Abstract
Bone marrow transplantation in human X-linked severe combined immunodeficiency (XSCID) without pretransplant conditioning results in engraftment of donor T cells and reconstitution of T-cell function but engraftment of few, if any, donor B cells and poor reconstitution of humoral immune function. Since bone marrow transplantation remains the most effective treatment of XSCID patients, better strategies are necessary to achieve optimum long-term results. Canine XSCID, like human XSCID, is due to mutations in the common γ chain (γc) gene and has clinical and immunologic features identical to those of human XSCID, making it a true homolog of the human disease. We have successfully performed bone marrow transplantation in three XSCID dogs without pretransplant conditioning, using untreated bone marrow cells from mixed lymphocyte culture–nonreactive normal littermates. Unlike the experience in human XSCID patients, all three dogs engrafted both donor B and T cells and attained full reconstitution of immunologic function. Normal percentages of T cells and T-cell mitogenic responses were attained by 3 months posttransplant. CD3+ T cells after transplantation expressed the CD45RA isoform indicating that the cells were recent thymic emigrants derived from immature progenitors. Serum IgG levels were within normal range by 5 months posttransplant. Immunization with the T-dependent antigen, bacteriophage φX174, demonstrated normal antibody titers, immunologic memory, and class-switching. Polymerase chain reaction (PCR) analysis of the γc locus showed that 100% of circulating T cells and 30% to 50% of circulating B cells were donor-derived. None of the dogs developed clinically evident graft-versus-host disease (GVHD). Thus, canine XSCID provides a model to determine the optimal conditions for bone marrow transplantation in human patients, and to develop and test strategies for somatic gene therapy.
SEVERE COMBINED immunodeficiency (SCID) is a heterogeneous group of genetic diseases characterized by a lack of both humoral and cellular immune responses that usually result in death during infancy.1,2 Based on the high prevalence of male cases, it has been proposed that X-linked SCID (XSCID) is the most common form of the disease.3,4 XSCID results from mutations in the common γ chain (γc) gene,5,6 so named because it is a component of the receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, and IL-15.7-13
Since the first successful bone marrow transplant in a child with SCID in 1968, histocompatible bone marrow transplantation has become the treatment of choice for all forms of SCID.4,14,15 Patients receiving a histocompatible (HLA-identical) bone marrow transplant have greater than 90% long-term survival rates.15-19 Histocompatible recipients generally show reconstitution of normal B- and T-cell function without the need for prior cytoreduction. However, the majority of SCID patients do not have a histocompatible donor. Advances in bone marrow transplantation techniques, namely prevention of graft-versus-host disease (GVHD) by T-cell depletion, have permitted the use of haploidentical (half-matched) bone marrow cells for immunologic reconstitution of SCID.19-27 Although this development makes possible the treatment of virtually every SCID patient, long-term immune reconstitution and survival is less favorable, ranging from 52% to 67%.14,15,19,27 The biologic problems associated with haploidentical bone marrow transplantation are a higher risk of nonengraftment; a delayed reconstitution of T-cell function compared with patients receiving histocompatible transplants; GVHD, which may occur despite T-cell depletion and may require posttransplant immunosuppressive therapy; and an absence of normal humoral immune function in over half of transplanted patients.4,15,17-19,21,28 The incomplete humoral immune reconstitution or lack of it following haploidentical bone marrow transplantation for SCID has been shown to be strongly associated with the failure to engraft donor B cells.15-20,29 The use of pretransplant conditioning has been reported to enhance donor B-cell engraftment following haploidentical bone marrow transplantation, thereby improving the likelihood of reconstituting normal humoral immune function.15,16,19,20,27 28
Although many of the transplanted SCID patients reported in previous bone marrow transplantation studies were males, only 18 documented XSCID patients who have survived more than 2 years following transplantation have been described in the literature.3,19,20,29-31 The reason for this discrepancy is that until the recent development of genetic tests for carrier detection and identification of the XSCID gene defect,5,6,32 it was impossible, in the absence of a positive family history, to document that male patients represented XSCID. All transplanted patients have engrafted donor T cells with reconstitution of T-cell function.3,19,20,27,29-31 The largest immunologic problem in human XSCID patients following bone marrow transplantation is the engraftment of few, if any, donor B cells with resultant poor reconstitution of humoral immune function. Such patients must be maintained indefinitely on prophylactic Ig therapy.3,19,20,27 29-31 Since bone marrow transplantation is presently the only treatment available to provide a cure for patients with XSCID, new strategies to improve donor B-cell engraftment are necessary to achieve optimal long-term survival.
Our laboratory has characterized a naturally occurring XSCID in the dog that is homologous to human XSCID.33-36 Canine XSCID is due to mutation of the γc and has clinical and immunologic features identical to those of human XSCID. This study demonstrates that it is possible to completely reconstitute T- and B-cell function in canine XSCID by bone marrow transplantation, and that canine XSCID therefore represents a unique experimental animal model to develop better strategies for treatment of human XSCID.
MATERIALS AND METHODS
Dogs.XSCID dogs used in this study were derived from a breeding colony established from a single carrier female.33,34 All of the affected males have the same γc mutation, a four-basepair (bp) deletion in exon 1, and were diagnosed shortly after birth by a polymerase chain reaction (PCR)-based mutation detection assay using DNA isolated from whole blood.36 Compatible donors for transplantation were determined by mixed lymphocyte culture.37 38
Bone marrow preparation.Bone marrow cells were collected from the normal donor following euthanasia by removing a segment of the femur, cracking open the bone, scraping the marrow into a sterile petri dish containing Hanks balanced salt solution (HBSS) without calcium and magnesium (Mediatech; Fisher Scientific, Philadelphia, PA), and mincing into a single cell suspension. The resulting suspension was filtered through a fine mesh filter and washed twice with HBSS. Following filtration, the cells were centrifuged and resuspended in ammonium chloride lysing buffer (Sigma Chemical, St Louis, MO) to remove erythrocytes. After a 5-minute incubation on ice, the cells were washed twice in HBSS, and the final pellet was resuspended in sterile saline.
Quantitation of serum IgG.Serum IgG concentrations were measured by radial immunodiffusion using affinity-purified heavy chain–specific antisera to canine IgG.33
Flow cytometry.Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by centrifugation over a discontinuous density gradient of Hypaque-Ficoll and stained for flow cytometric analysis as previously described.35 Negative control samples were stained only with the secondary antibody. Analysis gates were adjusted to 2% positive staining with negative controls. For each sample, 10,000 cells were analyzed using a FACScan (Becton Dickinson, Mountain View, CA). The murine monoclonal antibodies used in this study were CA17.3G9 (canine CD3), 12.125 (canine CD4), 4.78 (canine CD8), and CA4.1D3 (canine CD45RA).39-41 Fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 goat anti-dog IgG (heavy and light chain–specific) was purchased from Cappel (Durham, NC). The secondary antibodies (FITC-labeled anti-mouse IgG, phycoerythrin [PE]-labeled anti-mouse IgG, and FITC-labeled anti-mouse IgM) were purchased from Fisher Scientific (Pittsburgh, PA).
Proliferation assays.The response of peripheral blood lymphocytes to in vitro mitogenic stimulation with phytohemagglutinin-P (PHA-P; 5 μg/mL; Sigma) was determined as previously described.35 Mixed lymphocyte cultures were performed by incubating 105 responder cells with 105 irradiated (3,000 rad) stimulator cells in 96-well, round-bottom microtiter plates for 7 days.37 38 Sixteen hours before the end of the culture period, 0.5 mCi 3H-thymidine (6.8 Ci/mmol; New England Nuclear, Boston, MA) was added to each well. At the end of the incubation period, the cells were harvested onto glass-fiber filters, and the incorporation of radioactivity was measured by liquid scintillation spectrometry. The results are expressed as counts per minute.
Sorting of PBMCs.Purified B and T cells were obtained by direct sorting of surface Ig+ and CD3+ cells, respectively, using a FACStar Plus (Becton Dickinson). Sorting gates were placed on positive cells that had light-scatter characteristics of lymphocytes. Monocytes were sorted based on their characteristic light-scatter and lack of CD3 staining. The purity of B-cell and T-cell populations was greater than 98.5% as determined by flow cytometry.
PCR mutation analysis.Genomic DNA was prepared from heparinized whole blood (diagnosis of XSCID) or from the various sorted cell subpopulations by resuspending cell pellets in lysis buffer (4.5% Nonidet P-40, 4.5% Tween 20, 10 μg/mL RNase, and 0.125 μg/mL proteinase K), incubating at 55°C for 2 hours, and boiling for 10 minutes to denature enzymes. Oligonucleotide primers (cgam5UT, CTACACCCAGGGAACGAAGAGC; and c5pIVS1R, CCCCTTCCAGTCCCATGTTTCC) flanking the deletion in the γc gene were used to amplify 0.1 to 1.0 μg genomic DNA as previously described.36 Products were resolved on 9% nondenaturing polyacrylamide gels and visualized with ethidium bromide.
Assessment of specific antibody production.The transplanted dogs were immunized with the T-cell–dependent antigen, bacteriophage φX174, when their serum IgG concentrations reached normal levels. Bacteriophage φX174 was administered intravenously at a dose of approximately 3 × 109 PFU/kg. A secondary immunization was given 6 weeks after the primary immunization. Phage clearance and specific phage-neutralizing antibody activity, expressed as the rate of phage inactivation (K value [Kv]), was determined as previously described.42 43 IgG antibody was measured as antibody activity that was resistant to treatment with 2-mercaptoethanol.
RESULTS
Bone marrow transplantation.Three XSCID male dogs underwent bone marrow transplantation between 2 and 3 weeks of age using either a normal female or normal male littermate as the bone marrow donor. The choice of the normal littermate donor for each dog was based on a one-way mixed lymphocyte culture assay (donor × irradiated recipient cells), using the littermate with the lowest stimulation index as the donor. None of the dogs received any pretransplant conditioning. Untreated nucleated bone marrow cells containing 0.9% to 1.8% mature T cells were administered intravenously at a dose of 1.3 to 1.5 × 108 nucleated cells/kg. Table 1 describes the donor, the one-way mixed lymphocyte reaction between the donor and recipient performed at 1 to 2 weeks of age, the dose of bone marrow cells administered, and the percentage of mature T cells in the bone marrow preparation used for transplantation of each dog. R-350 and R-352 were transplanted with bone marrow cells from the same donor. The dogs were reared in a conventional environment following bone marrow transplantation and were maintained on prophylactic antibiotics for the first 2 to 3 months following transplantation.
Description of Bone Marrow Cells Used in the Bone Marrow Transplants
| Recipient . | Donor . | MLC* . | Dose of BM . | T cells . |
|---|---|---|---|---|
| . | . | . | (× 108/kg) . | (%) . |
| R-16 | F | 2.6 | 1.3 | 1.8 |
| R-350 | M | 1.6 | 1.5 | 0.9 |
| R-352 | M | 0.8 | 1.5 | 0.9 |
| Recipient . | Donor . | MLC* . | Dose of BM . | T cells . |
|---|---|---|---|---|
| . | . | . | (× 108/kg) . | (%) . |
| R-16 | F | 2.6 | 1.3 | 1.8 |
| R-350 | M | 1.6 | 1.5 | 0.9 |
| R-352 | M | 0.8 | 1.5 | 0.9 |
Abbreviations: MLC, mixed lymphocyte culture; BM, bone marrow.
Expressed as stimulation index.
Lymphocyte recovery following transplantation.The phenotype of peripheral blood lymphocytes of the three transplanted XSCID dogs prior to and following transplantation is illustrated in Table 2. Before transplantation, all three dogs had the typical XSCID phenotype of low to absent peripheral T cells and an elevated percentage of B cells. The first dog, R-16, was not evaluated until 3 months posttransplantation. At this time, the percentage and absolute number of peripheral T cells had normalized. The majority of T cells were CD4+, resulting in a CD4:CD8 ratio of 12:1. At 5 months posttransplantation, the percentage and absolute number of T cells was still normal and the CD4:CD8 ratio had normalized. The next two dogs, R-350 and R-352, were evaluated monthly following transplantation. Both dogs exhibited an increase in peripheral T cells at 1 month posttransplantation and a normal percentage and absolute number of peripheral T cells at 2 months posttransplantation. As in R-16, the CD4:CD8 ratio was elevated in both dogs at this time. The CD4:CD8 ratio in both dogs was within normal limits for age-matched normal dogs by 3 months posttransplantation.
Lymphocyte Profile Before and After Bone Marrow Transplantation
| Parameter . | Pretransplantation . | Months Posttransplantation . | ||||
|---|---|---|---|---|---|---|
| . | . | 1 . | 2 . | 3 . | 4 . | 5 . |
| Lymphocytes (per μL) | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | 3,658 | ND | ND | 4,154 | ND | 3,962 |
| 734 | ND | ND | 10,492 | ND | 12,366 | |
| 1,062 | 1,050 | 8,624 | 8,786 | 7,708 | 7,100 | |
| 874 | 986 | 8,316 | 4,251 | 4,408 | 4,464 | |
| Phenotype (%) | ||||||
| B | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | 17.5 | ND | ND | 8.1 | ND | 11.2 |
| 46.5 | ND | ND | 1.8 | ND | 4.1 | |
| 83.7 | 37.5 | 4.7 | 3.5 | 5.5 | 6.3 | |
| 42.8 | 40.8 | 3.7 | 6.8 | 5.2 | 7.1 | |
| CD3 | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | 54.6 | ND | ND | 76.1 | ND | 78.7 |
| 3.4 | ND | ND | 89.9 | ND | 90.6 | |
| 0 | 36.8 | 91.8 | 90.4 | 83.9 | 92.2 | |
| 0 | 28.9 | 90.2 | 89.3 | 88.5 | 90.8 | |
| CD4 | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | ND | ND | ND | 58.4 | ND | 62.1 |
| ND | ND | ND | 89.1 | ND | 74.6 | |
| ND | 20.5 | 79.6 | 67.2 | 74.2 | 73.7 | |
| ND | 23.3 | 75.3 | 71.5 | 74.3 | 74.9 | |
| CD8 | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | ND | ND | ND | 13.2 | ND | 8.3 |
| ND | ND | ND | 7.2 | ND | 19.1 | |
| ND | 17.9 | 8.7 | 15.6 | 14.7 | 16.4 | |
| ND | 12.5 | 10.9 | 11.7 | 16.2 | 12.4 | |
| CD45RA+ T cells | ||||||
| R-350 | ||||||
| R-352 | ND | 82.1 | 98.2 | 95.4 | 97.5 | 86.9 |
| ND | 85.2 | 97.2 | 94.3 | 98.1 | 84.9 | |
| Parameter . | Pretransplantation . | Months Posttransplantation . | ||||
|---|---|---|---|---|---|---|
| . | . | 1 . | 2 . | 3 . | 4 . | 5 . |
| Lymphocytes (per μL) | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | 3,658 | ND | ND | 4,154 | ND | 3,962 |
| 734 | ND | ND | 10,492 | ND | 12,366 | |
| 1,062 | 1,050 | 8,624 | 8,786 | 7,708 | 7,100 | |
| 874 | 986 | 8,316 | 4,251 | 4,408 | 4,464 | |
| Phenotype (%) | ||||||
| B | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | 17.5 | ND | ND | 8.1 | ND | 11.2 |
| 46.5 | ND | ND | 1.8 | ND | 4.1 | |
| 83.7 | 37.5 | 4.7 | 3.5 | 5.5 | 6.3 | |
| 42.8 | 40.8 | 3.7 | 6.8 | 5.2 | 7.1 | |
| CD3 | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | 54.6 | ND | ND | 76.1 | ND | 78.7 |
| 3.4 | ND | ND | 89.9 | ND | 90.6 | |
| 0 | 36.8 | 91.8 | 90.4 | 83.9 | 92.2 | |
| 0 | 28.9 | 90.2 | 89.3 | 88.5 | 90.8 | |
| CD4 | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | ND | ND | ND | 58.4 | ND | 62.1 |
| ND | ND | ND | 89.1 | ND | 74.6 | |
| ND | 20.5 | 79.6 | 67.2 | 74.2 | 73.7 | |
| ND | 23.3 | 75.3 | 71.5 | 74.3 | 74.9 | |
| CD8 | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | ND | ND | ND | 13.2 | ND | 8.3 |
| ND | ND | ND | 7.2 | ND | 19.1 | |
| ND | 17.9 | 8.7 | 15.6 | 14.7 | 16.4 | |
| ND | 12.5 | 10.9 | 11.7 | 16.2 | 12.4 | |
| CD45RA+ T cells | ||||||
| R-350 | ||||||
| R-352 | ND | 82.1 | 98.2 | 95.4 | 97.5 | 86.9 |
| ND | 85.2 | 97.2 | 94.3 | 98.1 | 84.9 | |
“Normal” is the normal age-matched littermate of R-16.
Abbreviation: ND, not determined.
Because T cells derived from the transplanted stem cells would be expected to be naive, we examined the CD45 isoform usage following bone marrow transplantation. The majority of peripheral T cells were CD45RA+, similar to those observed in normal age-matched dogs (CD45RA+ T cells in normal 2-month-old puppies, 90.7% ± 5.9%; in normal 1-year-old dogs, 72.0% ± 6.0%). The lymphocyte phenotype has remained normal in all three dogs through 1 year following transplantation.
Reconstitution of immune function.Table 3 illustrates the peripheral T-cell response to mitogenic stimulation and serum IgG concentrations before and after bone marrow transplantation. The blastogenic response to PHA was normalized in R-16 by 3 months posttransplantation. The other two dogs, R-350 and R-352, who were evaluated monthly, showed a significant increase in the blastogenic response to PHA at 1 month posttransplantation and a normal blastogenic response by 2 months following transplantation. The blastogenic response to PHA has remained normal throughout the first year posttransplantation in all three dogs.
Lymphocyte Function Before and After Bone Marrow Transplantation
| Parameter . | Pretransplantation . | Months Posttransplantation . | ||||
|---|---|---|---|---|---|---|
| . | . | 1 . | 2 . | 3 . | 4 . | 5 . |
| PHA (cpm) | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | 25,286 | 26,312 | ND | 28,942 | ND | 20,614 |
| 826 | 1,508 | ND | 24,654 | ND | 17,920 | |
| 1,300 | 9,600 | 72,400 | 104,400 | 38,800 | 41,100 | |
| 100 | 7,700 | 31,000 | 70,700 | 24,500 | 25,100 | |
| IgG (mg/dL) | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | ND | ND | ND | 1,750 | ND | 1,800 |
| ND | ND | ND | 280 | ND | 960 | |
| ND | <100 | 145 | 210 | 230 | 570 | |
| ND | <100 | 320 | 405 | 545 | 710 | |
| Parameter . | Pretransplantation . | Months Posttransplantation . | ||||
|---|---|---|---|---|---|---|
| . | . | 1 . | 2 . | 3 . | 4 . | 5 . |
| PHA (cpm) | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | 25,286 | 26,312 | ND | 28,942 | ND | 20,614 |
| 826 | 1,508 | ND | 24,654 | ND | 17,920 | |
| 1,300 | 9,600 | 72,400 | 104,400 | 38,800 | 41,100 | |
| 100 | 7,700 | 31,000 | 70,700 | 24,500 | 25,100 | |
| IgG (mg/dL) | ||||||
| Normal | ||||||
| R-16 | ||||||
| R-350 | ||||||
| R-352 | ND | ND | ND | 1,750 | ND | 1,800 |
| ND | ND | ND | 280 | ND | 960 | |
| ND | <100 | 145 | 210 | 230 | 570 | |
| ND | <100 | 320 | 405 | 545 | 710 | |
“Normal” is the normal age-matched littermate of R-16.
Serum IgG concentrations did not attain normal levels until 5 months posttransplantation (serum IgG in normal adult dogs > 1 year of age is ≥700 mg/dL). Serum IgG continued to increase to greater than 1,000 mg/dL by 10 to 12 months posttransplantation in all three dogs.
Between 5 and 6 months posttransplantation, when serum IgG concentrations were approaching normal levels, all three transplanted XSCID dogs and a normal age-matched littermate were immunized with bacteriophage φX174. Table 4 shows that the transplanted dogs were capable of producing an antigen-specific primary and secondary antibody response similar to that of the normal dog and that they were capable of class-switching to IgG. This is in contrast to nontransplanted gnotobiotic XSCID dogs, in which the antibody titers not only were very low but the antibody produced was almost exclusively IgM.
Antibody Responses to Bacteriophage ΦX174
| Dog . | Weeks Postimmunization . | |||||
|---|---|---|---|---|---|---|
| . | Primary . | Secondary . | . | . | ||
| . | 1 . | 2 . | 4 (pre-2) . | 2 . | . | . |
| Age-matched normal dog | 12 (51%) | 12 (48%) | 24 (100%) | 456 (76%) | ||
| R-16 | 12 (27%) | 9 (46%) | 7 (100%) | 146 (83%) | ||
| R-350 | ND | 10 (28%) | 30 (37%) | 402 (61%) | ||
| R-352 | ND | 19 (29%) | 24 (68%) | 232 (71%) | ||
| Gnotobiotic XSCID (n = 4) | 3 (ND) | 5 (7%) | 5 (12%) | 15 (13%) | ||
| Gnotobiotic control (n = 2) | 10 (ND) | 23 (38%) | 42 (76%) | 909 (96%) | ||
| Dog . | Weeks Postimmunization . | |||||
|---|---|---|---|---|---|---|
| . | Primary . | Secondary . | . | . | ||
| . | 1 . | 2 . | 4 (pre-2) . | 2 . | . | . |
| Age-matched normal dog | 12 (51%) | 12 (48%) | 24 (100%) | 456 (76%) | ||
| R-16 | 12 (27%) | 9 (46%) | 7 (100%) | 146 (83%) | ||
| R-350 | ND | 10 (28%) | 30 (37%) | 402 (61%) | ||
| R-352 | ND | 19 (29%) | 24 (68%) | 232 (71%) | ||
| Gnotobiotic XSCID (n = 4) | 3 (ND) | 5 (7%) | 5 (12%) | 15 (13%) | ||
| Gnotobiotic control (n = 2) | 10 (ND) | 23 (38%) | 42 (76%) | 909 (96%) | ||
Values in parentheses are expressed as neutralizing antibody titer, Kv (% IgG).
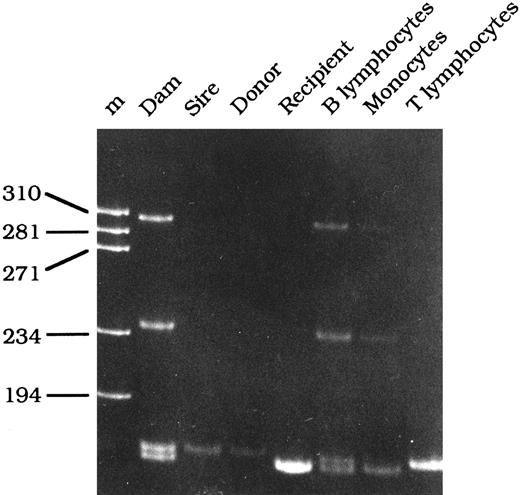
Engraftment of donor B and T cells.The origin of the various circulating mononuclear cell populations was determined at 6 months following transplantation by assessing the genotype of the γc gene using the PCR-based mutation assay described for diagnosing XSCID dogs. If the cell population is derived from the donor, only the 169-bp fragment should be observed, whereas if the cells are still of host origin, they will contain the 165-bp fragment. The presence of heteroduplexes, similar to those observed in carrier females, indicates the presence of cells with both the mutant (host) γc gene and the normal (donor) γc gene.36 The B- and T-cell populations were purified by FACS to purities greater than 98.5%. The T-cell population in all three transplanted dogs was exclusively of donor origin, since the T cells demonstrated only the 169-bp fragment. However, the B-cell population demonstrated split chimerism, since the PCR resembled that of a carrier female. Comparison of the B-cell PCR results of the three dogs to the PCR results of a mixing panel ranging from 95% XSCID cells and 5% normal cells to 5% XSCID cells and 95% normal cells provided an estimate of donor B-cell engraftment for each dog. The B-cell population of R-16 following transplantation was approximately 50% donor B cells, and the B-cell populations in R-350 and R-352 were estimated to consist of approximately 30% to 40% donor B cells. Figure 1 illustrates the PCR results of R-16 6 months posttransplantation. Before transplantation, PBMCs contained only the 165-bp (mutant γc) fragment (recipient lane). Following transplantation, the T-cell fraction still contains a single band, but it now consists of the 169-bp fragment, suggesting that the T cells are exclusively donor-derived, whereas the B-cell population demonstrates split chimerism with both donor- and host-derived cells.
PCR-based mutation analysis of the γc gene in genomic DNA isolated from the mother (dam), father (sire), normal female littermate donor (donor), R-16 before bone marrow transplantation (recipient), and B lymphocytes, monocytes, and T lymphocytes obtained from R-16 6 months following bone marrow transplantation.
PCR-based mutation analysis of the γc gene in genomic DNA isolated from the mother (dam), father (sire), normal female littermate donor (donor), R-16 before bone marrow transplantation (recipient), and B lymphocytes, monocytes, and T lymphocytes obtained from R-16 6 months following bone marrow transplantation.
Clinical outcome.All three dogs remain clinically healthy 1 year following transplantation. None of the dogs have demonstrated any clinical evidence of GVHD. Although the dogs experienced the same bacterial and parasitic infections as normal dogs, they responded to appropriate treatment.
DISCUSSION
This report documents the first successful bone marrow transplantation in canine XSCID. T-cell reconstitution achieved in the transplanted XSCID dogs was similar to that observed in successfully transplanted XSCID boys. Although normal mitogen responses may be observed in HLA-identical human transplants within 2 to 3 weeks following transplantation, this appears to be due to the significant number of mature donor T cells contained within the bone marrow preparation.14,27 Depletion of mature T cells from either HLA-identical or haploidentical bone marrow preparations results in a delay of approximately 90 to 100 days for generation of normal numbers of peripheral blood T cells and approximately 120 days for normal T-cell mitogenic responses.21,27 The basis for this delay is unknown, but it is consistent with recapitulation of fetal thymopoiesis.44 45 This suggests that the T cells generated in patients receiving T-cell–depleted bone marrow must develop from transplanted hematopoietic stem cells (HSCs), rather than representing mature T cells derived from the transplanted marrow.
In the transplanted XSCID dogs, peripheral T cells were evident by 30 days posttransplantation, with normal numbers and normal mitogen responses present by 60 days. This delay in T-cell development and the fact that the majority of T cells were CD45RA+ is consistent with recapitulation of thymic ontogeny in the dog. The delay in the appearance of T cells in the dog is shorter than in humans following transplantation due to the difference in the time for normal thymopoiesis to occur in the two species. Normal canine thymopoiesis occurs in 25 to 30 days,46,47 versus 90 to 100 days proposed for human thymopoiesis. Although none of the transplanted XSCID dogs received bone marrow that was actively depleted of T cells, the technique used for obtaining bone marrow cells resulted in transplantation of a low number of mature T cells. The standard dose of unfractionated bone marrow used in human HLA-identical bone marrow transplants for SCID is approximately 5 × 107 nucleated cells/kg, which contains significant contamination with peripheral blood cells as evidenced by the presence of 13% to 25% mature T cells in the usual bone marrow preparations.22 26 The mean dose of unfractionated bone marrow used in our studies was 14.5 × 107 nucleated cells/kg and contained a mean of 1.3% mature T cells, suggesting that essentially all the nucleated cells used were bone marrow–derived.
Although the transplanted dogs received approximately 1.8 × 106 T cells/kg at the time of bone marrow transplantation, it is unlikely that CD45RA+ T cells that appeared following transplantation were derived from mature T cells in the bone marrow inoculum. The number of CD45RA+ T cells observed 2 months after transplantation, without considering T cells present in the lymphoid tissues, would require at least a 1,000-fold expansion of CD45RA+ T cells in the original inoculum. Extrathymic expansion of mature T cells in the inoculum to these levels would require antigen-driven proliferation that would result in conversion from a CD45RA+ phenotype to a CD45RO+ phenotype, which is CD45RA−.48-52 Additional evidence comes from the murine bone marrow transplant studies of Mackall et al53 showing that extrathymic expansion of mature T cells in the inoculum used for transplant of thymectomized hosts results in a predominant murine equivalent of a CD45RO+ (memory) T-cell phenotype, and that this phenotype remained constant over the 6-month period of the study. In contrast, transplantation of thymus-bearing mice with an identical inoculum resulted in a predominant murine equivalent of a CD45RA+ (naive) T-cell phenotype. Their conclusions were that expansion of peripheral T cells is downregulated in the presence of a functional thymus such that in thymus-bearing animals the predominant source of T-cell regeneration is through production of “naive” T cells within the thymus. More recently, similar findings have been described in humans. T-cell regeneration in infants recovering from chemotherapy or following HLA-identical bone marrow transplantation exhibits a predominantly CD45RA+ (naive) phenotype, whereas adults show a predominantly CD45RO+ (memory) phenotype, suggesting that extrathymic maturation of mature T cells is the major source of T-cell regeneration in humans after the thymus begins its involution.44,45 54 Therefore, although a minimal amount of extrathymic expansion of the mature T cells may have occurred during the first few weeks posttransplant, the presence of predominantly CD45RA+ T cells in the canine recipients strongly suggests they were derived from progenitors and not the mature T cells in the inoculum. The similarity in the delay in T-cell function between dog and human transplant patients suggests that the dog XSCID model represents a valuable tool for studying the mechanism of delayed T-cell function after bone marrow transplantation and for evaluating strategies to accelerate development of normal T-cell function.
We also demonstrated normal B-cell development in the transplanted dogs. B-cell engraftment following nonconditioned bone marrow transplants in human XSCID patients has been equivocal, with many patients not achieving donor B-cell reconstitution and consequently remaining antibody-deficient.3,19,20,27 29-31 Pretransplant cytoablative chemotherapy has been recently shown to enhance donor B-cell engraftment and reconstitution of humoral immune function in XSCID patients (KI Weinberg, unpublished data, December 1996).
The failure of human XSCID patients to engraft donor B cells without cytoablation is poorly understood, but may be due to the lack of selective and/or proliferative advantage of donor B-cell progenitors expressing a normal γc gene. The observation that XSCID boys have normal or elevated numbers of IgM+ B cells and that peripheral IgM+ B cells from carrier females exhibit random X-chromosome inactivation suggests that a mutant γc does not interfere with the early stages of human B-cell development.3,4 55-57 Thus, B-cell precursors expressing a normal γc seem to have little selective advantage over those expressing a mutant γc, especially during the early stages of differentiation. The observation that pretransplant cytoablation enhances donor B-cell engraftment in human patients is most likely due to reducing the competition between donor and host stem cells.
The PCR and φX174 studies demonstrate that successful donor B-cell engraftment and reconstitution of normal humoral immune function were achieved in transplanted XSCID dogs without cytoablation. The question remains as to why XSCID dogs engraft donor B cells without cytoablation and human XSCID patients have difficulty in doing so, especially since the role of the γc in B-cell development appears to be similar in humans and dogs.
One explanation is that normal canine B-cell progenitors have more of a selective advantage over γc-deficient cells than human B-cell progenitors. Alternatively, it is conceivable that the number of B-cell progenitors expressing a normal γc gene is critical to the successful engraftment of donor B cells in nonconditioned bone marrow transplants. The XSCID dogs in this study were transplanted with high doses of untreated bone marrow from donors known to be normal with respect to the γc gene that contained little contamination with peripheral blood cells. The dose of bone marrow stem cells used in our studies was nearly three to four times greater than that used in human HLA-identical transplants. Thus, transplantation of significantly greater numbers of genotypically normal γc stem cells may reduce the competition between donor and host B-cell progenitors, resulting in engraftment of donor B cells in transplanted XSCID dogs.
Another possibility is the age of the donors we used in our transplant experiments. All donors were newborn puppies, whereas the majority of human donors were older sisters or adults. Studies comparing the thymic maturation potential of murine fetal and adult HSCs have shown that murine HSCs lose some of their developmental potential during ontogeny and that adult bone marrow HSCs may no longer be pluripotent.58,59 Recent studies have also demonstrated extensive ontogeny-related differences between human fetal cord blood and adult bone marrow progenitor cells.60 61 It is possible that the B-cell engraftment observed in transplanted XSCID dogs was dependent on the young age of the donors, whose HSCs may have greater potential for B-cell differentiation than older marrow donors.
Historically, the dog has been a valuable model for bone marrow transplantation, with many of the advances achieved in the dog being directly transferable to human clinical bone marrow transplantation protocols.62 Since canine and human XSCID are due to mutations of the same gene, canine XSCID provides a unique animal model to test which variable(s) contribute to the successful engraftment of donor B cells, and to develop better strategies for human XSCID bone marrow transplantation. Although similar studies could be proposed using the recently developed “γc-deficient” mice,63-65 there is at least one major difference between these mice and XSCID boys and dogs. In humans and dogs, a mutant γc appears to have little effect on B-cell development through the IgM+ stage, with the result that XSCID boys and dogs have normal or elevated numbers of peripheral B cells. On the other hand, a mutant γc in the mouse blocks early stages of B-cell development such that peripheral B cells are not observed. This suggests that γc-dependent cytokines have differing roles in human and canine B-cell development than in the mouse, and that the XSCID dog therefore represents a more appropriate model of human XSCID.
Elucidation of the conditions allowing full immunologic reconstitution and engraftment of both donor B and T cells in transplanted XSCID dogs without pretransplant conditioning needs to be explored, since cytoablation increases the risk of life-threatening infections during the posttransplant period and requires lengthy hospitalization, thus increasing the cost of bone marrow transplantation. In addition, possible long-term sequelae, including growth retardation, sterility, learning disabilities, and malignancies, have to be considered following the use of pretransplant conditioning.
Supported by Grants No. RO1 AI 26103, RO1AI33177, and RO1 HL 52971 from the National Institutes of Health.
Address reprint requests to Peter J. Felsburg, UMD, PhD, Department of Clinical Studies-Philadelphia, School of Veterinary Medicine, University of Pennsylvania, 3850 Spruce St, Philadelphia, PA 19104.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal