Abstract
Bax is a proapoptotic member of the Bcl-2 protein family. The incidence and prognostic significance of Bax protein expression in diffuse non-Hodgkin's lymphomas with a large cell component (DLCL) was determined by an immunohistochemical method by using paraffin-embedded tumors from a cohort of patients treated uniformly with combination chemotherapy (n = 139). All patients were between 16 and 70 years of age and had advanced stage disease of diffuse large cell type (diffuse mixed, diffuse large cell, immunoblastic, or anaplastic large cell). Paraffin sections from diagnostic biopsies were successfully immunostained for Bax in 113 cases. Of these, 7 (6%) tumors were scored as Bax immunonegative (<1% Bax-stained tumor cells), 42 (37%) as low (1% to 10%), 9 (8%) as low-intermediate (11% to 30%), 25 (22%) as high-intermediate (31% to 70%), and 30 specimens (27%) as high for Bax expression (<70%). Of the 7 Bax-immunonegative lymphomas, all also scored low (≤10% immunostained tumor cells) for Bcl-2 expression, whereas 78 of the 106 (74%) Bax-immunopositive tumors had low Bcl-2 expression. By itself, Bax expression was not of prognostic significance in univariate analysis, although there was a clear trend for patients with Bax-immunonegative lymphomas (n = 7) to relapse sooner and to die faster than patients whose tumors contained Bax-immunopositive malignant cells (n = 106; 8-year overall survival 29% versus 55%; P = .06). When combined with Bcl-2 immunostaining data, Bax provided additional prognostic information. Among patients with Bcl-2 low-expressing DLCLs, for example, Bax immunonegativity was associated with lower 8-year relapse-free survival (RFS; 29% v 61%; P < .01) and lower 8-year overall survival (OS; 29% v 63%; P < .05), suggesting that absence of Bax protein connotes a more aggressive phenotype when Bcl-2 protein is also not expressed at high levels. In contrast, low Bax expression was associated with improved 8-year disease-free survival (52% v 16%; P < .02), RFS (47% v 11%; P < .02), and OS (64% v 11%; P < .01) in patients whose tumors expressed Bcl-2 at high levels, suggesting that the combination of high levels of Bax and Bcl-2 expression is more deleterious than high levels of Bcl-2 expression alone. Bax expression failed to provide additional prognostic information beyond Bcl-2 expression in multivariate analysis that included the clinical International Prognostic Index factors (age, stage, lactate dehydrogenase, performance status, and number of extranodal sites) and immunophenotype. Taken together, the results suggest that Bax expression is not a major prognostic marker in DLCL. However, the interactions of the Bcl-2 and Bax expression data with respect to clinical outcome may shed new insights into the biological significance of Bcl-2/Bax protein heterodimerization.
MEMBERS OF THE Bcl-2 family of proteins regulate a distal step in an evolutionarily conserved pathway for programmed cell death and apoptosis.1-3 The first identified member of this family, Bcl-2, is a blocker of cell death that was discovered by virtue of its involvement in t(14; 18) chromosomal translocations commonly found in B-cell lymphomas.4 High levels of Bcl-2 expression confer resistance to apoptosis induced by chemotherapeutic drugs and x-irradiation,5,6 presumably accounting for the association of Bcl-2 with poor prognosis in patients with diffuse large cell lymphoma (DLCL) and some other types of cancer.7-11
The antiapoptotic function of the Bcl-2 protein appears to be modulated at least in part by its ability to heterodimerize with another member of this family, Bax.12 In contrast to Bcl-2, overexpression of Bax protein typically promotes apoptosis.13 A relation between Bax and apoptotic responses of cancer cells to chemotherapeutic drugs has been supported by the observations that (1) drug-induced damage to DNA can result in p53-dependent increases in Bax expression and (2) tumor cell lines with gene transfer-mediated elevations in Bax protein are reported to be more sensitive to the cytotoxic actions of anticancer drugs.14-18 Bax protein immunostaining has shown evidence of potential prognostic use in solid tumors, whereas reduced expression of Bax has been reported in ∼35% of adenocarcinomas of the breast in association with poor responses to chemotherapy and shorter overall survival.19 A higher Bcl-2:Bax ratio (based on polymerase chain reaction [PCR] analysis of mRNA) has also been associated with progressive disease in patients with chronic lymphocytic leukemia (CLL).20 However, to date Bax expression, either alone or in combination with Bcl-2, has not been evaluated as a prognostic marker in non-Hodgkin's lymphomas (NHL).
DLCL and related disorders represent the second most common type of NHL, with approximately 12,000 new cases diagnosed annually in the United States alone.21 These neoplasms represent a diverse spectrum of lymphoid malignancies, with variable clinical, histological, immunophenotypical, cytogenetic, and molecular genetic features.21,22 With currently available therapy, long-term cure is possible for approximately half of DLCL patients.23,24 Nevertheless, 40% to 50% of DLCL patients are not cured with combination chemotherapy, suggesting a need to develop prognostic models that accurately recognize high-risk patients at the time of diagnosis so that alternate treatment strategies can be attempted. In this regard, survival for patients with DLCL can often be predicted by using the established International Prognostic Index, which includes age, stage, serum lactate dehydrogenase (LDH), performance status, and number of extranodal sites.25 However, these clinical variables are surrogates for the underlying biological heterogeneity of DLCLs. A search for biological correlates should improve our understanding of the clinical behavior of these neoplasms. Immunophenotype has been shown to predict for survival in DLCLs by using multivariate analysis. Those patients with a T-cell phenotype do worse than their B-cell counterparts. In addition, high levels of Bcl-2 protein expression, as defined by immunohistochemical methods, have been reported to be an independent predictor of shorter disease-free survival (DFS) in at least three separate studies of patients with DLCL, but a significant effect on overall survival has only been documented in one study in which lengthy follow-up data (8 years) were available and uniform therapy was applied.7,8,26 In this report, we characterized the expression of Bax by immunostaining in DLCLs, by using tumor specimens from the same cohort of DLCL patients for whom high levels of Bcl-2 immunostaining have previously been associated with shorter overall survival (OS) in multivariate analysis.26
MATERIALS AND METHODS
Patients.This study included 145 consecutive patients with DLCLs (diffuse mixed [DM], diffuse large cell [DL], immunoblastic, and anaplastic large cell lymphoma [ALCL]) diagnosed between April 1981 and June 1989 at the British Columbia Cancer Agency (Vancouver, Canada). Of these 145 patients, 6 were excluded because no blocks were available for analysis. Eligibility criteria included age from 16 to 70 years, diffuse lymphoma with a large cell component (Working Formulation categories F, G, or H), advanced disease with stage III or IV or having stage II with either B symptoms or a mass greater than 10 cm, no prior chemotherapy or radiotherapy, and absence of congestive heart failure.23,26 Lymphomas related to acquired immunodeficiency syndrome or organ transplantation were excluded. Patients with antecedent low grade lymphoma or discordant lymphoma at diagnosis were also excluded. Eligible patients were treated with MACOP-B (n = 121) or VACOP-B (n = 18). The therapy consisted of a 12-week outpatient regimen of doxorubicin and cyclophosphamide alternating with vincristine plus either bleomycin or moderate-dose methotrexate with leucovorin rescue.23 Prednisone was given daily along with prophylactic cotrimoxazole, ketoconazole, and an H2-receptor blocker, usually cimetidine. Patients treated with VACOP-B had etoposide substituted for methotrexate, but were otherwise treated the same. Dose reductions were based on the absolute granulocyte count on the day of treatment. Most (90%) of the patients received more than 80% of their planned dose. Details of the patients' characteristics, treatment delivery, and outcome have been previously published.23
Histology and immunohistochemistry.Pretreatment tissue biopsy specimens were fixed in buffered formalin or B5 fixative, embedded in paraffin, sectioned (3 μm), and stained with hematoxylin and eosin. T, B, or null cell lineage was assigned by using paraffin section immunostaining and a panel of monoclonal antibodies, CD20 (L26), MB-2, CD45RO (A6, UCHL-1), CD45 (LCA), CD30 (Ber-H2), EMA, and a polyclonal anti-CD3 antiserum (Dako, Carpinteria, CA) as described.26 Bcl-2 immunostaining data have been reported.26 Bax immunostaining was performed on diagnostic biopsy specimens by using polyclonal antihuman Bax antiserum after microwave antigen retrieval as previously described.19 Sections were scored as negative if no large neoplastic cells stained; +1 (1% to 10% positive cells); +2 (11% to 30%); +3 (31% to 70%); and +4 (>70% positive large tumor cells). Of the 139 total cases, 26 were excluded for lack of any Bax-immunopositive normal lymphocytes or other normal cells that serve as positive internal controls (n = 113).
Statistical analysis.OS was calculated from the date of diagnosis until the patient's death from any cause or last follow-up. DFS was calculated as the interval between diagnosis and relapse or failure to enter complete remission (CR) or death due to toxicity. Relapse-free survival (RFS) was calculated only for patients achieving CR as the interval between diagnosis and relapse of the disease or death due to toxicity. Survival curves were calculated by the method of Kaplan and Meier. Statistical comparison between curves was made by the log-rank test. Determination of the distribution of clinical prognostic factors between groups was by χ2 analysis. Multivariate analysis was performed with the use of a stepwise proportional hazards model.
RESULTS
A total of 139 DLCL patients were identified for whom adequate biopsy material was available for analysis. Of these, 121 (87%) achieved a CR. At a median follow-up of 104 months (range 1 to 183 months), the 8-year overall DFS and RFS were 55%, 58%, and 51%, respectively. Histological subclassification showed 23 (16%) DM, 87 (63%) DL, 19 (14%) immunoblastic, and 10 (7%) ALCLs (confirmed by CD30 immunostaining). Paraffin section immunophenotyping was successful in assigning lineage in 125 of the 139 (90%) cases, showing 115 (83%) B-cell, 10 (7%) T-cell, and 14 (10%) null cell immunophenotype. By using a cut-off of >10% Bcl-2 immunopositive cells, as used for previous studies,7,8 88 (76%) of the 139 cases were scored as negative, whereas 28 (24%) were positive and 23 indeterminate for expression of Bcl-2 protein.26
Immunostaining for Bax was successful in 113 of the 139 paraffin specimens. The clinical characteristics of these patients are listed in Table 1. Among these 113 tumors, 106 (94%) contained at least 1% Bax-immunopositive malignant cells, whereas 7 were Bax immunonegative (6%; Fig 1). Of the Bax-immunopositive cases, 42 (40%) contained only a small percentage of Bax-positive tumor cells (1% to 10%), 9 (8%) had low-intermediate percentages of Bax-stained cells (11% to 30%), 25 (22%) had high-intermediate percentages of Bax-stained cells (31% to 70%), and 30 (28%) highly expressed Bax (>70% Bax-immunopositive tumor cells). All 7 (100%) of the Bax-immunonegative tumors were also negative for Bcl-2 immunostaining, compared with 78 of the 106 (74%) Bax-immunopositive tumors ( P = .26). Among patients with no or only low levels of Bax (≤10%), 9 of 49 (18%) were Bcl-2 positive. The relationship between Bax and Bcl-2 protein expression for all percent cut-offs used for scoring is shown in Table 2.
Clinical Characteristics of the 113 Patients With DLCL
| Characteristic . | Number of Patients (%) . |
|---|---|
| Age | |
| ≤60 yrs | |
| >60 yrs | 81 (72) |
| 32 (28) | |
| Sex | |
| Male | |
| Female | 72 (64) |
| 41 (36) | |
| Site | |
| Nodal | |
| Extranodal | 76 (67) |
| 37 (33) | |
| Stage | |
| IIB | |
| III | |
| IV | 44 (39) |
| 27 (24) | |
| 42 (37) | |
| B symptoms | 55 (49) |
| Serum LDH | |
| Normal | |
| Elevated | 27 (24) |
| 86 (76) | |
| Performance status (ECOG) | |
| 0, 1 | |
| 2-4 | 91 (81) |
| 22 (19) | |
| Extranodal sites | |
| 0, 1 | |
| >1 | 81 (72) |
| 32 (28) | |
| BM involvement | 10 (9) |
| Complete remission | 96 (85) |
| Characteristic . | Number of Patients (%) . |
|---|---|
| Age | |
| ≤60 yrs | |
| >60 yrs | 81 (72) |
| 32 (28) | |
| Sex | |
| Male | |
| Female | 72 (64) |
| 41 (36) | |
| Site | |
| Nodal | |
| Extranodal | 76 (67) |
| 37 (33) | |
| Stage | |
| IIB | |
| III | |
| IV | 44 (39) |
| 27 (24) | |
| 42 (37) | |
| B symptoms | 55 (49) |
| Serum LDH | |
| Normal | |
| Elevated | 27 (24) |
| 86 (76) | |
| Performance status (ECOG) | |
| 0, 1 | |
| 2-4 | 91 (81) |
| 22 (19) | |
| Extranodal sites | |
| 0, 1 | |
| >1 | 81 (72) |
| 32 (28) | |
| BM involvement | 10 (9) |
| Complete remission | 96 (85) |
Abbreviations: DLCL, diffuse large cell lymphoma; LDH, lactate dehydrogenase; ECOG, Eastern Cooperative Oncology Group; BM, bone marrow.
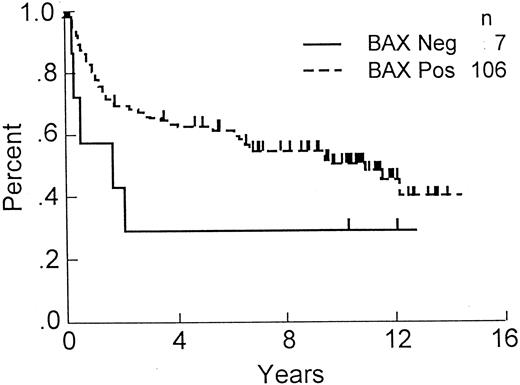
OS of DLCL patients with Bax-immunonegative and -immunopositive tumors. OS, comparing patients whose tumors contained less than 1% Bax-immunostained malignant cells (n = 7) with those that were Bax-immunopositive (n = 106; P = .06).
OS of DLCL patients with Bax-immunonegative and -immunopositive tumors. OS, comparing patients whose tumors contained less than 1% Bax-immunostained malignant cells (n = 7) with those that were Bax-immunopositive (n = 106; P = .06).
Relationship of Bax and Bcl-2 Protein Immunostaining for the 113 Tumors Successfully Immunostained for Bax
| Bax Immuno Score . | Number* . | Bcl-2 Positive† . |
|---|---|---|
| Negative | 7 | 0 |
| 1%-10% | 42 | 9 |
| 11%-30% | 9 | 2 |
| 31%-70% | 25 | 5 |
| >70% | 30 | 12 |
| Total | 113 | 28 |
| Bax Immuno Score . | Number* . | Bcl-2 Positive† . |
|---|---|---|
| Negative | 7 | 0 |
| 1%-10% | 42 | 9 |
| 11%-30% | 9 | 2 |
| 31%-70% | 25 | 5 |
| >70% | 30 | 12 |
| Total | 113 | 28 |
Number of specimens with the indicated Bax scores of <1% (negative), 1%-10%, 11%-30%, 31%-70%, and >70%.
The number of Bcl-2 “immunopositive” cases as defined by >10% immunostained tumor cells is presented for each category of Bax scoring.
Correlations of Bax-immunostaining results with clinical outcome showed a trend toward shorter OS among the small subgroup (n = 7) of patients with Bax-immunonegative tumors (8-year OS 29% v 55%; Fig 1). However, the difference in outcome for the Bax-positive versus -negative subgroups failed to reach statistical significance ( P = .06) presumably, at least in part, because of the small size of the Bax-negative subgroup. Comparisons of clinical outcome among the subgroups of patients whose tumors contained low (1% to 10%), low-intermediate (11% to 30%), high-intermediate (31% to 70%), or high (>70%) percentages of Bax-immunopositive malignant lymphoma cells failed to show clear trends or statistically significant differences (not shown). Moreover, combining the Bax immunonegative (<1%) and low-Bax (1% to 10%) tumors (n = 49) also failed to dichotomize the patients into groups with significantly different outcomes, unlike Bcl-2 data in which ≤10% versus >10% separates prognostically distinct patient subgroups.26
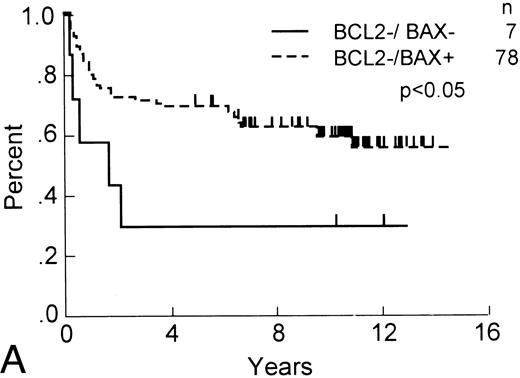
Although Bax expression by itself was not of apparent prognostic significance, we explored the possibility that it might contribute additional information when combined with Bcl-2 immunostaining data. Among patients with Bcl-2-negative tumors (ie, ≤10%) that stained positive for Bax protein expression (n = 85), the subpopulation of patients with Bax-positive tumors (ie, >1%) enjoyed longer OS ( P < .05) and RFS ( P < .01) compared with those whose tumors were both Bcl-2 negative and Bax negative (Fig 2). Dichotomizing the Bax data by using other percent cut-offs failed to show additional prognostically significant subgroups among those patients whose tumors were Bcl-2 immunonegative.
OS and RFS of Bcl-2–immunonegative DLCL patients with Bax-immunonegative and -immunopositive tumors. (A) OS and (B) RFS, comparing Bcl-2–immunonegative patients (n = 85) whose tumors contained (less than 1%) Bax-immunostained malignant cells (n = 7) with those that were Bax-immunopositive (n = 78; P < .05 and <.01, respectively).
OS and RFS of Bcl-2–immunonegative DLCL patients with Bax-immunonegative and -immunopositive tumors. (A) OS and (B) RFS, comparing Bcl-2–immunonegative patients (n = 85) whose tumors contained (less than 1%) Bax-immunostained malignant cells (n = 7) with those that were Bax-immunopositive (n = 78; P < .05 and <.01, respectively).
For the patients with Bcl-2-positive tumors (n = 28), in contrast, dichotomization based on Bax immunopositivity versus Bax immunonegativity was impossible, because no Bax-immunonegative (<1% stained cells) tumors were found within this subgroup. However, segregating these Bcl-2-immunopositive tumors for low (1% to 10%) versus intermediate/high percentages (>10%) of Bax-immunopositive lymphoma cells showed improved 8-year OS (68% v 11%; P < .01; Fig 3). The analysis of DFS (52% v 16%; P < .02) and RFS (47% v 11%; P < .02) showed similar results (not shown). No statistically significant differences were found among the Bax-negative and Bax-low subgroups compared with Bax intermediate/high patients with regards to age, sex, number of extranodal sites of disease, stage, B symptoms, performance status, LDH, frequency of bone marrow involvement, CR rate, or T versus B lineage (not shown). Dichotomization based on other percent cut-offs failed to show statistically significant differences based on the relative percentages of Bax-stained tumor cells.
OS of Bcl-2–immunopositive DLCL patients with Bax-low and Bax-intermediate/high tumors. OS, comparing Bcl-2–immunopositive patients (n = 28) whose tumors contained low percentages (≤10%) of Bax-immunostained malignant cells (n = 9) with those that contained higher percentages (<10%) of Bax-immunopositive patients (n = 19; P < .01).
OS of Bcl-2–immunopositive DLCL patients with Bax-low and Bax-intermediate/high tumors. OS, comparing Bcl-2–immunopositive patients (n = 28) whose tumors contained low percentages (≤10%) of Bax-immunostained malignant cells (n = 9) with those that contained higher percentages (<10%) of Bax-immunopositive patients (n = 19; P < .01).
In multivariate models that included the International Prognostic Index factors, T or B immunophenotype, and Bcl-2 protein expression, Bcl-2 immunopositivity (>10%) was independently associated with shorter DFS ( P < .001), RFS ( P < .001), and OS ( P < .01), as reported previously.26 However, addition of Bax to the model failed to provide additional prognostic information.
DISCUSSION
In this study we sought to address three questions: (1) What is the frequency of Bax expression in DLCLs? (2) Is Bax immunostaining of prognostic significance for patients with DLCL? and (3) Do Bax data enhance the predictive power of Bcl-2 immunostaining data? To accomplish this, we studied a uniform cohort of DLCL patients from a single institution with lengthy follow-up. The findings show that Bax expression varies widely among DLCLs and fails to correlate with several other clinical or laboratory variables examined here. Despite this wide variability, Bax immunostaining was not of clear prognostic use, either in univariate or multivariate analysis of clinical outcome, at least when several arbitrary cut-off percentages of Bax-immunostained tumor cells were employed. Thus, unlike Bcl-2, immunohistochemical analysis of Bax expression is evidently not predictive of DFS, RFS, or OS.
Nevertheless, some interesting relationships between Bcl-2 and Bax immunostaining data were shown during attempts to establish correlations with outcome. For example, patients whose tumors were both Bax and Bcl-2 negative experienced much lower 8-year RFS (29% v 61%; P < .01) and OS (29% v 63%; P < .05) when compared with patients whose tumors were Bax immunopositive but Bcl-2 negative. Because both Bcl-2 and Bax are commonly expressed in normal B cells,27 one potential interpretation of these results is that the loss of both Bcl-2 and Bax expression is indicative of a highly deregulated state of apoptosis control and, thus, reflects a more aggressive stage of tumor progression. These findings are also generally consistent with the notion that Bax can function both as a tumor suppressor gene by promoting cell turnover28 and as a radio and chemosensitizer.16-18 However, one caveat about conclusions drawn from the Bax-immunonegative DLCLs is that this represents a small subgroup of cases.
In contrast, lower percentages of Bax-immunostained tumor cells (≤10%) were associated with improved DFS (52% v 16%; P < .02), RFS (47% v 11%; P < .02), and OS (68% v 11%; P < .01) among the subgroup of patients with Bcl-2-positive tumors. This finding seems counter-intuitive based on the notion that Bax promotes apoptosis. However, previous studies have suggested that the functional as well as physical interactions of Bcl-2 and Bax may be complex and modulated by cellular context.29-33 One potential interpretation of these findings is that Bcl-2 and Bax may collaborate in the suppression of cell death, presumably by forming heterodimers. In this regard, recent structural and electrophysiological data suggest that both Bcl-2 and Bax are pore forming molecules that can create ion channels in lipid bilayers.34-37 Although highly speculative, it is possible that Bcl-2 and Bax transport ions or proteins in opposite directions across membranes, thus explaining their opposing effects on cell life and death. Moreover, Bcl-2/Bax heterodimers theoretically may create unique types of pores that may differ from those formed by Bcl-2/Bcl-2 or Bax/Bax homodimers or homooligomers and that have distinct biological functions. Alternatively, the answer to the paradoxical association of lower levels of Bax expression with better outcome among Bcl-2-immunopositive DLCLs could reside in the observation that Bcl-2 over-expression can slow cell cycling in G1 phase of the cell cycle in at least some types of cells.38-41 Because Bax has been reported to overcome this G1 checkpoint function of Bcl-2,39 conceivably those tumors that coexpress Bcl-2 and Bax may on balance manifest a growth advantage. This would be particularly true if the relative ratios of Bcl-2 and Bax were such that sufficient Bax was produced to offset the cell cycle arrest effects of Bcl-2 without completely interfering with Bcl-2's cell death suppressing action.
In summary, this study shows that Bax gene expression as determined by routine histochemical methods is probably not a major independent prognostic variable for patients with DLCL. However, statistically significant interactions observed between Bax and Bcl-2 immunostaining data with regards to clinical outcome are consistent with the known ability of these two apoptosis-regulating proteins to heterodimerize and may provide novel insights into the functional significance of Bcl-2 and Bax coexpression in DLCLs and possibly other types of normal and malignant cells.
ACKNOWLEDGMENT
The authors thank Drs P. Hoskins, P. Klimo, and S.E. O'Reilly for their assistance with providing clinical data for this study; Drs C. Coppin and A. Coldman for help with the statistical analysis; C. Wong for data collection; and H. Gallant for manuscript preparation.
Supported by a grant from the National Cancer Institute (CA-60421) and the British Columbia Health Research Foundation.
Address reprint requests to John C. Reed, MD, PhD, The Burnham Institute, 10901 N Torrey Pines Rd, La Jolla, CA 92037.

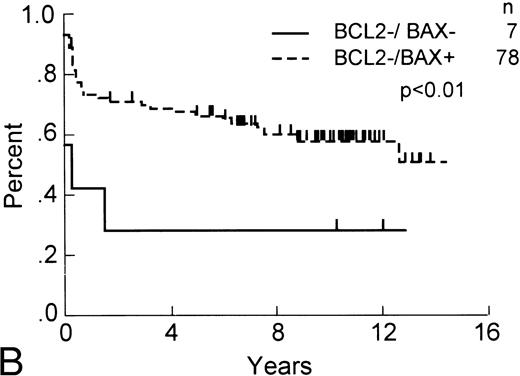
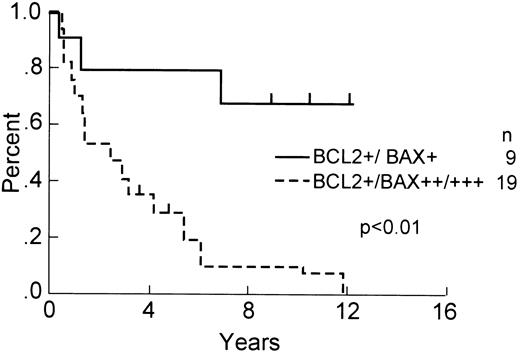
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal