Abstract
Multiple myeloma remains an incurable malignancy because of marked resistance of tumor cells to conventional chemotherapeutic agents. Alternative strategies are needed to solve these problems. To develop a new strategy, we have generated a monoclonal antibody (MoAb), which detects a human plasma cell-specific antigen, HM1.24. In this report, we evaluated the in vivo antitumor effect of unconjugated anti-HM1.24 MoAb on human myeloma xenografts implanted into severe combined immunodeficiency (SCID) mice. Two models of disseminated or localized tumors were established in SCID mice by either intravenous or subcutaneous injection of human myeloma cell lines, ARH-77 and RPMI 8226. When mice were treated with a single intraperitoneal injection of anti-HM1.24 MoAb 1 day after tumor inoculation, the development of disseminated myeloma was completely inhibited. In mice bearing advanced tumors, multiple injections of anti-HM1.24 MoAb reduced the tumor size and significantly prolonged survival, including tumor cure, in a dose-dependent manner. The proliferation of cultured human myeloma cells was inhibited in vitro by anti-HM1.24 IgG-mediated complement-dependent cytotoxicity, but not by the antibody alone. Moreover, spleen cells from SCID mice mediated antibody-dependent cell cytotoxicity against RPMI 8226 cells. These results indicate that anti-HM1.24 MoAb can be used for immunotherapy of multiple myeloma and related plasma cell dyscrasias.
MULTIPLE MYELOMA REMAINS an incurable malignancy despite advances in chemotherapy. Although partial remissions of up to 60% are obtained with a variety of chemotherapeutic protocols, the overall survival has not improved over the past three decades.1 Myeloablative chemotherapy followed by allogeneic or autologous hematopoietic stem cell transplantation has increased the incidence of complete remission,2 3 but relapses are still observed even after remission. Because these chemotherapies have only limited value, alternative strategies are needed to solve these problems.
Numerous applications of monoclonal antibodies (MoAb) have been proposed in cancer therapy.4,5 Several investigators have reported immunotherapeutic approaches targeting to cell surface antigens on myeloma cells, using anti-CD38 antibody,6-8 anti-CD54 antibody,9 or soluble CD16.10 However, because these molecules are also expressed on normal tissues including hematopoietic stem cells,11,12 these approaches may induce side effects related to crossreactivity in vivo. Based on the fact that interleukin (IL)-6 is a major growth factor for myeloma cells functioning by an autocrine/paracrine fashion, immunotherapy targeting the IL-6–signaling system has also been reported.13-16 Clinical trials of anti-IL–6 MoAb showed transient tumor cytostasis, but did not result in tumor cure.13 14 Thus, the efficacy of immunotherapy for multiple myeloma has been disappointing.
To develop a new strategy, we have generated a MoAb, which detects a novel plasma cell-specific antigen, termed HM1.24.17 The HM1.24 is a transmembrane glycoprotein that has a molecular weight of 29 to 33 kD and is selectively expressed on terminally differentiated normal and neoplastic B cells, while its role in the growth or functions of plasma cells is unknown. Our previous study has shown that anti-HM1.24 MoAb is useful to detect and target human myeloma cells transplanted into severe combined immunodeficiency (SCID) mice in vivo.18 In this report, we describe the antitumor activity of the anti-HM1.24 MoAb against human myeloma xenografts in SCID mice. Our results indicate that the HM1.24 antigen serves as a target for immunotherapy of multiple myeloma and related plasma cell dyscrasias.
MATERIALS AND METHODS
Production of MoAb and F(ab′)2 fragment. Anti-HM1.24 MoAb (mouse IgG2aκ subclass) was produced by the fusion of mouse myeloma cells SP2/0 with spleen cells from Balb/c mice immunized with human myeloma cell line, KPC-32, as described previously.17 This MoAb recognizes 29 to 33 kD glycoprotein by immunoprecipitation assay under reducing conditions. Anti-HM1.24 MoAb and isotype-matched myeloma protein UPC-10 (Cappel, Malvern, PA) were purified from the ascites fluid by ammonium sulfate precipitation and a protein A-affinity chromatography kit (Ampure PA, Amersham Japan, Tokyo, Japan). F(ab′)2 fragment was prepared by pepsin (Sigma Chemical Co, St Louis, MO) digestion and purified on a PD-10 Sephadex G-25 column (Pharmacia, Uppsala, Sweden). The reactivity of F(ab′)2 fragment was determined by flow cytometry using RPMI 8226 cells and a fluorescein isothiocyanate-labeled rat antimouse κ light-chain antibody (Tago, Burlingame, CA)
Cell lines. Two human myeloma cell lines, RPMI 8226 and ARH-77, and T-cell line, HPB-ALL, were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan). HPB-ALL cells, which do not express the HM1.24 antigen, were used as control tumors. The cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin. Before inoculation to mice, cells were washed and resuspended in sterile phosphate-buffered saline (PBS) at a concentration of 1 × 108 cells/mL.
Animals. Five- to 6-week-old female C. B-17 SCID mice were purchased from Clea Japan Co, Inc. (Osaka, Japan) and were maintained in a specific pathogen-free facility in our Animal Resources Center. All experiments were approved by the Committee on Animals of the University of Tokushima. To eradicate residual natural killer (NK) cells, mice were injected intraperitoneally with 10 μL rabbit antiasialo GM1 antiserum (Wako Chemicals, Osaka, Japan) 1 day before tumor inoculation. The human myeloma cells (5 × 106 cells) were inoculated by either intravenous injection through the tail vein or subcutaneous injection in the back of mice. The tumor volume was determined by measuring the longest (a) and shortest (b) diameter using a caliper and calculated using the formula: ab2π/6 (mm3 ). Animals were killed by cervical dislocation and histologic examination was performed to confirm that they had developed myeloma. Excised tissues were fixed in 10% buffered formalin, paraffin-embedded, sectioned, and stained with hematoxylin and eosin, whereas additional tissue was snap-frozen for immunohistologic analysis. The subcutaneous tumors were removed and minced in RPMI 1640 medium and cells were analyzed for HM1.24 antigen expression by flow cytometry as described previously.18
In vivo antitumor experiments. To evaluate the ability of anti-HM1.24 MoAb in preventing tumor growth, immunotherapy was started 1 or 15 days after tumor inoculation. Groups of five mice received intraperitoneal injections of anti-HM1.24 MoAb in a volume of 100 μL of PBS with a daily dose of 20 μg after tumor inoculation. In further experiments, immunotherapy was started when high levels of human IgG had been detected in the sera or the subcutaneous tumors had grown to approximately 5 mm in diameter. Mice received intraperitoneal injections of anti-HM1.24 MoAb (100 μg/dose) once or twice a week. All mice were observed daily for the appearance of solid tumors and duration of survival.
Human immunoglobulin assay. The levels of human IgG in the culture supernatants and in the sera of SCID mice with ARH-77 tumors were measured by enzyme-linked immunosorbent assay (ELISA) as described previously.19
Cell proliferation assay. The effect of anti-HM1.24 MoAb on the proliferation of human myeloma cell lines was determined using a 3H-thymidine incorporation assay. The cells were cultured in 96-well plates at 1 × 105 cells/mL in 100 μL/well and incubated with various amounts of anti-HM1.24 MoAb or control IgG for 24 hours. The cells were then pulsed for 18 hours with 0.5 μCi 3H-thymidine (New England Nuclear, Boston, MA) and harvested onto glass filters using an automated cell harvester (Packard, Meriden, CT). The incorporated radioactivity was measured using a liquid scintillation counter.
Complement-dependent cytotoxicity. Cell lysis with baby rabbit complement was determined using a 51Cr-release assay. Myeloma cells were labeled with 0.1 mCi 51Cr-sodium chromate (New England Nuclear) at 37°C for 1 hour. The cells were then washed three times with RPMI 1640 medium. 51Cr-labeled cells (1 × 104 cells) were incubated with various concentrations of anti-HM1.24 MoAb or control IgG on ice for 30 minutes. The unbound antibody was removed by washing the cells three times with medium. The cells were then distributed into 96-well plates and incubated with serial dilutions of baby rabbit complement (Cedarlane, Ontario, Canada) at 37°C for 2 hours. After incubation, supernatants from each well (50 μL) were harvested and 51Cr was measured using a gamma counter. Spontaneous release of 51Cr was measured after incubating 51Cr-labeled cells with medium alone. The maximum release of 51Cr was determined after incubation of 51Cr-labeled myeloma cells with 1% NP-40. Percentage of cytotoxicity was calculated from the formula: specific cytotoxicity (%) = (A − C)/(B − C) × 100, where A = experimental 51Cr release, B = maximum 51Cr release, and C = spontaneous 51Cr release.
Antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC activity was determined by standard 4-hour 51Cr-release assay. Splenic mononuclear cells from SCID mice were used as effector cells and cultured in RPMI 1640 medium with or without 500 U/mL of recombinant mouse interleukin (IL)-2 (Genzyme, Cambridge, MA) for 6 days, then washed, and resuspended in medium before use. 51Cr-labeled target myeloma cells (1 × 104 cells) were placed in 96-well plates and various concentrations of anti-HM1.24 MoAb or control IgG were added to wells. Effector cells were then added to the plates at various effector to target (E/T) ratios. After 4 hours incubation, supernatants were removed and counted in a gamma counter. The percentage of cell lysis was determined as above.
Statistical analysis. The statistical significance in the data of in vitro experiments was determined by the unpaired t-test. The significant differences in survival data were evaluated using a log-rank test.
RESULTS
Growth and characteristics of human myeloma tumors in SCID mice. As reported previously,20 after intravenous injection of ARH-77 cells, SCID mice developed disseminated myeloma, which infiltrates the bone marrow, vertebral column, and femur. These mice showed hind leg paralysis 20 to 30 days after tumor inoculation due to compression of the spinal cord. SCID mice did not develop the disseminated myeloma by intravenous injection of RPMI 8226 cells. In contrast, after subcutaneous injection of ARH-77 or RPMI 8226 cells, mice developed localized subcutaneous tumors at the site of injections within 22 days. The localized tumors grew to approximately 2 to 3 cm in diameter 2 to 3 months after inoculation. Because ARH-77 cells produce human IgGκ in vitro, high levels of human IgG were detected in the sera of SCID mice bearing ARH-77 cells. There was a correlation between measured tumor mass and the concentration of human IgG in the serum. Thus, the serum human IgG provides a valuable measure of the presence of ARH-77 cells and estimation of the tumor burden. The expression of the HM1.24 antigen in the subcutaneous tumors was assessed by flow cytometry. The implanted cells uniformly expressed HM1.24 and CD38 as plasma cell markers similar to the original cells. The level of HM1.24 expression was similar in ARH-77 cells and RPMI 8226 cells.
In vivo antitumor experiments. We first determined whether anti-HM1.24 MoAb could prevent the long-term growth of human myeloma cell lines in vivo (Table 1). In SCID mice inoculated intravenously with ARH-77 cells, a single intraperitoneal injection of 20 μg anti-HM1.24 MoAb 1 day after tumor inoculation completely inhibited the development of disseminated myeloma. Serum human IgG levels were not increased in these mice until the end of the experimental period at day 180. When the treatment was delayed until day 15, serum levels of human IgG were significantly lower than those of control mice at day 28. However, serum IgG levels of anti-HM1.24 IgG-treated mice (n = 3) were increased to the same levels as those of control mice (n = 3) at day 42 (337 ± 91 and 333 ± 61, respectively) and no prolongation of survival was observed. In mice inoculated subcutaneously with ARH-77 cells, the same treatment had no antitumor effect on either serum level of human IgG or survival. We next determined whether the multiple injections of anti-HM1.24 MoAb could exhibit antitumor activity against localized RPMI 8226 tumors (Fig 1). After subcutaneous injection of RPMI 8226 cells, all mice developed localized tumors in 22 ± 4 (mean ± standard deviation [SD]) days. When mice were treated with 20 μg anti-HM1.24 MoAb 1 day after tumor inoculation, the mean tumor-free time was extended by 41 ± 18 days and survival was significantly prolonged compared with control IgG-treated group (P < .025). When the antibody was administered to mice for 4 consecutive days starting 1 day after tumor inoculation, the local tumor development was completely inhibited for 180 days (P < .005). In contrast, F(ab′)2 fragment of anti-HM1.24 MoAb had no therapeutic effect in vivo. The control IgG preparation did not show any tumor inhibitory effect compared with untreated mice. On the other hand, anti-HM1.24 MoAb did not improve the survival of SCID mice bearing HPB-ALL cells (T-cell line), which do not express HM1.24 antigen, showing that the antitumor effect of the antibody is mediated by a tumor-specific immune response.
Effect of Anti-HM1.24 MoAb on the Prevention of Human ARH-77 Myeloma Engraftment
| Tumor Inoculation . | Treatment (day) . | Tumor/Total Mice . | Serum Human IgG (μg/mL) . | Survival (days) . |
|---|---|---|---|---|
| IV | Control IgG (1, 15) | 5/5 | 273 ± 174 | 53 ± 21 |
| Anti-HM1.24 IgG (1) | 0/5 | 0 ± 0 | >180† | |
| Anti-HM1.24 IgG (15) | 5/5 | 60 ± 73* | 48 ± 12 | |
| SC | Control IgG (1, 15) | 5/5 | 373 ± 117 | 67 ± 19 |
| Anti-HM1.24 IgG (1) | 5/5 | 269 ± 160 | 61 ± 6 | |
| Anti-HM1.24 IgG (15) | 5/5 | 321 ± 180 | 65 ± 16 |
| Tumor Inoculation . | Treatment (day) . | Tumor/Total Mice . | Serum Human IgG (μg/mL) . | Survival (days) . |
|---|---|---|---|---|
| IV | Control IgG (1, 15) | 5/5 | 273 ± 174 | 53 ± 21 |
| Anti-HM1.24 IgG (1) | 0/5 | 0 ± 0 | >180† | |
| Anti-HM1.24 IgG (15) | 5/5 | 60 ± 73* | 48 ± 12 | |
| SC | Control IgG (1, 15) | 5/5 | 373 ± 117 | 67 ± 19 |
| Anti-HM1.24 IgG (1) | 5/5 | 269 ± 160 | 61 ± 6 | |
| Anti-HM1.24 IgG (15) | 5/5 | 321 ± 180 | 65 ± 16 |
SCID mice were treated with a single intraperitoneal injection of antibody (20 μg/day) after inoculation of ARH-77 cells. The concentrations of serum human IgG were measured at day 28 after tumor inoculation. Data represent the mean ± SD of serum human IgG or survival. P values are based on the comparison between the indicated experimental and the control IgG-treated groups.
Abbreviations: IV, intravenously; SC, subcutaneously.
P < .05.
P < .005.
Tumor-free survival curves of SCID mice bearing subcutaneous RPMI 8226 tumors. SCID mice were treated with 20 μg/dose of control IgG (○, day 1 to 4), anti-HM1.24 F(ab′)2 (□, day 1 to 4), anti-HM1.24 IgG (•, day 1), or anti-HM1.24 IgG (▪, day 1 to 4) after tumor inoculation. There were five animals per group.
Tumor-free survival curves of SCID mice bearing subcutaneous RPMI 8226 tumors. SCID mice were treated with 20 μg/dose of control IgG (○, day 1 to 4), anti-HM1.24 F(ab′)2 (□, day 1 to 4), anti-HM1.24 IgG (•, day 1), or anti-HM1.24 IgG (▪, day 1 to 4) after tumor inoculation. There were five animals per group.
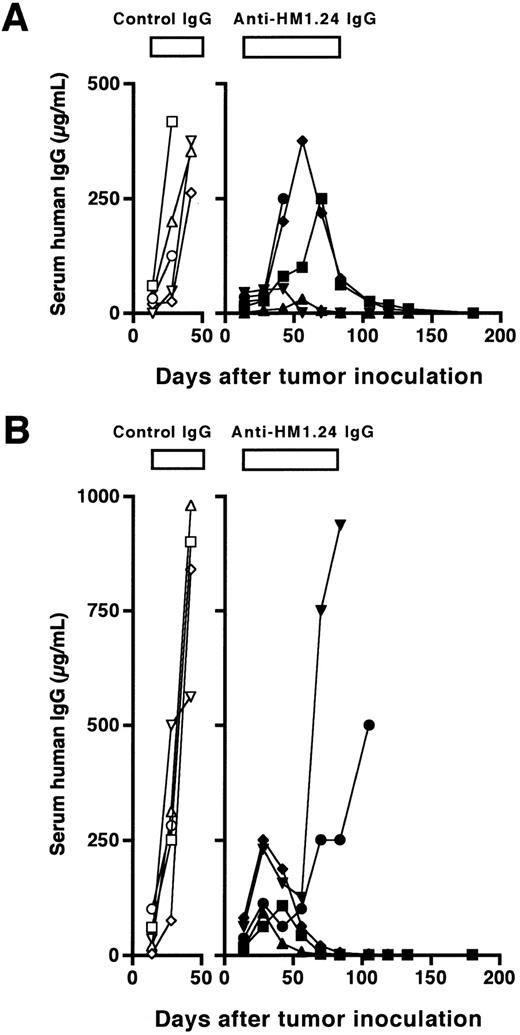
We next evaluated the therapeutic efficacy of anti-HM1.24 MoAb in SCID mice with advanced tumors. Weekly injections of a higher amount of the antibody (100 μg/dose) for 10 weeks were started 15 days after inoculation of ARH-77 cells. At that time, all mice had detectable human IgG in the sera and the levels were 6 ± 7 μg/mL (n = 10) in mice inoculated intravenously and 38 ± 33 μg/mL (n = 10) in mice inoculated subcutaneously. The course of serum concentrations of human IgG is shown in Fig 2. The levels of serum human IgG were increased in control mice inoculated intravenously or subcutaneously with ARH-77 cells, and the mice died within 53 ± 21 or 55 ± 9 days, respectively. In contrast, weekly treatment with the antibody reduced the levels of serum human IgG and prolonged the survival for up to 180 days in four of five mice bearing disseminated tumors and three of five mice bearing localized tumors. Although three of five mice bearing disseminated tumors showed an initial increase in serum human IgG, only one of them died within 50 days, and serum human IgG started to decrease by 70 days of tumor inoculation in the remaining two mice (Fig 2A). In localized tumor models, serum human IgG restarted to increase by 50 days in two of five mice after initial increase, and these mice died at day 114 and 115 (Fig 2B). Serum levels of human IgG at day 180 were undetectable (<0.1 μg/mL) in all the surviving mice, resulting in a complete remission rate of 80% and 60%, respectively. These effects on survival were statistically significant in both models as compared with the control groups (P < .01). Finally, we examined the dose-response of the effect of the antibody on in vivo antitumor activity in mice with more advanced RPMI 8226 tumors. The antibody treatment (100 μg/dose) was started 22 days after injection of RPMI 8226 cells for 50 days either once or twice a week. As shown in Fig 3, anti-HM1.24 MoAb inhibited the tumor growth in a dose-dependent manner. These results show that the effect of anti-HM1.24 MoAb is dose-dependent and that higher doses of the antibody can be effective in mice with advanced tumors. There were no treatment-related deaths with the antibody.
Course of serum levels of human IgG in SCID mice after (A) intravenous or (B) subcutaneous inoculation of ARH-77 cells. The treatment (100 μg/dose, total of 10 injections, once a week) was started 15 days after tumor inoculation. Data represent serum human IgG of each mouse treated with either control IgG (○, □, ⋄, ▵, ▿) or anti-HM1.24 MoAb (•, ▪, ♦, ▴, ▾).
Course of serum levels of human IgG in SCID mice after (A) intravenous or (B) subcutaneous inoculation of ARH-77 cells. The treatment (100 μg/dose, total of 10 injections, once a week) was started 15 days after tumor inoculation. Data represent serum human IgG of each mouse treated with either control IgG (○, □, ⋄, ▵, ▿) or anti-HM1.24 MoAb (•, ▪, ♦, ▴, ▾).
Antitumor effect of anti-HM1.24 MoAb on advanced RPMI 8226 tumors in SCID mice. Mice were injected subcutaneously with RPMI 8226 cells at day 0. The anti-HM1.24 MoAb treatment (100 μg/dose) was started 22 days after tumor inoculation for 50 days either once (•, n = 5) or twice (▪, n = 9) a week. Control IgG was used for negative controls (○, n = 9). Data represent the mean ± standard error of mean (SEM). *P < .05, †P < .005, ‡P < .0001, compared with the control IgG-treated group.
Antitumor effect of anti-HM1.24 MoAb on advanced RPMI 8226 tumors in SCID mice. Mice were injected subcutaneously with RPMI 8226 cells at day 0. The anti-HM1.24 MoAb treatment (100 μg/dose) was started 22 days after tumor inoculation for 50 days either once (•, n = 5) or twice (▪, n = 9) a week. Control IgG was used for negative controls (○, n = 9). Data represent the mean ± standard error of mean (SEM). *P < .05, †P < .005, ‡P < .0001, compared with the control IgG-treated group.
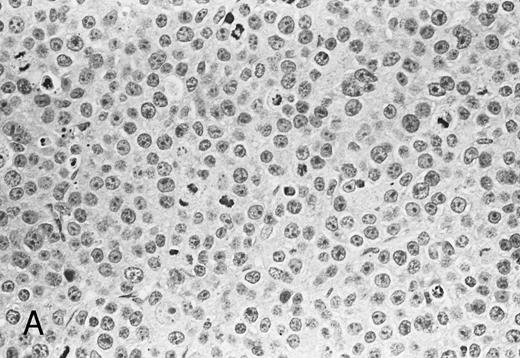
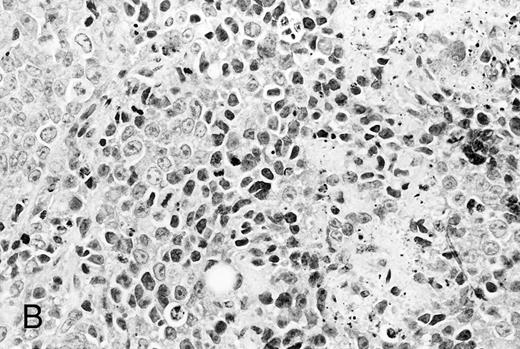
Histological analysis. The in vivo antitumor activity of anti-HM1.24 MoAb was further studied by histologic analysis of tumor-bearing mice that had been treated with either control IgG or anti-HM1.24 MoAb. In control groups of mice inoculated intravenously with ARH-77 cells, tumor cells proliferated mainly in the vertebral bodies and invaded the spinal cord and perivertebral tissues. In contrast, no tumor cells were found in the anti-HM1.24 MoAb-treated mice, surviving for 180 days. In SCID mice with advanced localized tumors, there was no evidence of metastases in other organs such as the liver, spleen, kidneys, lung, heart, stomach, and intestine by immunohistologic analysis using anti-HM1.24 MoAb. In the models with localized RPMI 8226 cells, tumor cells invaded the surrounding tissues including the muscle and showed typical morphologic features of neoplastic plasma cells, ie, irregular nuclear profiles with prominent nucleoli and abundant cytoplasm. Mitotic figures of the tumor cells were also identified. Neither necrotic areas nor infiltrating cells were found in the tumors of control groups (Fig 4A). In contrast, the tumors of anti-HM1.24 MoAb-treated mice showed many shrunk cells with picnotic nuclei. Necrotic areas and infiltrating cells were also observed in the tumors (Fig 4B). No tissue damage was found in the liver, spleen, kidney, lung, or heart in mice treated with anti-HM1.24 MoAb.
Histologic findings of RPMI 8226 tumors at day 25 after tumor inoculation. The subcutaneous tumors in SCID mice were removed 3 days after injection (100 μg/mouse) of (A) control IgG or (B) anti-HM1.24 MoAb. Necrotic areas and infiltrating cells were seen in the tumor from mice treated with anti-HM1.24 MoAb. Stained with hematoxylin and eosin. Original magnification ×130.
Histologic findings of RPMI 8226 tumors at day 25 after tumor inoculation. The subcutaneous tumors in SCID mice were removed 3 days after injection (100 μg/mouse) of (A) control IgG or (B) anti-HM1.24 MoAb. Necrotic areas and infiltrating cells were seen in the tumor from mice treated with anti-HM1.24 MoAb. Stained with hematoxylin and eosin. Original magnification ×130.
In vitro effects of anti-HM1.24 MoAb. Treatment with anti-HM1.24 MoAb alone did not inhibit either the proliferation of ARH-77 and RPMI 8226 cells or the IgG secretion from ARH-77 cells in culture even at a concentration of 100 μg/mL (data not shown).
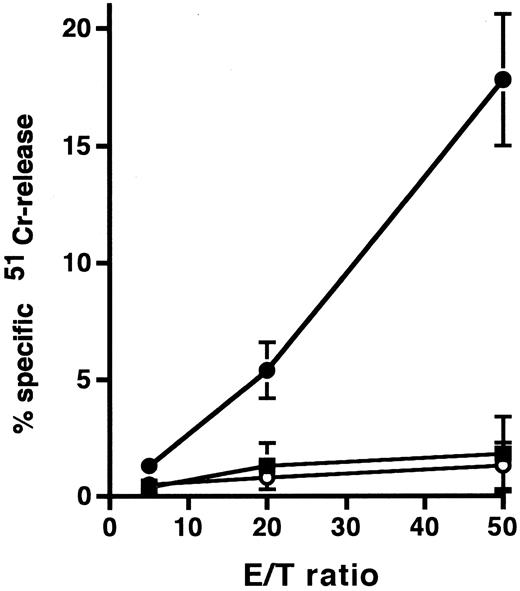
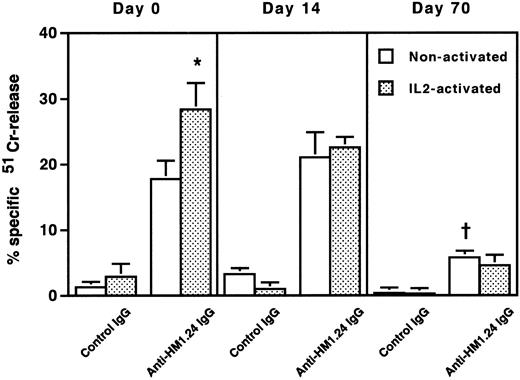
To clarify the mechanism of the cytotoxic effect of anti-HM1.24 MoAb on human myeloma cells, the effect of complements on RPMI 8226 cells was examined under different incubation conditions (Table 2). Whole IgG antibody, but not F(ab′)2 fragment, of anti-HM1.24 mediated complement-dependent cytotoxicity in vitro. Anti-HM1.24 IgG mediated the complement-dependent cytotoxicity (59.0 ± 5.8) against ARH-77 cells (complement dilution, 1:5). Anti-HM1.24 MoAb was tested for ADCC activity against RPMI 8226 cells using spleen cells from SCID mice as effector cells. Although control IgG and F(ab′)2 fragment of anti-HM1.24 had no ADCC activity in the presence of spleen cells, anti-HM1.24 IgG mediated ADCC activity and the extent of cytotoxicity was dependent on the E/T ratio (Fig 5). ADCC activity (43.8 ± 6.5) against ARH-77 cells was also observed (E/T ratio, 50). The complement-dependent cytotoxicity and ADCC were mediated by anti-HM1.24 IgG even at a low concentration of 0.01 μg/mL. The serum from myeloma patients caused no blocking of binding of anti-HM1.24 MoAb in ADCC assay (data not shown). We examined the differences of ADCC activity of spleen cells in SCID mice after tumor inoculation (Fig 6). ADCC activity of spleen cells was augmented by the stimulation with IL-2 (day 0). In the absence of anti-HM1.24 MoAb, natural killing activity of spleen cells was not observed. At day 14 after tumor inoculation, ADCC activity was not decreased, although the effect was not enhanced by IL-2. In mice with advanced RPMI 8226 tumors (day 70), ADCC activity of spleen cells was significantly reduced in the presence or absence of IL-2.
Complement-Dependent Cytotoxicity
| Antibody (1 μg/mL) . | Complement Dilution . | |||
|---|---|---|---|---|
| . | None . | 1:100 . | 1:20 . | 1:5 . |
| None | 1.0 ± 1.6 | 1.2 ± 0.5 | 0.4 ± 2.1 | 0.8 ± 0.9 |
| Control IgG | 0.5 ± 1.4 | 0.9 ± 1.6 | 0.6 ± 0.5 | 0.1 ± 1.0 |
| Anti-HM1.24 | ||||
| F(ab′)2 | 0.2 ± 0.5 | 1.1 ± 1.5 | 0.5 ± 0.9 | 0.5 ± 0.6 |
| Anti-HM1.24 IgG | 1.3 ± 1.1 | 3.8 ± 0.6 | 16.6 ± 1.0 | 68.6 ± 2.8 |
| Antibody (1 μg/mL) . | Complement Dilution . | |||
|---|---|---|---|---|
| . | None . | 1:100 . | 1:20 . | 1:5 . |
| None | 1.0 ± 1.6 | 1.2 ± 0.5 | 0.4 ± 2.1 | 0.8 ± 0.9 |
| Control IgG | 0.5 ± 1.4 | 0.9 ± 1.6 | 0.6 ± 0.5 | 0.1 ± 1.0 |
| Anti-HM1.24 | ||||
| F(ab′)2 | 0.2 ± 0.5 | 1.1 ± 1.5 | 0.5 ± 0.9 | 0.5 ± 0.6 |
| Anti-HM1.24 IgG | 1.3 ± 1.1 | 3.8 ± 0.6 | 16.6 ± 1.0 | 68.6 ± 2.8 |
Data represent the mean ± SD of the percentage of Cr-release.
ADCC activity against RPMI 8226 cells by spleen cells of SCID mice. In this experiment, mice were not treated with rabbit antiasialo GM1 antiserum. 51Cr-labeled RPMI 8226 cells were incubated with spleen cells in the presence of 1 μg/mL control IgG (○), anti-HM1.24 F(ab′)2 (▪), or anti-HM1.24 IgG (•). Data represent the mean ± SD of triplicates. Results are shown for a single experiment, but have been confirmed in two further experiments.
ADCC activity against RPMI 8226 cells by spleen cells of SCID mice. In this experiment, mice were not treated with rabbit antiasialo GM1 antiserum. 51Cr-labeled RPMI 8226 cells were incubated with spleen cells in the presence of 1 μg/mL control IgG (○), anti-HM1.24 F(ab′)2 (▪), or anti-HM1.24 IgG (•). Data represent the mean ± SD of triplicates. Results are shown for a single experiment, but have been confirmed in two further experiments.
ADCC activity of spleen cells in mice after tumor inoculation. 51Cr-labeled RPMI 8226 cells were incubated with nonactivated or IL-2–activated spleen cells at an E/T ratio of 50 in the presence of 1 μg/mL control IgG or anti-HM1.24 IgG. Data represent the mean ± SD of triplicate determinants. *P < .02, †P < .005, compared with the data of nonactivated spleen cells in the presence of anti-HM1.24 IgG. Results are shown for a single experiment, but have been confirmed in two further experiments.
ADCC activity of spleen cells in mice after tumor inoculation. 51Cr-labeled RPMI 8226 cells were incubated with nonactivated or IL-2–activated spleen cells at an E/T ratio of 50 in the presence of 1 μg/mL control IgG or anti-HM1.24 IgG. Data represent the mean ± SD of triplicate determinants. *P < .02, †P < .005, compared with the data of nonactivated spleen cells in the presence of anti-HM1.24 IgG. Results are shown for a single experiment, but have been confirmed in two further experiments.
DISCUSSION
In this report, we provide evidence that human myeloma in SCID mice can be successfully treated with a MoAb directed against a human plasma cell-specific antigen, HM1.24. In vivo antitumor activity of anti-HM1.24 MoAb in SCID mice was observed in both disseminated and localized human myeloma models. Treatment with a single injection of anti-HM1.24 MoAb was effective if the antibody was administered early in the course of disease. Moreover, tumors were cured by multiple injections of the antibody even when therapy was started at an advanced stage of disease.
Although several investigators have developed plasma cell-specific antibodies,21-23 the use of antibodies for immunotherapy of multiple myeloma has not been tested in vivo. In a similar SCID mouse model, Huang et al9 reported that treatment with four consecutive injections of anti-CD54 MoAb could prevent the development of disseminated ARH-77 tumors partially and prolong the survival of mice. However, CD54 is not a tumor-specific antigen and antitumor mechanisms were not explored. Because anti-HM1.24 MoAb is highly specific for human plasma cells, immunotherapy with this antibody has the potential to provide a new therapeutic approach to plasma cell dyscrasias.
As shown in our previous study,17 the HM1.24 is expressed on terminally differentiated normal plasma cells, as well as their malignant counterparts, although it was absent on unstimulated peripheral blood B lymphocytes. The major concern is that Ig secretion from plasma cells might be affected by the antibody treatment. Because anti-HM1.24 MoAb does not react with memory B cells or early stage B cells, anti-HM1.24 MoAb therapy would be expected to have only transient suppressive effect on normal Ig production. In fact, single injection of anti-HM1.24 MoAb inhibited the increase of serum human IgG in ARH-77 myeloma models without killing all tumor cells. It may be possible to allow B cells to recover by using a small amount of the antibody. Therefore, another indication of anti-HM1.24 MoAb could be an immunosuppressive therapy for patients with hypergammaglobulinemia and autoimmune diseases.
The precise mechanisms whereby the anti-HM1.24 MoAb exerts its antitumor activity remain elusive. Previous studies have reported that ADCC mediated by NK cells and macrophages with MoAb is the most important mechanism for antitumor effect in animal models.24 We have shown that anti-HM1.24 IgG can mediate complement-dependent cytotoxicity and ADCC against human myeloma cells in vitro. In contrast, F(ab′)2 fragment of the antibody had no antitumor activity either in vitro or in vivo. These results indicate that Fc-mediated effector functions are responsible for the antitumor activity and support the hypothesis that ADCC and complement-dependent cytotoxicity are important for the antitumor effect of the antibody in vivo. We have ruled out the possibility that antitumor activity is due to antiproliferative effects of the antibody because anti-HM1.24 MoAb had no effect on 3H-thymidine incorporation in ARH-77 and RPMI 8226 cells in vitro. However, the possibility cannot be ruled out that other mechanisms may be involved to exert the powerful in vivo antitumor effects of the antibody.
Vascularization of tumors, permeability of the antibody, infiltration of effector cells, and immune status of the treated animals may play an important role in the outcome of immunotherapy. We found that the therapeutic effect of anti-HM1.24 MoAb was different in two animal models of disseminated and localized tumors. Our data showed that low doses of anti-HM1.24 MoAb could inhibit the growth of disseminated, but not localized, ARH-77 tumors. Similar observations have been reported in animal models of lymphoma in which immunotherapy had limited therapeutic efficacy on solid tumors due to poor biolocalization of antibodies into the tumors.25 Thus, anti-HM1.24 MoAb may have limited value in the therapy of bulky solid tumors. However, in view of the fact that myeloma cells in the bone marrow are readily accessible to the antibody, immunotherapy with anti-HM1.24 MoAb can become a useful measure for the treatment of multiple myeloma.
It is well-known that tumor-bearing hosts exhibit a generalized immunosuppression including reduced antitumor immune responses.26 27 We have investigated the responsiveness of spleen cells at various tumor-bearing stages and found that ADCC activity of mice with advanced tumors was significantly reduced even after stimulation of spleen cells with IL-2. In fact, the efficacy of anti-HM1.24 MoAb appeared to be different in mice with advanced tumors. About 60% to 80% of the mice bearing ARH-77 tumors achieved complete remission, whereas the other mice died after treatment. This difference may be due to the variation of ADCC activity of each mouse. Although low ADCC activity might limit the effect of immunotherapy, there is a possibility that treatment with increased doses of anti-HM1.24 MoAb may be effective as shown in the present study with RPMI 8226 tumor model.
In considering the therapeutic use of anti-HM1.24 in humans, it is important to determine whether the anti-HM1.24 MoAb will mediate ADCC in myeloma patients. The variation of ADCC among individual patients with various types of cancers has been reported, as some patients have substantially suppressed ADCC activity.28 However, we and others have reported increased numbers of NK cells in the peripheral blood and bone marrow of myeloma patients,29,30 and strong NK activity was found in myeloma patients with a low tumor burden.31 These cells could act as effector cells to kill antibody-coated target cells by ADCC mechanism. Moreover, the ability to generate antibodies against therapeutic MoAb may be suppressed due to compromised B-cell function in myeloma patients.32 Secretion of the surface antigen would form a barrier to antibody attack and has been a problem in the use of antibodies. For the HM1.24 antigen, this appears not to occur because the serum from myeloma patients caused no blocking of binding of anti-HM1.24 MoAb in ADCC assay. Taken together, immunotherapy using anti-HM1.24 MoAb can be applicable to myeloma patients especially when significant tumor reduction has been achieved by conventional and/or high-dose chemotherapy with stem cell support. In addition, because administration of cytokines, such as IL-2,33 IL-10,34 IL-12,35 macrophage colony-stimulating factor (M-CSF ),36 has been shown to increase the levels of ADCC by the stimulation of effector cells, combinations of these cytokines along with the antibody may further potentiate the effect of the antibody.
In conclusion, the present study demonstrates that anti-HM1.24 MoAb specifically inhibits the growth and IgG secretion of human myeloma cells in SCID mouse models mainly by ADCC and complement-dependent cytotoxicity mechanisms. This MoAb can be used for immunotherapy of multiple myeloma and related plasma cell dyscrasias. Clinical trials using anti-HM1.24 MoAb are desired to improve the prognosis of these diseases.
ACKNOWLEDGMENT
We thank the technical staff from the animal department for excellent technical assistance.
Supported in part by a Grant for Cancer-induced Bone Diseases from the Ministry of Health and Welfare, Tokyo, Japan.
Address reprint requests to Masaaki Kosaka, MD, PhD, First Department of Internal Medicine, School of Medicine, University of Tokushima, 3-18-15 Kuramoto-cho, Tokushima 770, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal