Abstract
The influence of bone marrow transplantation (BMT) conditioning regimens on the incidence and severity of graft-versus-host disease (GVHD) has been suggested in clinical BMT. Using murine BMT models, we show here an increase in GVHD severity in several donor-recipient strain combinations after intensification of the conditioning regimen by increasing the total body irradiation (TBI) dose from 900 cGy to 1,300 cGy. Increased GVHD was mediated by systemic increases in tumor necrosis factor α (TNFα). Histologic analysis of gastrointestinal tracts showed synergistic damage by increased TBI and allogeneic donor cells that permitted increased translocation of lipopolysacharide (LPS) into the systemic circulation. In vitro, LPS triggered excess TNFα from macrophages primed by the GVH reaction. In addition, macrophages isolated within 4 hours of conditioning were primed in proportion to the TBI dose itself to secrete TNFα. Thus, the higher TBI dose increased macrophage priming and increased gut damage after allogeneic BMT, causing higher systemic levels of inflammatory cytokines and subsequent severe GVHD. These data highlight the importance of conditioning in GVHD pathophysiology and suggest that interventions to prevent LPS stimulation of primed macrophages may limit the severity of GVHD after intensive conditioning for allogeneic BMT.
BONE MARROW transplantation (BMT) is currently indicated in the treatment of a number of malignant and nonmalignant diseases, including acute and chronic leukemias, myelomas, lymphomas, aplastic anemia, solid tumors, and severe immunodeficiencies. Allogeneic BMT is associated with serious side effects and its most common complication is graft-versus-host disease (GVHD). Recently, the use of unrelated donors and HLA-nonidentical family members has resulted in increased frequencies of severe GVHD. Previous clinical studies have shown a correlation between GVHD incidence and radiation dose (<1,200 v ≥1,200 cGy)1,2 and conditioning regimens containing radiation compared with those containing only chemotherapy.3 The severity of conditioning-related toxicity has also been associated with GVHD incidence.4 However, these studies have concurrently demonstrated an inverse correlation between conditioning intensity and immunosuppression prophylaxis compliance, which may partially account for the increase in GVHD. Indeed, only one of these studies identified increased radiation intensity as an independent variable for GVHD,1 and there was no demonstrable association with severe disease (grade III-IV). By contrast, the association of increased GVHD with dose reductions in cyclosporine was maintained throughout all GVHD grades. It is thus difficult to separate the influence of conditioning intensity and suppression of T-cell function on subsequent GVHD severity in the clinical setting.
The variable of GVHD prophylaxis can be eliminated from consideration in experimental animal models. Although early animal studies suggested GVHD incidence and severity may be related both to irradiation5 and cytotoxic agents (cyclophosphamide),6 this relationship may be attributed to the immunosupressive nature of these agents. The effects of partial engraftment or the contribution of mixed chimerism to donor T-cell tolerance of the host was also not addressed in these studies. Mixed chimerism has been associated with reduced GVHD in experimental7,8 and clinical settings.9
The relationship of conditioning intensity to GVHD severity has therefore not been clearly established independent of immunosuppression or the tolerance induced by mixed donor-host chimerism. We therefore chose to examine this issue in murine BMT models in which these variables could be tightly controlled. We have compared two total body irradiation (TBI) doses (900 and 1,300 cGy), both of which provided complete donor engraftment and elimination of host lympho-hematopoetic cells. Our results confirm that high TBI doses increase GVHD severity and that they do so by amplifying the dysregulation of inflammatory cytokines. TBI and allogeneic immune cells synergize to damage the gastrointestinal (GI) tract, thereby permitting increased translocation of lipopolysacharide (LPS) into the systemic circulation. This damage, together with increased production of tumor necrosis factor α (TNFα) by host cells after TBI, leads to the increased morbidity and mortality of GVHD.
MATERIALS AND METHODS
Mice. Female C57BL/6 (B6, H-2b, Ly-5.2+), B6D2F1 (H-2b/d, Ly-5.2+)10, B6.C-H2bm-1 (Kbm-1 DbI-AbI-Eb), CBA/J (H-2k), and B10.BR (H-2k) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). B6 Ly-5a (H-2b, Ly-5.1+)10 mice were purchased from Frederick Cancer Research Facility (Frederick, MD). The age of mice used for experiments ranged between 10 and 14 weeks. Mice were housed in sterilized microisolator cages and received filtered water and normal chow or autoclaved hyper-chlorinated drinking water for the first 2 weeks post-BMT.
BMT. Mice were transplanted according to a standard protocol described previously.11 Briefly, recipients received 900 cGy or 1,300 cGy TBI (137Cs source), split into two doses separated by 3 hours to minimize GI toxicity. T-cell–depleted (anti-Thy 1.2 monoclonal antibody [MoAb] and rabbit complement) bone marrow cells (5 × 106) plus 2 × 106 nylon wool purified splenic T cells from respective allogeneic or syngeneic donors were resuspended (in 0.25 mL of Leibovitz's L-15 media; GIBCO BRL, Gaithersburg, MD) and injected intravenously into recipients on day 0. For engraftment experiments in the B6 → B6D2F1 system, Ly-5a (H-2b, Ly-5.1+) animals were used as donors. Survival was monitored daily, and recipient's body weights and GVHD clinical scores were measured weekly. Donor cell engraftment was determined by examining the percentage of Ly-5.1+ cells in peripheral blood at days 28 and 70 after transplantation.
Assessment of GVHD. The degree of systemic GVHD was assessed by a scoring system as previously described that incorporates five clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity.12 At the time of analysis, mice from coded cages were evaluated and graded from 0 to 2 for each criterion. A clinical index was subsequently generated by summation of the five criteria scores (maximum index = 10).12 This index included the percentage of weight change, a parameter that has been found to be a reliable indicator of systemic GVHD in our model and other murine models.13-15 Transplanted mice were ear-punched and individual weights were obtained and recorded on day 0 and weekly thereafter.
Fluorescence-activated cell sorting (FACS) analysis. Fluorescein isothiocyanate (FITC)-conjugated MoAbs to mouse Ly 5.1 and Ly 5.2 antigens were purchased from PharMingen (San Diego, CA). F4/80 antibody, specific for murine macrophages, was purchased from Caltag Laboratories (San Francisco, CA). For determining the extent of donor cell engraftment (anti-Ly 5.1 and anti-Ly 5.2 MoAb) or cell macrophage content (F4/80 MoAb), peripheral blood cells (PBCs) or peritoneal cells were analyzed, respectively. Cells were first incubated with MoAb 2.4G2 for 15 minutes at 4°C and then with the relevant FITC-conjugated MoAb for 30 minutes at 4°C. Finally, cells were washed twice with phosphate-buffered saline (PBS)/0.2% bovine serum albumin and fixed with PBS/1% paraformaldehyde. In control experiments, PBCs from donor B6 Ly-5a mice were 98.4% Ly-5.1+, and PBCs from recipient B6D2F1 mice were 98.9% Ly-5.2+. Peritoneal lavage cell suspensions varied from 25% to 70% F4/80 positive (data not shown).
Cytokine enzyme-linked immunosorbent assay (ELISA). The antibodies used in the assays were purchased from Genzyme (Cambridge, MA), and assays were performed according to the manufacturer's protocol. Briefly, samples were diluted 1:3 to 1:4 and TNFα or interleukin-1β (IL-1β) was captured by the specific primary monoclonal antibody (MoAb) and detected by horse radish peroxidase (TNFα) or biotin-labeled (IL-1β) secondary MoAbs. The assays were developed by strepavidin (IL-1β only) and substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Plates were read at 450 nm using a microplate reader (Model 3550; Bio-Rad Labs, Hercules, CA). Recombinant mTNFα and mIL-1β (Genzyme) were used as standards for ELISAs. Samples and standards were run in duplicate and the sensitivity of the assays was 16 to 20 pg/mL for both cytokines, depending on sample dilution.
Serum LPS estimation. For determination of endotoxin concentration in serum, the Limulus Amebocyte Lysate (LAL) assay (Bio Whittaker, Walkersville, MD) was performed according to the manufacturer's protocol. Briefly, serum samples were collected and analyzed using pyrogen-free materials, diluted 10% (vol/vol) in LAL reagent water, and heated to 70°C for 5 minutes to remove any nonspecific inhibition to the assay. Samples were then incubated with equal volumes of LAL for 10 minutes at 37°C and developed with equal volumes of substrate solution for 6 minutes. The absorbance of the assay plate was read at 405 nm using the same microplate reader used in cytokine assays. Samples and standards were run in duplicate and the lower limit of detection was 0.15 U/mL. All units expressed are relative to the US reference standard EC-6.
Cell culture. All culture media reagents were purchased from GIBCO BRL (Gaithersburg, MD). Peritoneal macrophages were lavaged and pooled from individual animals within a treatment group before culture at 2 × 105 (day 0) or 1 × 105 (day 7) cells per well in flat-bottomed 96-well Falcon plates (Lincoln Park, NJ) with or without LPS. Cell culture was performed in 3% fetal calf serum (FCS)/RPMI 1640 (day-0 cultures) or 3% FCS/Dulbecco's Modified Eagle Medium (day-7 cultures) supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 0.1 mmol/L nonessential amino acid, 0.02 mmol/L β-mercaptoethanol, and 10 mmol/L HEPES, pH 7.75, at 37°C in a humidified incubator supplemented with 7% CO2 . Supernatants were collected at 4 hours for TNFα and 48 hours for day-7 IL-1β analysis by ELISA. In preliminary experiments, no TNFα or IL-1β was produced by macrophages cultured in serum-free media. The addition of serum resulted in the production of small quantities of these cytokines only at the above-mentioned time points.
TNFR:Fc treatment. Recombinant human TNF receptor bound to the Fc portion of human Ig (TNFR:Fc) was supplied by Immunex (Seattle, WA).16 Mice were injected intraperitoneally with TNFR:Fc (100 μg/animal) on days −2, −1, and 0 then on alternate days up to and including day 14 (10 injections total). TNFR:Fc was diluted in normal saline before injection, as recommended by the manufacturer. Mice from the control group received an injection of control human Ig.
Histology. Formalin-preserved liver and small and large bowel were embedded in paraffin, cut into 5-μm–thick sections, and stained with haematoxylin and eosin for histologic examination. Slides were coded without reference to prior treatment and examined in a blinded fashion by a pathologist (J.M.C.). A semiquantitative scoring system was used to assess the following abnormalities known to be associated with GVHD17,18: small intestine: villous blunting, crypt regeneration, loss of enterocyte brush border, luminal sloughing of cellular debri, crypt cell apoptosis, outright crypt destruction, and lamina propria lymphocytic infiltrate; colon: crypt regeneration, surface coloncytes, colonocyte vacuolization, surface colonocyte attenuation, crypt cell apoptosis, outright crypt destruction, and lamina propria lymphocytic infiltrate. The scoring system denoted 0 as normal, 0.5 as focal and rare, 1.0 as focal and mild, 2.0 as diffuse and mild, 3.0 as diffuse and moderate, and 4.0 as diffuse and severe. Scores were added to provide a total score for each specimen. Only after scoring was performed were codes broken and data compiled.
Statistical analysis. The Mann-Whitney U test was used for the statistical analysis of cytokine data, LPS levels, clinical scores, weight loss, and histology, whereas the Wilcoxon rank test was used to analyze survival data. P ≤ .05 was considered statistically significant.
RESULTS
Reduction of conditioning TBI intensity reduces GVHD mortality and morbidity. We first studied two lethal doses of TBI, 900 cGy and 1,300 cGy, for their effects on GVHD. At both TBI doses animals receiving syngeneic marrow had 100% survival, whereas animals receiving no marrow had 0% survival. The T-cell dose of 2 × 106 cells was chosen to induce acute GVHD in a well-established murine model (B6 → B6D2F1). As shown in Fig 1, animals receiving the higher TBI dose had 40% survival at day 70, associated with weight loss and clinical features of GVHD that were seen in all animals. Clinical signs of GVHD were quantified by summing the scores (0-2) for each of five parameters: mobility, posture, fur texture, skin, and weight loss, as previously reported.12 Significant differences in clinical scores relative to syngeneic animals were observed from day 28 onwards. By contrast, animals transplanted after 900 cGy TBI had greater than 90% survival, although they displayed significant differences in body weight and clinical parameters of GVHD relative to syngeneic controls; these clinical signs were significantly less than those in animals conditioned with 1,300 cGy TBI from day 7 after BMT (P < .01).
Reduction of GVHD mortality and morbidity in BMT (B6 → B6D2F1) after reduced conditioning TBI. Results represent a combination of two experiments in which allogeneic arms are represented by solid and syngeneic arms are represented by open symbols: 1,300 cGy TBI (•), n = 15; 900 cGy TBI (▪), n = 15; 1,300 cGy TBI (○), n = 8. The relative bone marrow and T-cell inoculum were identical in all groups. (A) Percentage of survival: • v ▪, P < .001 by Wilcoxon signed-rank test. (B) Weight after BMT as a percentage of pretransplant weight; • v ▪, P < .02 from day 28 onward; ▪ v ○, P < .05 from day 49 onward. (C) Clinical score: • v ▪, P < .005 from day 7 onward; ▪ v ○, P < .005 from day 35 onward.
Reduction of GVHD mortality and morbidity in BMT (B6 → B6D2F1) after reduced conditioning TBI. Results represent a combination of two experiments in which allogeneic arms are represented by solid and syngeneic arms are represented by open symbols: 1,300 cGy TBI (•), n = 15; 900 cGy TBI (▪), n = 15; 1,300 cGy TBI (○), n = 8. The relative bone marrow and T-cell inoculum were identical in all groups. (A) Percentage of survival: • v ▪, P < .001 by Wilcoxon signed-rank test. (B) Weight after BMT as a percentage of pretransplant weight; • v ▪, P < .02 from day 28 onward; ▪ v ○, P < .05 from day 49 onward. (C) Clinical score: • v ▪, P < .005 from day 7 onward; ▪ v ○, P < .005 from day 35 onward.
Reduction in the conditioning TBI dose to 900 cGy is not associated with mixed donor/host chimerism. We evaluated donor engraftment because mixed donor/host chimerism has been associated with tolerance of donor T cells to host tissues and reduced GVHD.19 Donor engraftment was documented at days 28 and 70 in allogeneic recipients by phenotyping of Ly 5 alleles as detailed in the Materials and Methods. All animals (n = 5 to 6) had 100% donor (Ly5.1+) cells in the peripheral blood at day 28 and at the study endpoint (day 70). No (0%) host (Ly 5.2+) peripheral blood cells were observed in any animal, ruling out mixed chimerism as a cause for the reduction in GVHD after conditioning with 900 cGy TBI.
The reductions in GVHD mortality and morbidity after a lower conditioning TBI dose is observed in several donor/host combinations. We next investigated whether this association between GVHD severity and higher TBI dose was observed when different histocompatibility antigens were used to stimulate GVHD. Figure 2 presents the data for survival, weight loss, and GVHD clinical scores when donors and host differ at a single class I major histocompatibility complex (MHC) antigen (bm1 → B6, Fig 2A) or at multiple minor histocompatibility (H) antigens (B10.BR → CBA, Fig 2B). The doses of TBI, T cells, and bone marrow were identical to those in the experiments in Fig 1. This last model was particularly important because studies in a similar model of GVHD to minor H antigens (B10.BR → AKR) had not shown significant differences in GVHD severity between 900 and 1,200 cGy TBI.8 Our data clearly show that a difference of 400 cGy in the dose of TBI increased GVHD in all parameters measured in all donor/recipient strain combinations. The total clinical GVHD score was again noted to be a more sensitive index than weight loss alone, capable of identifying significant GVHD earlier after BMT.
Reduction of GVHD mortality and morbidity in BMT across both isolated MHC class I (bm1 → B6; A) and minor histocompatible antigen barriers ( B10.BR → CBA; B) after reduced conditioning TBI. Solid symbols represent allogeneic groups 1,300 cGy TBI (•; n = 10) and 900 cGy TBI (▪; n = 10) and open symbols represent the syngeneic group 1,300 cGy TBI (○; n = 4 to 5). Relative bone marrow and T-cell inoculums were identical in all groups. (A) The percentage of survival: • v ▪ and ○, P < .001 by Wilcoxon signed rank-test, weight after BMT as a percentage of pretransplant weight; • v ▪, P < .01 from day 28 onward and • v ○, P < .02 from day 21 onward, clinical score; • v ▪, P < .01 from day 7 onward; ▪ v ○, P < .02 from day 28 onward. (B) The percentage of survival: • v ▪ and ○, P < .001 by Wilcoxon signed rank test, weight after BMT as a percentage of pretransplant weight; • v ▪, P < .01 from day 21 onward and • v ○, P < .02 from day 28 onward, clinical score; • v ▪, P < .01 from day 7 onward; ▪ v ○, P < .01 from day 14 onward.
Reduction of GVHD mortality and morbidity in BMT across both isolated MHC class I (bm1 → B6; A) and minor histocompatible antigen barriers ( B10.BR → CBA; B) after reduced conditioning TBI. Solid symbols represent allogeneic groups 1,300 cGy TBI (•; n = 10) and 900 cGy TBI (▪; n = 10) and open symbols represent the syngeneic group 1,300 cGy TBI (○; n = 4 to 5). Relative bone marrow and T-cell inoculums were identical in all groups. (A) The percentage of survival: • v ▪ and ○, P < .001 by Wilcoxon signed rank-test, weight after BMT as a percentage of pretransplant weight; • v ▪, P < .01 from day 28 onward and • v ○, P < .02 from day 21 onward, clinical score; • v ▪, P < .01 from day 7 onward; ▪ v ○, P < .02 from day 28 onward. (B) The percentage of survival: • v ▪ and ○, P < .001 by Wilcoxon signed rank test, weight after BMT as a percentage of pretransplant weight; • v ▪, P < .01 from day 21 onward and • v ○, P < .02 from day 28 onward, clinical score; • v ▪, P < .01 from day 7 onward; ▪ v ○, P < .01 from day 14 onward.
Higher TBI doses are associated with significant increases in serum inflammatory cytokines 1 week after BMT both in vivo and in vitro. Inflammatory cytokines (TNFα and IL-1β) have been implicated in the pathogenesis of acute GVHD both clinically and experimentally (reviewed in Holler and Ferrara20 ). We therefore hypothesized that higher TBI doses would be associated with increased serum levels of these cytokines. As shown in Fig 3, both TNFα and IL-1β serum levels were significantly higher at day 7 after BMT in allogeneic animals conditioned with 1,300 cGy TBI, whereas TNFα levels at later time points were similar between groups. This early increase in TNFα is consistent with the kinetics observed after clinical BMT.21 Increased TNFα was also observed after 1,300 cGy TBI in acute GVHD induced to minor H antigens (B10.BR → CBA: 310 ± 134 pg/mL v 113 ± 14 pg/mL, P < .05) and isolated class I antigens (bm1 → B6: 46 ± 23 pg/mL v levels below detection, P < .05). No syngeneic BMT recipient had measurable serum levels of TNFα or IL-1β from day 3 onward (n = 8, data not shown). Indeed, the only TNFα detectable in the serum after syngeneic BMT was at day +1 and was similar to levels in allogeneic BMT recipients (data not shown). Increased TNFα and IL-1β were also secreted by macrophages isolated from allogeneic BMT recipients at day 7 post-BMT and stimulated in vitro with LPS (Table 1). Interestingly, syngeneic BMT recipients also showed significantly elevated TNFα and IL-1β production after 1,300 cGy TBI, although these were below levels secreted by macrophages from allogeneic BMT recipients after 900 cGy TBI. Small amounts of TNFα were produced (<10 pg) by macrophages in the absence of LPS stimulation, probably reflecting the presence of trace amounts of LPS in the fetal bovine serum used in culture media.
Increased serum TNFα and IL-1β levels after higher TBI doses. Mice were bled at the above-mentioned times post-BMT after conditioning with 900 cGy (□) or 1,300 cGy TBI (▪). Data represent the mean ± standard error. At day 7, the TNFα data represent n = 15 and 18 in the 900 and 1,300 cGy groups, respectively, with between 5 and 9 animals in each group at the remaining time intervals. For IL-1β, n = 13 and 20, respectively. * P < .01 and < .001 at days 6 and 7, respectively, for TNFα and P < .02 at day 7 for IL-1β.
Increased serum TNFα and IL-1β levels after higher TBI doses. Mice were bled at the above-mentioned times post-BMT after conditioning with 900 cGy (□) or 1,300 cGy TBI (▪). Data represent the mean ± standard error. At day 7, the TNFα data represent n = 15 and 18 in the 900 and 1,300 cGy groups, respectively, with between 5 and 9 animals in each group at the remaining time intervals. For IL-1β, n = 13 and 20, respectively. * P < .01 and < .001 at days 6 and 7, respectively, for TNFα and P < .02 at day 7 for IL-1β.
Day 7 In Vitro Cytokine Production After BMT
| . | Conditioning Regimen . | |
|---|---|---|
| . | 900 cGy TBI . | 1,300 cGy TBI . |
| TNFα* | ||
| Allogeneic | 1,938 ± 151 | 3,110 ± 219† |
| Syngeneic | 749 ± 9 | 1,046 ± 47† |
| IL-1β* | ||
| Allogeneic | 87 ± 5.5 | 154 ± 3.1† |
| Syngeneic | 6 ± 4.1 | 72 ± 5.1† |
| . | Conditioning Regimen . | |
|---|---|---|
| . | 900 cGy TBI . | 1,300 cGy TBI . |
| TNFα* | ||
| Allogeneic | 1,938 ± 151 | 3,110 ± 219† |
| Syngeneic | 749 ± 9 | 1,046 ± 47† |
| IL-1β* | ||
| Allogeneic | 87 ± 5.5 | 154 ± 3.1† |
| Syngeneic | 6 ± 4.1 | 72 ± 5.1† |
Macrophages were pooled from 4 animals in each group and plated at 105 cells per well in the presence of LPS (0.1 μg/mL). Supernatants were taken at 4 (TNFα) and 48 (IL-1β) hours and analyzed by ELISA. Data represent the mean ± standard error of quadruplicate wells standardized for macrophage number.
In vitro TNFα and IL-1β production (in picograms) per 105 macrophages.
P < .05 v 900 cGy TBI.
TNFα is a significant effector of early GVHD mortality in the groups conditioned with 1,300 cGy TBI. To confirm that TNFα was causally related to increased GVHD mortality observed after 1,300 cGy TBI, we studied the effect of cytokine neutralization on clinical GVHD in this model. Recombinant human TNF receptor:Fc (TNFR:Fc), a bivalent soluble form of the TNFα receptor bound to the heavy portion of human Ig molecule, was administered to animals intraperitoneally on days −2, −1, and 0 and then on alternate days up to and including day 14. This agent is known to have a serum half life of greater than 20 hours in mice after intravenous administration,22 enabling alternate day treatment. TNFR:Fc administration largely abrogated the mortality from GVHD that was observed in animals receiving control human Ig (Fig 4) and significantly reduced the clinical score of GVHD throughout the 50-day observation period.
TNFα blockade reduces GVHD mortality and morbidity. B6D2F1 mice received recombinant human TNFR:Fc (▴; n = 10) or control human Ig (•; n = 9) 100 μg/d intraperitoneally on days −2, −1, and 0 (0 is day of BMT) and then on alternate days up to day 14. (A) The percentage of survival: ▴ v •, P < .001 by Wilcoxon rank-test. (B) Clinical score: ▴ v •, P < .05 by Mann-Whitney U test from day 7 up to 35.
TNFα blockade reduces GVHD mortality and morbidity. B6D2F1 mice received recombinant human TNFR:Fc (▴; n = 10) or control human Ig (•; n = 9) 100 μg/d intraperitoneally on days −2, −1, and 0 (0 is day of BMT) and then on alternate days up to day 14. (A) The percentage of survival: ▴ v •, P < .001 by Wilcoxon rank-test. (B) Clinical score: ▴ v •, P < .05 by Mann-Whitney U test from day 7 up to 35.
Increased TBI dose is associated with elevated serum levels of LPS and increased GI tract damage at day 7 in allogeneic animals. Endotoxin (LPS) has been shown to stimulate excessive inflammatory cytokine release from macrophages during GVHD,23,24 and we next examined whether the increased TNFα and IL-1β serum levels associated with the increased TBI were also associated with greater systemic LPS levels. As shown in Fig 5, there was significantly more serum LPS in allogeneic BMT recipients conditioned with 1,300 cGy than with 900 cGy TBI. The increased TBI in and of itself was not enough to cause the increase because syngeneic BMT recipients conditioned with 1,300 cGy TBI had approximately 30-fold lower serum LPS concentrations than allogeneic recipients conditioned with the same TBI dose. We hypothesize that this surprising result was due to the action of allogeneic donor cells that contributed to greater systemic LPS levels, probably through a direct toxic effect of donor T and NK cells on the GI tract.25 26 The GI tract (particularly the large bowel) is colonized by gram-negative bacteria and is a potent source of LPS. Because injury by GVHD and/or TBI would facilitate LPS translation into the circulation, we examined both the upper and lower bowel for histologic changes at day 7 post-BMT. Representative samples from allogeneic and syngeneic groups are shown in Fig 6. A semiquantitative index was developed in which features of GI damage were scored on a scale of 0 to 4 and summed (see the Materials and Methods). As seen in Table 2, allogeneic BMT recipients after 1,300 cGy TBI had significantly greater GI tract pathology than those after 900 cGy TBI. Enhanced apoptosis, lymphocytic infiltrate, brush border loss, and sloughing into the lumen were all observed in the small bowel of 1,300 cGy TBI allogeneic recipients. Greater degrees of epithelial pathology and crypt regeneration were also observed in the large bowel of these animals. The low level of these abnormalities in syngeneic animals transplanted after 1,300 cGy TBI corroborates the finding of very low levels of LPS in the systemic circulation and underscores the fact that higher TBI doses synergized with allogeneic reactions of donor cells to damage the GI tract of the host.
Higher TBI doses are associated with detectable serum LPS at day 7. Data represent the mean ± standard error of 22 animals in the 1,300 cGy TBI allogeneic group, 21 in the 900 cGy TBI allogeneic group, and 21 in the syngeneic group. * P < .01 for 1,300 cGy TBI allogeneic versus the 900 cGy TBI allogeneic group and P < .05 1,300 cGy TBI allogeneic versus the syngeneic group.
Higher TBI doses are associated with detectable serum LPS at day 7. Data represent the mean ± standard error of 22 animals in the 1,300 cGy TBI allogeneic group, 21 in the 900 cGy TBI allogeneic group, and 21 in the syngeneic group. * P < .01 for 1,300 cGy TBI allogeneic versus the 900 cGy TBI allogeneic group and P < .05 1,300 cGy TBI allogeneic versus the syngeneic group.
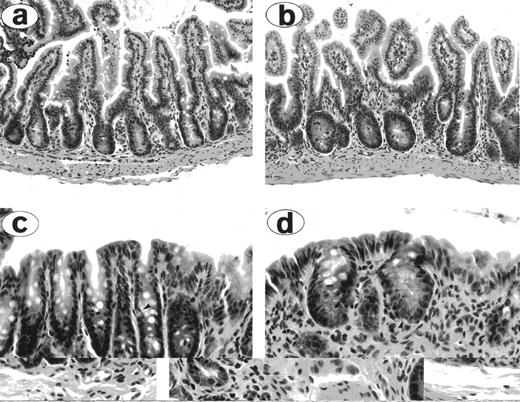
High TBI doses increase the severity of early GI tract GVHD. Bowel histology in allografted mice treated with 900 cGy TBI (a and c) and 1,300 cGy TBI (b and d). The small bowel of the 900 cGy mouse (a) exhibits mild villous blunting and crypt regenerative changes; crypts in the large bowel (c) exhibit mitotic figures and a rare apoptotic cell (arrowhead). In contrast, small bowel of the 1,300 cGy mouse (b) exhibits more marked villous blunting and crypt regeneration with mitotic figures. The large bowel (d) exhibits crypt atrophy, abundant apoptotic cells (arrowhead), and marked depletion of surface epithelial mucous. Original magnification: (a and b) × 200; (c and d) × 400.
High TBI doses increase the severity of early GI tract GVHD. Bowel histology in allografted mice treated with 900 cGy TBI (a and c) and 1,300 cGy TBI (b and d). The small bowel of the 900 cGy mouse (a) exhibits mild villous blunting and crypt regenerative changes; crypts in the large bowel (c) exhibit mitotic figures and a rare apoptotic cell (arrowhead). In contrast, small bowel of the 1,300 cGy mouse (b) exhibits more marked villous blunting and crypt regeneration with mitotic figures. The large bowel (d) exhibits crypt atrophy, abundant apoptotic cells (arrowhead), and marked depletion of surface epithelial mucous. Original magnification: (a and b) × 200; (c and d) × 400.
Day 7 GI Tract Damage
| . | Conditioning Regimen . | ||
|---|---|---|---|
| . | 1,300 cGy TBI Syngeneic . | 900 cGy TBI Allogeneic (n = 4) . | 1,300 cGy TBI Allogeneic . |
| . | (n = 2) . | . | (n = 4) . |
| Small bowel* | 2.0 ± 0.5 | 4.0 ± 1.3 | 10.9 ± 1.9† |
| Large bowel‡ | 4.0 ± 1.0 | 4.6 ± 0.7 | 7.6 ± 0.6† |
| . | Conditioning Regimen . | ||
|---|---|---|---|
| . | 1,300 cGy TBI Syngeneic . | 900 cGy TBI Allogeneic (n = 4) . | 1,300 cGy TBI Allogeneic . |
| . | (n = 2) . | . | (n = 4) . |
| Small bowel* | 2.0 ± 0.5 | 4.0 ± 1.3 | 10.9 ± 1.9† |
| Large bowel‡ | 4.0 ± 1.0 | 4.6 ± 0.7 | 7.6 ± 0.6† |
Scores (0-4) for each parameter below were given to coded slides and summed.
Villous blunting, crypt regeneration, loss of enterocyte brush border, luminal sloughing of cellular debri, crypt cell apoptosis, outright crypt destruction, and lamina propria lymphocytic infiltrate.
P < .03 v 900 cGy TBI allo.
Crypt regeneration, surface colonocytes, colonocyte vacuolization, surface colonocyte attenuation, crypt cell apoptosis, outright crypt destruction, and lamina propria lymphocytic infiltrate.
Conditioning primes macrophages to produce increased TNFα after LPS stimulation. The increased LPS level noted in allogeneic BMT recipients conditioned with 1,300 cGy TBI might itself explain the higher serum concentrations of TNFα and IL-1β in these animals. However, data from other model systems suggest that low radiation doses (<400 cGy) are capable of priming cells to increased production upon LPS challenge,27-29 although there are no data regarding the relative effects of larger radiation doses used in BMT conditioning schedules. Increased TNFα and IL-1β secretion by macrophages taken from animals conditioned with 1,300 cGy TBI 7 days after syngeneic BMT suggested that this difference in radiation might itself amplify responses to LPS. We therefore analyzed the in vitro responses of host peritoneal macrophages to LPS within 4 hours of the completion of different conditioning regimens without bone marrow rescue. Interestingly, macrophages exposed to 1,300 cGy TBI in vivo produced significantly more TNFα after LPS stimulation in vitro than the same cells exposed to 900 cGy TBI, whereas both regimens result in priming greater than that of naive macrophages (Fig 7).
Conditioning regimes prime macrophages to secrete TNFα after LPS stimulation. Peritoneal macrophages were pooled from a minimum of 4 B6D2F1 animals in each group ([▪] naive; [□] 900cGy TBI; [▧] 1,300 cGy TBI) within 4 hours of the completion of TBI and stimulated with LPS (0.1 μg/mL). TNFα was determined by ELISA in the culture supernatants taken 4 hours after stimulation. Data represent the mean ± standard error of quadruplicate wells standardized for 105 macrophages by FACS analysis using F4/80 positivity. Data represent one of three similar experiments. *P < .03 v naive and 1,300 cGy TBI groups.
Conditioning regimes prime macrophages to secrete TNFα after LPS stimulation. Peritoneal macrophages were pooled from a minimum of 4 B6D2F1 animals in each group ([▪] naive; [□] 900cGy TBI; [▧] 1,300 cGy TBI) within 4 hours of the completion of TBI and stimulated with LPS (0.1 μg/mL). TNFα was determined by ELISA in the culture supernatants taken 4 hours after stimulation. Data represent the mean ± standard error of quadruplicate wells standardized for 105 macrophages by FACS analysis using F4/80 positivity. Data represent one of three similar experiments. *P < .03 v naive and 1,300 cGy TBI groups.
DISCUSSION
This study shows an increase in GVHD severity after allogeneic BMT in several donor-recipient strain combinations after intensification of the TBI dose from 900 to 1,300 cGy. GVHD, as measured by both clinical morbidity and mortality, was more severe after the intensified conditioning, regardless of the histocompatibilities between donor and host. GVHD was mediated by a systemic increase in TNFα that was induced both by greater sensitivity of macrophages to LPS and higher circulating LPS levels in allogeneic BMT recipients conditioned with the higher TBI dose. Allogeneic donor cells and increased radiation synergized to cause GI damage that was associated with greater amounts of LPS entering the systemic circulation. These studies show that increased inflammatory cytokine levels after high TBI doses occur independently of reductions in immunosuppression or the presence of mixed chimerism that might effect donor T-cell responses to the host. These data contrast with a previous study that did not see differences in GVHD mortality after two TBI doses (900 v 1,200 cGy).8 This discordance may be attributable to differences in TBI administration (single fraction v two fractions) in addition to the difference in higher TBI dose (1,200 v 1,300 cGy).
Inflammatory cytokines are known to be important in the pathogenesis of experimental acute GVHD30-32 and in clinical GVHD.33 It is of particular interest to compare the dramatic benefit of TNFα blockade in allogeneic recipients after 1,300 cGy TBI and the lack of such a benefit in a GVHD model using 800 cGy TBI and a high T-cell dose (25 × 106 splenocytes).34 These data suggest that TNFα blockade may prevent GVHD most effectively when used in conjunction with intensive conditioning regimens containing large TBI doses. The partial protection afforded by TNFα blockade suggests that other cytokines (eg, IL-1 and interferon γ) and cellular mechanisms (eg, Fas and perforin pathways35,36 ) may play additive roles in this process. The prevention of critical target organ damage that occurs in response to TNFα (particularly the gastrointestinal tract30 and liver37 ) may also contribute to the reduction in GVHD and may account for the reduction of GVHD observed when the injection of the donor inoculum is delayed until the initial inflammatory cytokine burst from host tissues has subsided.38
We were unable to consistently detect TNFα and IL-1β in host serum within 72 hours after BMT, although it has been seen at this time after 400 cGy TBI in SCID mice.38 This discrepancy may relate to the radiation hypersensitivity of SCID animals39 and the subsequent translocation of LPS across their tenuous GI mucosal barrier. Clinical studies have shown that conditioning regimens can induce early TNFα release to varying degrees, which probably reflects differences in assay techniques, patient populations, and the conditioning schedules themselves. Holler et al21 found abnormal TNFα levels in 24% of patients during conditioning with either cyclophosphamide and TBI or busulphan and cyclophosphamide, but Girinsky et al40 found elevated TNFα levels only in patients who received TBI as a single 10-Gy fraction. Whether inflammatory cytokines are initially released in response to radiation alone or the combination of radiation and LPS stimulation, as is suggested by our data, remains to be determined.
The increased TNFα and IL-1β serum levels after 1,300 cGy TBI led us to hypothesize that greater amounts of LPS would be present in the systemic circulation of the more intensively conditioned recipients. Our study confirms this hypothesis and provides evidence that allogeneic donor cells contribute to the entry of LPS into the systemic circulation. Increased LPS levels associated with high TBI doses occurred only in the setting of enhanced damage to the GI tract (Table 2) during severe GVHD, which may have permitted LPS translation from the gut lumen into the portal and ultimately into the systemic circulation. It should be noted that the scoring system used to grade GI tract damage in this study is semiquantative and may not be linear, with a tendency to understate differences between groups. Interestingly, endotoxin in the gut lumen itself may be capable of inducing local epithelial apoptosis after irradiation,41 and the presence of systemic TNFα may also directly induce gut damage.42 Combined with T-cell and NK cell effectors of allogeneic damage,25 these mechanisms may perpetuate the transit of LPS into the systemic circulation.
It has been known for some time that the gut microflora plays an important role in GVHD pathogenesis. Early animal studies showed that mortality after BMT was prevented if mice received antibiotics to decontaminate the gut; normalization of the gut flora at or before day 20 abrogated this effect.43 In the clinical setting, gram-negative gut decontamination has also been shown to reduce GVHD.44,45 In an interesting study, fetal GI tracts from parental or F1 hybrids were implanted into F1 recipients that were then transplanted with parental bone marrow with or without GI tract decontamination.46 GVHD crypt lesions in implanted GI tracts were most severe in the allogeneic implants, but lesions were also noted in syngeneic recipients. This study is consistent with our finding of synergy between TBI and allogeneic cells in inducing GI tract damage. The mucosal damage in syngeneic implants can be interpreted as a bystander effect from cytokines released by monocytes that have been primed by interferon γ released by allogeneic donor T cells.23
Our finding that conditioning schedules directly influence macrophages and their response to subsequent LPS exposure was perhaps most surprising. A 50% increase in TNFα response to LPS was seen in macrophages after 1,300 cGy as compared with 900 cGy TBI, although the lower TBI dose was still capable of significant macrophage priming (Fig 3). The ability of radiation to induce TNFα mRNA without protein production is known,29,47 but the ability of TBI to prime macrophages has not been previously described. Indeed, the production of TNFα after in vitro irradiation without LPS stimulation has been described only in malignant cell lines.48 However, the ability of in vitro irradiation to synergize with LPS and potentiate TNFα production has been shown in peritoneal macrophage27,29 and brain cells,28 with dramatic synergy seen at 100 to 200 cGy. In addition, clinical studies have shown enhanced monocyte responses (including TNFα production) after BMT that persisted for up to 6 weeks, with greater responses observed in allogeneic compared with autologous recipients.49 This phenomenon of macrophage priming may eventually be useful in predicting inflammatory cytokine dysregulation in clinical BMT, although the additional effects of chemotherapy will need to be studied.
Our findings suggest that early prevention of the LPS-macrophage interaction may reduce GVHD after the intensive conditioning schedules required in clinical BMT. This prevention may be achieved at a number of different steps. First, attempts to eradicate or significantly reduce the load of gram-negative organisms from the GI tract is current practice in a number of transplant centers. Unfortunately, endotoxemia has been noted to be common after BMT even with gut decontamination, occurring in association with biochemical parameters of gut damage.50 Although gut decontamination can reduce GVHD,44 the effect is at best partial and problems with compliance and organism resistance are likely to limit this approach. Secondly, strengthening the GI mucosal barrier before TBI may prevent LPS entry into the circulation. Because shielding the GI tract from TBI is not feasible, this effort will rely on pharmacologic agents that reduce mucosal sensitivity to radiation and/or chemotherapy. This approach is attractive because it blocks the axis responsible for inflammatory cytokine dysregulation itself. Finally, the efficacy of systemic LPS inactivation by neutralizing proteins has been demonstrated in inflammatory systems51 and should now be studied in GVHD. These agents have the potential to allow the more intensive conditioning schedules desired to control malignancy without the accompanied increase in GVHD. If these approaches limit the antigen nonspecific inflammatory component of GVHD without interfering with the antigen-specific compartment, further progress in separating graft-versus-leukemia from GVHD may be achieved.
Supported by National Institutes of Health Grants No. CA39542 and HL55162 (J.L.M.F.). J.L.M.F. is a scholar of the Leukemia Society of America.
Address reprint requests to James L.M. Ferrara, MD, Department of Pediatric Oncology, Dana Farber Cancer Institute, Room 1630, 44 Binney St, Boston, MA 02115.






![Fig. 7. Conditioning regimes prime macrophages to secrete TNFα after LPS stimulation. Peritoneal macrophages were pooled from a minimum of 4 B6D2F1 animals in each group ([▪] naive; [□] 900cGy TBI; [▧] 1,300 cGy TBI) within 4 hours of the completion of TBI and stimulated with LPS (0.1 μg/mL). TNFα was determined by ELISA in the culture supernatants taken 4 hours after stimulation. Data represent the mean ± standard error of quadruplicate wells standardized for 105 macrophages by FACS analysis using F4/80 positivity. Data represent one of three similar experiments. *P < .03 v naive and 1,300 cGy TBI groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3204/4/m_bl_0004f7.jpeg?Expires=1765883440&Signature=DTQrw0XPimXV0CjFoqBASTxUpspgUZKaz6OyqDiNOxVmdbTEpRZG5Mbi85JsYfJVmWZzyDiYOqeW8hb3CqqPjY~twGOvIGdYuI-hM7jwsGa9x9HRo4gAbStfq9cdfWODTBxUKAuxi-cjmO6LfyNhMTvRLtSSUDdOC5JnKza0boQL2H~Md76AHmfi8C-D5WSZfvNNGkqY7mMmHF2IhW3IC0z6Ve-7oaNICH5NPgxhl8c1rcl1M-Bq5QQsSbtagvL9WoAuhP8BthwPkFG4SLkXaNYy2nBTcYiaA0wt9bf3eW~raa2V0~bjJD5WkGxjWeFLxTSyeLW2-L8TzM-d8tpWFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal