDNA was synthesized by the addition of random hexamer primers and reverse transcriptase. The presence of specific PCR products was detected by Southern blot using a TPO specific 32P-labeled internal probe as indicated.
Southern blot analysis of PCR products from stromal cultures initiated by FACS sorted single cells. (A) RNA was extracted from six fetal bone marrow stromal cultures that had been initiated by CD34+ or CD34− FACS sorted single cells. (+) cDNA was synthesized by the addition of random hexamer primers and reverse transcriptase. (−) The same reaction conditions lacking reverse transcriptase were used. The presence of specific PCR products was detected by Southern blot using a Fibronectin, SCF, CD34 32P-labeled specific probes as indicated. (B) RNA was extracted from 4 unselected stromal cultures of adult bone marrow stromal cells. cDNA was synthesized by the addition of random hexamer primers and reverse transcriptase. The presence of specific PCR products was detected by Southern blot using a FL 32P-labeled internal probe as indicated. (C) RNA was extracted from 10 stromal cultures initiated by FACS sorted single cells with the CD34+ or CD34− phenotype. cDNA was synthesized by the addition of random hexamer primers and reverse transcriptase. The presence of specific PCR products was detected by Southern blot using a TPO specific 32P-labeled internal probe as indicated.
TPO, SCF, and IL-3 protein production of fetal and adult bone marrow stromal cells detected by ELISA. TPO content was measured by a standard capture ELISA. We analyzed six samples of adult bone marrow in which both the CD34+ and CD34− subsets were cultured. The range of values for TPO in the culture media was 4 to 104 pg/mL. Overall, in 13 of 24 culture supernatants tested, the level of TPO was greater than 16 pg/mL, a level at which the OD from the TPO ELISA was well within the linear range in the standard curve. In fetal bone marrow stromal cultures initiated by CD34+ cells, two of six culture supernatants had greater than 16 pg/mL TPO, with a median of 11 pg/mL and a mean of 26 ± 39 pg/mL; in cultures initiated by CD34− fetal bone marrow cells, two of six culture supernatants had greater than 16 pg/mL TPO, with a median of 12 pg/mL and a mean of 17 ± 12 pg/mL. In adult bone marrow stromal cultures initiated by CD34+ bone marrow cells, five of six culture supernatants had greater than 16 pg/mL TPO, with a median of 20 pg/mL and a mean of 27 ± 19 pg/mL; in adult bone marrow stromal cultures initiated by CD34− bone marrow cells, four of six culture supernatants had greater than 16 pg/mL TPO, with a median of 24 pg/mL and a mean of 24 ± 10 pg/mL. In conclusion, no substantial differences in the frequency of TPO-positive cultures were detected between the CD34+ and CD34− subsets of either fetal or adult bone marrow cells (Table 5).
TPO Is Present in the Conditioned Media of Bone Marrow Stromal Cell Cultures
| Sample No. . | FBM . | ABM . | ||
|---|---|---|---|---|
| . | CD34+ . | CD34− . | CD34+ . | CD34− . |
| 1 | 1045-150 | 11 | 655-150 | 355-150 |
| 2 | 4 | 13 | 205-150 | 13 |
| 3 | 11 | 8 | 165-150 | 15 |
| 4 | 7 | 11 | 15 | 355-150 |
| 5 | 205-150 | 165-150 | 265-150 | 205-150 |
| 6 | 11 | 405-150 | 195-150 | 27 |
| Mean ± SD | 26 ± 39 | 17 ± 12 | 27 ± 19 | 24 ± 10 |
| Median | 11 | 12 | 20 | 24 |
| Sample No. . | FBM . | ABM . | ||
|---|---|---|---|---|
| . | CD34+ . | CD34− . | CD34+ . | CD34− . |
| 1 | 1045-150 | 11 | 655-150 | 355-150 |
| 2 | 4 | 13 | 205-150 | 13 |
| 3 | 11 | 8 | 165-150 | 15 |
| 4 | 7 | 11 | 15 | 355-150 |
| 5 | 205-150 | 165-150 | 265-150 | 205-150 |
| 6 | 11 | 405-150 | 195-150 | 27 |
| Mean ± SD | 26 ± 39 | 17 ± 12 | 27 ± 19 | 24 ± 10 |
| Median | 11 | 12 | 20 | 24 |
We used ELISA to quantify the amount of TPO protein secreted from fetal and adult bone marrow.
Samples with TPO content >16 pg/mL.
We analyzed SCF content by a standard capture ELISA in eight samples of conditioned media from stromal cultures of fetal bone marrow and in 10 samples of adult bone marrow in which both the CD34+ and CD34− subsets were isolated. The range of values for SCF in the culture media was 9 to 230 pg/mL (Table 6). Eight samples of conditioned media from fetal bone marrow stromal cultures had more than 32 pg SCF/mL, with an overall median value of 79 pg SCF/mL and a mean of 88.5 ± 55.5 pg/mL. In contrast, samples of conditioned media from adult bone marrow stromal cultures had lower amounts of SCF, and only one of eight samples had greater than 32 pg/mL, for an overall median of 16 pg/mL and a median of 18 ± 9.5 pg/mL (range, 23 to 230). While substantial differences existed between SCF present in the conditioned media from either fetal or adult bone marrow stromal cultures, no substantial differences were noted in the level of SCF detected in the conditioned media of stromal cultures initiated by either CD34+ or CD34− subsets of fetal bone marrow cells (Table 6).
SCF Is Present in the Conditioned Media of Fetal Bone Marrow Stromal Cell Cultures
| Sample No. . | FBM . | ABM . | ||
|---|---|---|---|---|
| . | CD34+ . | CD34− . | CD34+ . | CD34− . |
| 1 | 806-150 | 1006-150 | 12 | 13 |
| 2 | 506-150 | 906-150 | 406-150 | 12 |
| 3 | 30 | 706-150 | 20 | 25 |
| 4 | 2306-150 | 556-150 | 18 | 11 |
| 5 | 15 | 12 | ||
| 6 | 13 | 9 | ||
| Mean ± SD | 98 ± 91 | 79 ± 20 | 21 ± 12 | 15 ± 7 |
| Median | 65 | 93 | 19 | 13 |
| Sample No. . | FBM . | ABM . | ||
|---|---|---|---|---|
| . | CD34+ . | CD34− . | CD34+ . | CD34− . |
| 1 | 806-150 | 1006-150 | 12 | 13 |
| 2 | 506-150 | 906-150 | 406-150 | 12 |
| 3 | 30 | 706-150 | 20 | 25 |
| 4 | 2306-150 | 556-150 | 18 | 11 |
| 5 | 15 | 12 | ||
| 6 | 13 | 9 | ||
| Mean ± SD | 98 ± 91 | 79 ± 20 | 21 ± 12 | 15 ± 7 |
| Median | 65 | 93 | 19 | 13 |
We used ELISA to quantify the amount of SCF protein secreted from fetal and adult bone marrow.
Samples with SCF content >32 pg/mL.
IL-3 content was measured by a standard capture ELISA. We analyzed eight samples of conditioned media from stromal cultures of fetal and adult bone marrow. The range of values on IL-3 in the culture media from all cultures of adult and fetal stromal cells was less than the lower limit of the ELISA assay (<32 pg/mL), and not significantly above the background values (data not shown).
DISCUSSION
We have previously characterized a subset of CD34+ cells from fetal bone marrow that formed stromal cell colonies in vitro.1,2,11,28 In the current report, we compared the frequency, phenotype, and cytokine profiles of stromal progenitors in fetal and adult bone marrow. We found that approximately one in 5,000 cells from both the CD34+ and CD34− subsets gave rise to stromal colonies. We noted that cultures of 100 to 10,000 CD34+ cells from bone marrow often gave rise to mixed populations of stromal and hematopoietic cells. Previous experiments from this laboratory using single cell-sorting experiments demonstrated that the CD34+, CD38−, HLADR− fraction of bone marrow contained separate populations of hematopoietic and stromal progenitors.28 The presence of hematopoietic cells in 21-day cultures initiated by a mixed population of CD34+ bone marrow cells and maintained in vitro without addition of exogenous hematopoietic cytokines suggested that local production of hematopoietic growth factors by stromal cells might be important in maintaining the survival and supporting the growth of hematopoietic progenitor cells in vitro. To test this idea, we examined the CFC activity of nonadherent cells present in mixed hematopoietic/stromal cell cultures. In two experiments, significant CFC activity was present in the nonadherent hematopoietic cell population after 3 weeks of culture in the absence of hematopoietic cytokines, including CFU-GEMM and CFU-MEG activity (Table 2). In addition, heterologous cocultures of CD34+ nonadherent cells freshly isolated from bone marrow with preestablished stromal cultures that had been initiated by either CD34+ or CD34− cells supported the survival and expansion of CD34+ hematopoietic cells in vitro. Though serum growth factors present in the culture media may have contributed to the survival of undifferentiated hematopoietic progenitor, we reasoned that the synthesis of TPO and early-acting cytokines such as FL and SCF by stromal cells could be important in the growth and self-renewal of pluripotent hematopoietic stem cells.29 We have previously demonstrated that hematopoietic and stromal progenitors are present in separate bone marrow populations, and that cultures initiated by a single stromal progenitor cell failed to yield hematopoietic progenitors.28 In the present study, generation of hematopoietic cells in stromal cultures depended on the addition of hematopoietic CD34+ progenitors. In particular, FACS analysis of the rare nonadherent cells generated by stromal cultures initiated by single CD34+ or CD34− stromal progenitor cells showed the absence of cells expressing hematopoietic markers, particularly CD14 and HLA-DR, two markers associated with adherent monocytes and dendritic cells that could potentially contaminate stromal cell cultures.
On the basis of these data, we explored whether stromal cultures generated from phenotypically distinct populations have different functional capacities relevant to hematopoiesis. We used RT-PCR to measure mRNA for IL-3, SCF, CD34, FL, and TPO and measured the synthesis of TPO, SCF, and IL-3 using an ELISA. Our hypothesis was that stromal subsets defined by the presence or absence of the CD34 antigen would show significant differences in the expression of cytokines that regulate hematopoiesis, particularly those that act on primitive or pluripotent hematopoietic progenitor cells. However, we found that the pattern of cytokine mRNA expression did not differ significantly between stromal cell cultures initiated by single stromal progenitor cells isolated from fetal bone marrow and by CD34+ and CD34− marrow stromal cell subsets of adult bone marrow. The failure to distinguish clear functional differences between stromal cell subsets based on the presence or absence of the CD34 antigen does not support the “specific stromal cell subset/specific function” hypothesis. While SCF mRNA was detected only in CD34+ fetal bone marrow cells, the protein was secreted in significant amounts by stromal cultures initiated by both CD34+ and CD34− fetal bone marrow cells and was not detected in the conditioned media of adult bone marrow cells. In contrast, TPO mRNA was nearly always found in bone marrow stromal cell cultures. We confirmed that significant amounts of SCF and TPO were secreted into the media by cultures derived from both CD34+ or CD34− stromal cells (Tables 5 and 6).
The lack of a correlation between phenotypically defined stromal cell subsets and their functional properties could be due to a number of different reasons. First, CD34 expression may not be a sensitive or specific discriminator of functional variation among different stromal cell populations. The biologic significance of CD34 expression by stromal cells is not known. In contrast to differentiation of pluripotent CD34+ hematopoietic progenitors, in which lineage commitment is associated with the downregulation of CD34 expression,30 it is not clear whether CD34+ cells precede CD34− cells in stromal cell ontogeny.11 In addition, stromal cell differentiation may not proceed along a hierarchic, unidirectional pattern, in which different cell lineages have different functional capacities. In contrast to hematopoiesis, stromal cell differentiation may be more consistent with an alternative view of ontogeny in which differentiated stromal cells retain the potential to “de-differentiate” into other phenotypes.31 Indeed, the surface phenotype of stromal cells obtained after 21 days of culture was similar, and the majority of cultured stromal cells had the phenotype of CD34−, CDw90+, irrespective of the original phenotype of the cells sorted into culture (data not shown). Alternatively, heterogeneity within both the CD34+ and CD34− subsets with variable expression of SCF and FL could obscure functional differences between these two stromal subsets.
The selective expression of CD34 on hematopoietic progenitor cells and some stromal cells has led to the suggestion that homotypic adhesion between CD34+ hematopoietic and CD34+ stromal cells may be important in regulating hematopoiesis, although other speculation has focused on the function of CD34 as an antiadhesion molecule.30 32 The equivalent abilities of stromal cultures initiated by either CD34+ or CD34− bone marrow cells to support hematopoiesis suggests that CD34 expression by stromal cells is not critical to their role in hematopoietic stem cell adhesion and self-renewal, and that other as yet uncharacterized molecules may be more important in this regard.
TPO, SCF, IL-3, and FL are involved as early cytokines in the proliferation of more primitive progenitor cells.33,34 While SCF and FL have been previously shown to be synthesized by bone marrow stromal cells, the ubiquitous expression of TPO by all stromal cell cultures is a new finding. We found that TPO was expressed at the mRNA and protein level by nearly all stromal cell cultures (Tables 4 and 5). TPO synthesis by bone marrow stromal cells may be relevant to regulating hematopoiesis in the bone marrow microenvironment.35,36 Experiments in vivo with sublethally irradiated Balb/c male mice, with a single dose of PEG-rHuMGDF demonstrated the effect of TPO on the recovery of both myeloid cells and platelets, probably through expansion of both primitive and committed hematopoietic progenitors.37 The biologic significance of low TPO synthesis by stromal cells and its role in maintenance and differentiation of hematopoietic progenitor cells is difficult to assess.17,38,39 The level of TPO present in tissue culture media conditioned by stromal cells varied between 4 and 104 pg/mL. In four of 12 samples of conditioned media from fetal bone marrow stromal cultures, the level was greater than 16 pg/mL, and in nine of 12 samples of conditioned media from adult bone marrow stromal cultures it was greater than 16 pg/mL; in one case of CD34+ fetal bone marrow stromal cells, the concentration of TPO in the culture media was greater than 100 pg/mL. The amounts of TPO in the conditioned media measured by ELISA were similar to the amount of SCF in the culture supernatants (Tables 5 and 6). In tissue culture using PEG-rHuMGDF added to CD34+ hematopoietic progenitors, the optimal concentration of TPO to support megakaryocytopoiesis is in the range of 10 to 50 ng/mL,20 while the optimal concentration of soluble SCF to maintain and expand hematopoietic progenitor cells in vitro is 100 ng/mL.40 The level of TPO we found to be secreted by stromal cells was more than two logs less than the level that produces a maximal proliferative response of CFU-MEG in vitro, but was in the same range as the physiologic concentration of TPO found in platelet-poor human plasma.41 In addition, TPO may be present on the cell surface of stromal cells (as is SCF) and thus may have a local role in regulating megakaryocytopoiesis and acting on primitive hematopoietic progenitors as a membrane-bound cytokine.33,34 42 Our data on TPO expression by stromal cells raise a question as to whether clinically significant variations in the rate of platelet engraftment after high-dose chemotherapy and bone marrow transplantation may be related to variation in the integrity and function of stromal cells in the bone marrow microenvironment. In studying patients who underwent autologous BMT, there was a similar correlation between the CD34+, CD38− hematopoietic progenitor cell content of the autograft and the kinetics of platelet engraftment, but only one third of the variance in the engraftment data was explained by variation in the hematopoietic progenitor cell content (E.K.W., unpublished observations, March 1997), indicating that factors other than stem cell content influence megakaryocytopoiesis.
These data suggest the potential relevance of stromal cells in the regulation of both megakaryocytopoiesis and stem cell renewal by local production of TPO, SCF, and FL. Local cytokine production in the bone marrow microenvironment may be physiologically more significant than cytokines that are produced at distant sites and secreted into the bloodstream.37,39 43 The survival of relatively undifferentiated and pluripotent hematopoietic progenitors in mixed stromal/ hematopoietic cultures supports the idea that the local production of cytokines by stromal cells may be important in maintaining the growth potential of primitive CD34+ hematopoietic progenitors. Future efforts will be needed to determine whether identifiable subsets of bone marrow stromal cells have different regulating functions of hematopoiesis.
ACKNOWLEDGMENT
The authors thank Janet L. Nichol and Alex C. Hornkohl (Amgen, Thousand Oaks, CA) for providing anti-MPL ligand antibodies and human MPL ligands. Dr Noel Warner (Becton Dickinson Immunocytometry Systems, San Jose, CA) and Janet L. Nichol (Amgen) are gratefully acknowledged for their careful reading of the manuscript, Sylvia Ennis for editing the manuscript, the Microchemical Facility at the Winship Center for oligonucleotide synthesis, and Dave Houck (Becton Dickinson Immunocytometry Systems) for providing excellent technical assistance in sorting bone marrow cells.
Supported in part by National Institutes of Health Grant No. CA62593.
Address reprint requests to Edmund K. Waller, MD, PhD, Emory University School of Medicine, Division of Hematology and Oncology, 1639 Pierce Dr, Atlanta, GA 30322.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal