Abstract
Recombinant human interferon-γ (rhIFN-γ) decreases the frequency of serious infections in patients with chronic granulomatous disease (CGD) through an unknown mechanism. To test the hypothesis that it exerts a beneficial effect by enhancing clearance of microbes from the bloodstream and tissues, normal human subjects were treated in vivo with rhIFN-γ. Phagocyte opsonic receptor expression, serum opsonin levels, and phagocytosis of bacteria were then measured. A 4.7-fold increase in neutrophil expression of the high-affinity Fcγ-receptor (FcγRI) was observed that peaked 48 hours after the initiation of rhIFN-γ treatment (P < .05). Monocyte expression of FcγRI, FcγRII, FcγRIII, CD11a, CD11b, CD18, and HLA-DR also significantly increased with peak expression at 48 hours. Phagocytosis by neutrophils of killed Staphylococcus aureus opsonized with heat-inactivated pooled human serum significantly improved after rhIFN-γ treatment (P < .05) and correlated with FcγRI expression by neutrophils (r = .8, P < .001). This increase in ingestion could be inhibited by anti-FcγRI monoclonal antibodies. Levels of the serum opsonin lipopolysaccharide-binding protein also significantly increased after in vivo rhIFN-γ (P < .05). These results suggest that the protective effect of rhIFN-γ in patients with CGD may involve improved microbial clearance. Moreover, improved phagocyte trafficking may occur secondary to increased expression of monocyte β2 -integrins. Because these IFN-γ–related improvements in host defense were seen in normal hosts, rhIFN-γ may have broader applications in the treatment of various disorders of immunity in addition to its demonstrated efficacy in CGD.
RECOMBINANT HUMAN interferon-γ (rhIFN-γ), a lymphokine that exerts pleiotropic effects on the immune system, has been shown to reduce the incidence of severe infections in patients with chronic granulomatous disease (CGD), although the mechanism by which it exerts a beneficial clinical effect in these patients is not known.1-3 The multicenter phase III study of rhIFN-γ therapy in patients with CGD failed to show an improvement in neutrophil oxidative metabolism as determined by nitroblue tetrazolium reduction, neutrophil superoxide formation, and in vitro Staphylococcus aureus killing.1 Subsequent studies further showed no change in the levels of cytochrome b558 and no change in the activities of cytosol and membrane NADPH oxidase components using a cell free activation system.2,3 These findings were congruous with the fact that all the major genetic forms of CGD were equally responsive to rhIFN-γ. In addition, specific mutations found to be responsible for cases of CGD in recent years (including deletions, missense mutations, and stop codons that lead to structural defects in NADPH oxidase) predictably would not be amenable to correction with rhIFN-γ therapy.4-7 One hypothesis to explain its beneficial effect in patients with CGD is that it improves clearance of microbes from the bloodstream and tissues.
This postulated augmentation of microbial clearance by rhIFN-γ could be mediated, at least in part, by its ability to induce increased expression of FcγRI (or CD64, the high-affinity Fc receptor for IgG) in phagocytes. This effect has been demonstrated both in vitro using cultured neutrophils, monocytes, and myeloid cells8-13 and in vivo in patients with CGD, cancer, and arthritis.14-17 However, the study of the immunomodulatory effects of IFN-γ in these patients is complicated by polypharmacy and intercurrent illnesses. To eliminate these confounding variables, the effects of in vivo rhIFN-γ in normal human subjects were examined in this study. The expression of opsonic receptors on phagocytes was measured, as were levels of serum opsonins, and correlated with the extent of ingestion of bacteria by neutrophils after the treatment of normal human subjects with rhIFN-γ. It was found that in vivo treatment of normal human subjects with rhIFN-γ led to increased expression of FcγRI by their phagocytes and a related improvement in FcγR-mediated phagocytosis by neutrophils. In addition, rhIFN-γ treatment was associated with increased expression on monocytes of the opsonic receptors FcγRII, FcγRIII, and CR3 (CD11b/CD18) as well as increased serum levels of the opsonin lipopolysaccharide-binding protein (LBP).
MATERIALS AND METHODS
Study design. Volunteers were recruited from the normal donor pool of the General Clinical Research Center of Scripps Clinic and Research Foundation (La Jolla, CA). Eight women and eight men, aged 26 to 42 years, participated in the study. Medical history, physical examination, and routine laboratory investigations were normal in all subjects. The subjects did not use medication, were nonsmokers, and had no febrile illnesses 1 month before the study. Informed consent was obtained according to the rules of the Scripps Clinic and Research Foundation Human Subjects Committee. rhIFN-γ (Genentech, South San Francisco, CA) was administered at a dose of 0.05 mg/m2/dose subcutaneously (SC) daily for 2 days (the dose used in the phase III study of IFN-γ in patients with CGD was 0.05 mg/m2 SC administered three times weekly).1 Prophylactic acetaminophen (650 to 1,000 mg orally) was administered before rhIFN-γ and then every 4 hours as needed to minimize the incidence of fever and myalgia secondary to rhIFN-γ. Subjects were under medical observation during the first 48 hours of the trial. No significant toxicity was observed. Mild adverse reactions were limited to headache (4 subjects), myalgias (2 subjects), and nausea (1 subject).
Monoclonal antibodies (MoAbs) and antibody fragments. The following MoAbs and antibody fragments were used in direct immunofluorescent staining and phagocytosis inhibition studies. F(ab′)2 to FcγRI (32.2) and MoAbs to FcγRI (22, IgG1; 197, IgG2a), FcγRII (IV.3, IgG2b), and FcγRIII (3G8, IgG1) were purchased from Medarex (Annandale, NJ). MoAb to CD14 (MEM-15, IgG1), CD11a (MEM-25, IgG1), CD11b (CR3, IgG1), CD18 (MEM-48, IgG1), and HLA-DR (HL38, IgG1) and isotypic control MoAbs were obtained from Caltag (South San Francisco, CA). Control murine F(ab′)2 fragments were purchased from Cappel (Durham, NC). All of the antibodies listed above were obtained in their fluorescein isothiocyanate (FITC)-conjugated form except the antibodies used in the phagocytosis inhibition studies (197, 32.2, and their respective isotype controls).
Quantitative measurement of phagocyte opsonic receptor expression. A previously described method was used for measuring the binding of FITC-labeled MoAbs and isotype control murine MoAbs to leukocytes prepared from erythrocyte-lysed whole blood.14,18,19 Briefly, 100 μL of EDTA-anticoagulated whole blood diluted with phosphate-buffered saline (PBS) to a concentration of 2.5 × 106 leukocytes/mL were incubated with 5 μL of saturating amounts of MoAb. Erythrocytes were lysed, and then leukocytes were stabilized and fixed using the Coulter Q-Prep lysis procedure (Coulter Electronics, Hialeah, FL). This method allowed rapid analysis of small aliquots of whole blood with minimal sample manipulation.18 19 All samples were washed with PBS and then resuspended in 300 μL of PBS. Flow cytometric analysis was performed on 10,000 leukocytes per sample using a FACScan with Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, CA). Neutrophils and monocytes were identified by forward and right angle light scattering properties. In pilot experiments using double staining with anti-CD14/CD45 MoAbs, this gating technique identified neutrophil and monocyte populations that were greater than 97% and greater than 90% pure, respectively. Expression of surface markers was quantitated using Quantum FITC-standardized beads and accompanying software (Flow Cytometry Standards Corp, San Juan, Puerto Rico). Results were reported as FITC mean equivalent soluble fluorescence (MESF ) units. Quantitation of surface marker expression in FITC MESF units included the correction of MESF values for nonspecific binding, as detected by isotypic control antibody.
Leukocyte purification for phagocytosis assay. Leukocytes were purified by a whole-blood lysis method.20 Whole blood (10 mL) was collected into Vacutainer tubes containing sodium heparin (Becton Dickinson, Rutherford, NJ). To each milliliter of blood was added 14 mL of room temperature NH4Cl lysing solution (168 mmol/L NH4Cl, 10 mmol/L KHCO3 , and 0.1 mmol/L tetrasodium EDTA, pH 7.4). The mixture was allowed to stand for 3 to 5 minutes at room temperature, after which the samples were centrifuged at 300g for 5 minutes. Residual erythrocytes were lysed for 3 more minutes. The remaining leukocytes were washed twice in PBS at 4°C and resuspended in assay buffer (PBS containing 0.1% gelatin, 5 mmol/L glucose, and 0.5 mmol/L MgCl2 ) at a concentration of 1 × 107 leukocytes/mL. Viability of cells as determined by trypan blue exclusion was greater than 92%.
Phagocytosis of S aureus by neutrophils.S aureus-BODIPY BioParticles (Molecular Probes, Eugene, OR) were opsonized with pooled human serum (PHS) or PHS that had been heated to 56°C for 30 minutes to inactivate complement (HI-PHS). The following procedure was followed to estimate FcγR-mediated phagocytosis by neutrophils. At time 0, HI-PHS–opsonized BioParticles (2 × 107 in 200 μL of assay buffer) were added to leukocytes (1 × 106 in 100 μL of assay buffer). Samples were brought to a final volume of 400 μL and incubated in the dark at 37°C. At 0 and 120 minutes, 50-μL aliquots were removed and added to 400 μL of trypan blue 0.1% in PBS to quench external fluorescence.21-24 Linear mean fluorescence intensity (MFI) of the gated neutrophil population was determined by flow cytometry. Phagocytosis was calculated as MFI (subject)/MFI (control) × 100. The normal control was the same person for the entire course of the regimen for each given subject. A similar procedure was followed to determine complement-mediated phagocytosis by neutrophils, except that half the number of BioParticles was used and the incubation time was 30 minutes instead of 120 minutes. For the inhibition studies, leukocytes were preincubated with 20 μg/mL of the following MoAb or F(ab′)2 fragments for 1 hours at 4°C: murine IgG2a, anti-FcγRI MoAb (197), murine nonspecific F(ab′)2 , and anti-FcγRI F(ab′)2 (32.2). At time 0, BioParticles (2 × 107 in 200 μL of assay buffer) were added to leukocytes (1 × 106) with or without antibody or antibody fragments in assay buffer. The assay was then performed as described above.
Measurement of serum opsonins and acute-phase reactants. Complement, Ig, and C-reactive protein (CRP) levels were measured in the Scripps Clinic clinical laboratory. Soluble CD14 (sCD14) and LBP levels were determined by enzyme-linked immunosorbent assay (ELISA) as previously described using MoAb pairs raised against recombinant human LBP and sCD14.25 Serum levels of neopterin and β2 -microglobulin (β2 -m) were assessed using commercially available radioimmunoassay kits from ICN Biomedicals (Costa Mesa, CA) and T Cell Diagnostics (Cambridge, MA), respectively. The neopterin standard curve was linear from 1.56 to 100 ng/mL; the β2 -m standard curve was linear from 20 to 400 ng/mL. Soluble intercellular adhesion molecule (sICAM) was assessed using a commercially available ELISA kit (Endogen, Boston, MA).
Measurement of peripheral blood leukocyte counts and differentials. Peripheral blood leukocyte counts were determined in the Scripps Clinic hematology laboratory by an automated STKS Analyzer (Coulter, Miami, FL). A manual differential was performed in addition to the automated complete blood count.
Statistical analysis. Groups before and 24 hours, 48 hours, 72 hours, and 7 days after rhIFN-γ administration were compared by repeated measures ANOVA and P < .05 was considered significant. Group data are expressed as the mean ± standard error (SE), unless otherwise stated.
RESULTS
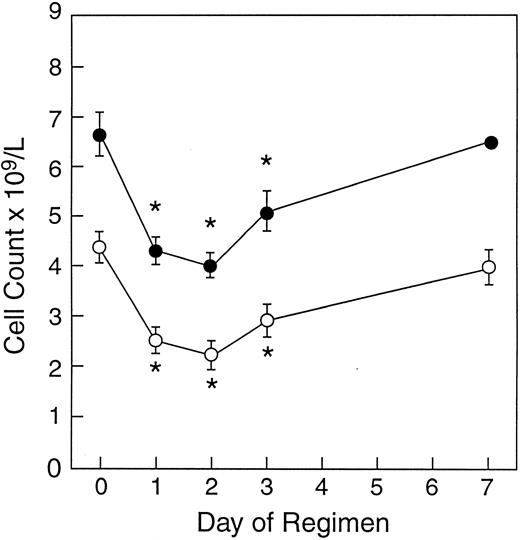
Leukocyte and absolute neutrophil counts after in vivo rhIFN-γ treatment. Peripheral leukocyte counts and neutrophil counts decreased significantly after in vivo rhIFN-γ. Leukocyte and neutrophil counts reached their nadir 48 hours after the administration of the first dose of rhIFN-γ, with troughs of 4.02 ± 0.96 × 109/L and 2.24 ± 1.06 × 109/L, respectively. Both returned to near pretreatment values by day 7 (Fig 1).
Effect of rhIFN-γ on peripheral blood leukocyte counts. Mean ± SE leukocyte (•) and neutrophil (○) counts after in vivo rhIFN-γ (0.05 mg/m2 SC daily for 2 doses) administered to 16 healthy volunteers are shown. Asterisks indicate statistical significance (P < .05).
Effect of rhIFN-γ on peripheral blood leukocyte counts. Mean ± SE leukocyte (•) and neutrophil (○) counts after in vivo rhIFN-γ (0.05 mg/m2 SC daily for 2 doses) administered to 16 healthy volunteers are shown. Asterisks indicate statistical significance (P < .05).
Expression of opsonic receptors by phagocytes after in vivo rhIFN-γ administration. In vivo rhIFN-γ treatment was associated with a significant increase in FcγRI expression on neutrophils (Fig 2), although expression of FcγRII, FcγRIII, CR3 (CD11b/CD18), and CD14 did not vary significantly from baseline at any time point (data not shown). FcγRI expression on neutrophils significantly increased from baseline by 24 hours after initiating in vivo rhIFN-γ treatment (P < .05). Peak expression of FcγRI by neutrophils was seen at 48 hours, with a mean 4.7-fold increase in expression. Before rhIFN-γ treatment, 36% of neutrophils from study subjects were positive for FcγRI expression when compared with an isotypic control (SD = 24; n = 12), whereas 95% were positive at 48 hours (SD = 4; n = 12). A substantial decrease in FcγRI expression was seen within 48 hours of the last rhIFN-γ dose. By day 7 of the treatment regimen, FcγRI expression by neutrophils returned to pretreatment levels.
Effect of in vivo rhIFN-γ administration on FcγRI expression on circulating neutrophils. FcγRI expression was measured by flow cytometry as described in the Materials and Methods. Expression is depicted as FITC MESF ± SE for 12 healthy volunteers (•) and 6 untreated controls (○). Asterisks indicate statistical significance (P < .05)
Effect of in vivo rhIFN-γ administration on FcγRI expression on circulating neutrophils. FcγRI expression was measured by flow cytometry as described in the Materials and Methods. Expression is depicted as FITC MESF ± SE for 12 healthy volunteers (•) and 6 untreated controls (○). Asterisks indicate statistical significance (P < .05)
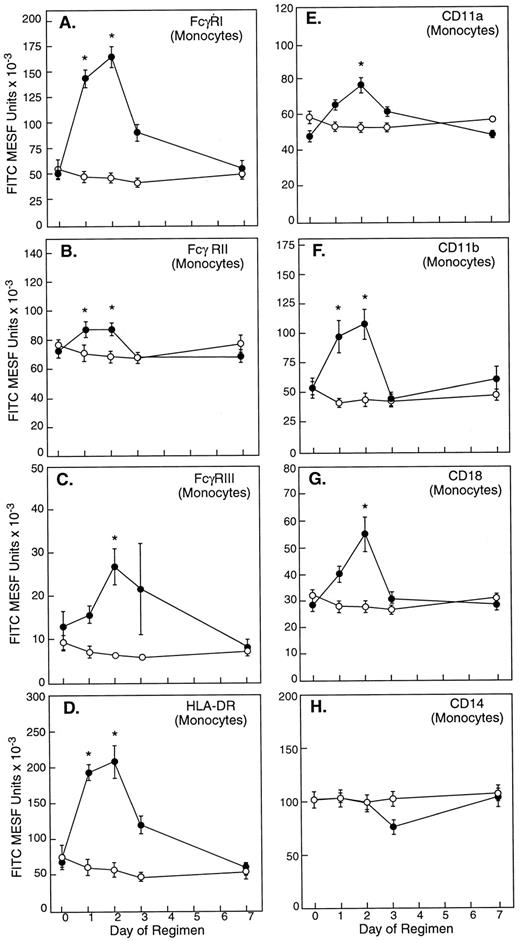
Consistent with its known role as an activator of monocytes, in vivo rhIFN-γ treatment was associated with a significant increase in the expression on monocytes of Fc γ receptors FcγRI, FcγRII, and FcγRIII; leukocyte integrin subunits CD11a, CD11b, and CD18; and HLA class II antigen HLA-DR (Fig 3). At 48 hours, monocytes exhibited maximal 3.3-, 1.2-, 2.1-, 3.1-, 1.6-, 2.0-, and 1.9-fold increases from baseline expression of FcγRI, FcγRII, FcγRIII, HLA-DR, CD11a, CD11b, and CD18, respectively (all P values < 0.05); monocyte expression of CD14 did not vary significantly from baseline after rhIFN-γ administration (Fig 3).
The effect of in vivo rhIFN-γ administration on monocyte surface molecule expression. In vivo rhIFN-γ was associated with a significant increase in expression of FcγRI (A), FcγRII (B), FcγRIII (C), HLA-DR (D), CD11a (F ), CD11b (G), and CD18 (H). Monocyte expression of CD14 (E) failed to change significantly. Results are reported as FITC MESF ± SE from patients (•; n = 12) who received rhIFN-γ per protocol and untreated normal controls (○; n = 6). Asterisks indicate statistical significance (P < .05).
The effect of in vivo rhIFN-γ administration on monocyte surface molecule expression. In vivo rhIFN-γ was associated with a significant increase in expression of FcγRI (A), FcγRII (B), FcγRIII (C), HLA-DR (D), CD11a (F ), CD11b (G), and CD18 (H). Monocyte expression of CD14 (E) failed to change significantly. Results are reported as FITC MESF ± SE from patients (•; n = 12) who received rhIFN-γ per protocol and untreated normal controls (○; n = 6). Asterisks indicate statistical significance (P < .05).
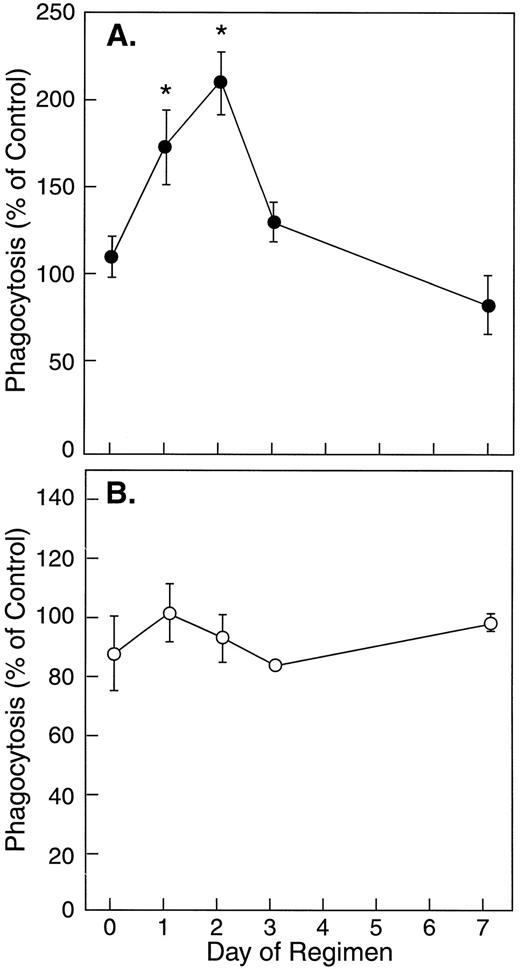
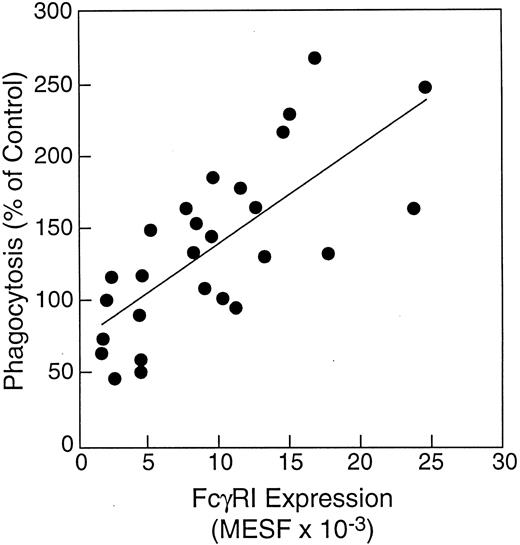
The effect of in vivo rhIFN-γ administration on phagocytosis of S aureus by neutrophils. In vivo rhIFN-γ treatment was associated with a significant improvement in the ability of neutrophils to ingest S aureus-BODIPY BioParticles when opsonized with complement-depleted HI-PHS, but not with pooled human serum (Fig 4). Neutrophils obtained from normal subjects 48 hours after initiating in vivo rhIFN-γ treatment displayed a 91% increase in their capacity to ingest HI-PHS–opsonized killed S aureus compared with baseline. Because the kinetics and magnitude of complement-mediated phagocytosis of S aureus by human neutrophils in this system are faster and greater than those seen with FcR-mediated phagocytosis, it is possible that the presence of complement in the experiment depicted in Fig 4B masked the increase in FcR-mediated phagocytosis. This increase in FcR-mediated phagocytosis (Fig 4A) significantly correlated with FcγRI expression by neutrophils (Fig 5; r = .8, P < .001).
The effect of in vivo rhIFN-γ on bacterial ingestion by neutrophils. These graphs show the mean ± SE phagocytosis by neutrophils of S aureus-BODIPY BioParticles opsonized with complement-depleted serum (A; •; n = 6) or pooled human serum with intact complement (B; ○; n = 4). Phagocytosis by neutrophils from rhIFN-γ–treated subjects was calculated as MFI (subject)/MFI (control) × 100. Asterisks indicate statistical significance (P < .05).
The effect of in vivo rhIFN-γ on bacterial ingestion by neutrophils. These graphs show the mean ± SE phagocytosis by neutrophils of S aureus-BODIPY BioParticles opsonized with complement-depleted serum (A; •; n = 6) or pooled human serum with intact complement (B; ○; n = 4). Phagocytosis by neutrophils from rhIFN-γ–treated subjects was calculated as MFI (subject)/MFI (control) × 100. Asterisks indicate statistical significance (P < .05).
Correlation of FcγRI expression by neutrophils with phagocytosis by neutrophils of S aureus opsonized with complement-depleted pooled human serum. FcγRI expression by neutrophils from study subjects was determined by direct immunostaining before and after in vivo rhIFN-γ administration (0.05 mg/m2 SC daily for 2 days). Correlation of FcγRI expression by neutrophils with their ability to perform phagocytosis of HI-PHS–opsonized S aureus BioParticles was determined by regression analysis. FcγRI expression by neutrophils correlated directly with ability to ingest HI-PHS–opsonized S aureus (r = .8, P < .05).
Correlation of FcγRI expression by neutrophils with phagocytosis by neutrophils of S aureus opsonized with complement-depleted pooled human serum. FcγRI expression by neutrophils from study subjects was determined by direct immunostaining before and after in vivo rhIFN-γ administration (0.05 mg/m2 SC daily for 2 days). Correlation of FcγRI expression by neutrophils with their ability to perform phagocytosis of HI-PHS–opsonized S aureus BioParticles was determined by regression analysis. FcγRI expression by neutrophils correlated directly with ability to ingest HI-PHS–opsonized S aureus (r = .8, P < .05).
Two antibodies that specifically bind to human FcγRI, MoAb 197 and F(ab′)2 32.2, inhibited phagocytosis of HI-PHS–opsonized S aureus by neutrophils by 55% and 31%, respectively, when neutrophils were obtained from subjects 48 hours after the initiation of rhIFN-γ treatment (Fig 6). Moreover, nonspecific murine IgG2a that binds preferentially to Fc receptor FcγRI blocked the ingestion of S aureus by 62% (Fig 6). None of these antibody preparations was significantly inhibitory when added to neutrophils obtained from subjects before rhIFN-γ treatment (Fig 6).
The effects of FcγRI antagonists on rhIFN-γ–enhanced bacterial ingestion by neutrophils. The graph shows the effect of anti-FcγRI MoAb (197) and F(ab′)2 (32.2), and control murine IgG2a (mIgG2a) and murine F(ab′)2 [mF(ab′)2 ] on phagocytosis of HI-PHS–opsonized S aureus-BODIPY BioParticles by neutrophils. The mean MFI of neutrophils obtained from two different study subjects (□) before in vivo rhIFN-γ administration (0.05 mg/m2/dose SC daily for 2 days) and (▪) 48 hours after the initiation of in vivo rhIFN-γ treatment are depicted. Asterisks indicate statistically significant inhibition by the MoAb or F(ab′)2 when compared with the corresponding 48-hour control in which no MoAb or F(ab′)2 was present (Student's t-test, P < .05).
The effects of FcγRI antagonists on rhIFN-γ–enhanced bacterial ingestion by neutrophils. The graph shows the effect of anti-FcγRI MoAb (197) and F(ab′)2 (32.2), and control murine IgG2a (mIgG2a) and murine F(ab′)2 [mF(ab′)2 ] on phagocytosis of HI-PHS–opsonized S aureus-BODIPY BioParticles by neutrophils. The mean MFI of neutrophils obtained from two different study subjects (□) before in vivo rhIFN-γ administration (0.05 mg/m2/dose SC daily for 2 days) and (▪) 48 hours after the initiation of in vivo rhIFN-γ treatment are depicted. Asterisks indicate statistically significant inhibition by the MoAb or F(ab′)2 when compared with the corresponding 48-hour control in which no MoAb or F(ab′)2 was present (Student's t-test, P < .05).
Serum opsonin and acute phase reactant levels after in vivo rhIFN-γ. The serum levels of neopterin (an intermediate product of the biosynthesis of tetrahydrobiopterin)26-29 and β2 -m (a short polypeptide chain located extracellularly in the class I major human histocompatibility complex)29 have been used to monitor in vivo responses to therapeutic IFN-γ.28-30 In the normal subjects treated with rhIFN-γ in this study, both neopterin and β2 -m levels peaked at 48 hours after the first dose of rhIFN-γ, with a 3.1-fold increase over pretreatment seen for neopterin and a 1.7-fold increase for β2 -m (Table 1). Similarly, the serum level of an opsonic protein LBP peaked at 24 hours, with a 39% increase over baseline (Table 1). LBP is a glycoprotein that binds to lipopolysaccharide (LPS) with high affinity and enhances LPS effects on CD14+ cells.31 None of the other serum opsonins or acute-phase reactants measured (ie, serum IgG, IgM, complement C3, CRP, sICAM, and sCD14) increased significantly after in vivo rhIFN-γ (Table 1).
Serum Levels of Opsonins and Acute-Phase Reactants After In Vivo Treatment With rhIFN-γ
| . | 0 h . | 24 h . | 48 h . | 72 h . | 168 h . |
|---|---|---|---|---|---|
| LBP (mg/mL, n = 12) | 17.8 ± 8.2 | 24.8 ± 7.7* | 23.8 ± 9.7 | 19.6 ± 6.5 | 16.2 ± 5.2 |
| Neopterin (ng/mL, n = 15) | 2.1 ± 0.5 | 4.7 ± 0.9† | 6.6 ± 1.4† | 5.4 ± 1.1† | 2.1 ± 0.4 |
| β2-m (ng/mL, n = 15) | 33.5 ± 4.7 | 49.0 ± 9.7† | 58.1 ± 10.7† | 50.5 ± 11.3† | 33.0 ± 7.0 |
| sCD14 (mg/mL, n = 12) | 0.96 ± 0.18 | 1.00 ± 0.18 | 1.02 ± 0.15 | 1.07 ± 0.3 | 0.94 ± 0.18 |
| sICAM (ng/mL, n = 15) | 16.8 ± 3.7 | 18.7 ± 3.3 | 20.8 ± 5.8 | 19.8 ± 3.2 | 18.3 ± 4.3 |
| C3 (mg/dL, n = 12) | 107 ± 24 | ND | 121 ± 30 | ND | 111 ± 18 |
| C4 (mg/dL, n = 12) | 28 ± 8 | ND | 31 ± 6 | ND | 29 ± 5 |
| CRP (mg/dL, n = 10) | 0.5 ± 0.2 | ND | 1.1 ± 1.4 | ND | 0.4 ± 0.1 |
| IgG (mg/dL, n = 7) | 990 ± 189 | ND | 991 ± 246 | ND | 996 ± 218 |
| IgM (mg/dL, n = 7) | 108 ± 26 | ND | 106 ± 25 | ND | 117 ± 31 |
| . | 0 h . | 24 h . | 48 h . | 72 h . | 168 h . |
|---|---|---|---|---|---|
| LBP (mg/mL, n = 12) | 17.8 ± 8.2 | 24.8 ± 7.7* | 23.8 ± 9.7 | 19.6 ± 6.5 | 16.2 ± 5.2 |
| Neopterin (ng/mL, n = 15) | 2.1 ± 0.5 | 4.7 ± 0.9† | 6.6 ± 1.4† | 5.4 ± 1.1† | 2.1 ± 0.4 |
| β2-m (ng/mL, n = 15) | 33.5 ± 4.7 | 49.0 ± 9.7† | 58.1 ± 10.7† | 50.5 ± 11.3† | 33.0 ± 7.0 |
| sCD14 (mg/mL, n = 12) | 0.96 ± 0.18 | 1.00 ± 0.18 | 1.02 ± 0.15 | 1.07 ± 0.3 | 0.94 ± 0.18 |
| sICAM (ng/mL, n = 15) | 16.8 ± 3.7 | 18.7 ± 3.3 | 20.8 ± 5.8 | 19.8 ± 3.2 | 18.3 ± 4.3 |
| C3 (mg/dL, n = 12) | 107 ± 24 | ND | 121 ± 30 | ND | 111 ± 18 |
| C4 (mg/dL, n = 12) | 28 ± 8 | ND | 31 ± 6 | ND | 29 ± 5 |
| CRP (mg/dL, n = 10) | 0.5 ± 0.2 | ND | 1.1 ± 1.4 | ND | 0.4 ± 0.1 |
| IgG (mg/dL, n = 7) | 990 ± 189 | ND | 991 ± 246 | ND | 996 ± 218 |
| IgM (mg/dL, n = 7) | 108 ± 26 | ND | 106 ± 25 | ND | 117 ± 31 |
All results are expressed as the mean ± SD.
Abbreviation: ND, not done.
P < .05 when compared with pretreatment levels.
P < .0001 when compared with pretreatment levels.
DISCUSSION
Administration of rhIFN-γ has been established by an international, double-blinded, placebo-controlled study as an effective prophylactic treatment regimen in patients with CGD for reducing the number of serious bacterial and fungal infections.1 Unexpectedly, in that study, administration of rhIFN-γ failed to correlate with enhanced neutrophil or monocyte superoxide production or in vitro S aureus bacterial killing.2,3 Because no laboratory test correlated with administration of rhIFN-γ, it has been suggested that expression of FcγRI be used as a measure of in vivo biologic activity of IFN-γ.14,16 Increased FcγRI expression secondary to IFN-γ has previously been shown in vivo in patients with cancer, arthritis, and CGD.14-17 However, patients with underlying infections or inflammatory conditions may not be the ideal models for studying FcγRI expression, because it is known that inflammation and infection upregulate FcγRI.14,32 33 In this present study, in vivo rhIFN-γ treatment of normal human subjects (in the absence of inflammation and infection) induced increased FcγRI expression by neutrophils and monocytes.
This study extends previous IFN-γ studies by showing a functional correlate to enhanced expression of FcγRI: improved FcγR-mediated phagocytosis by neutrophils. In vivo rhIFN-γ treatment was associated with a significant improvement in the ability of neutrophils to ingest killed S aureus when opsonized with complement-depleted pooled human serum (HI-PHS). The importance of Fcγ-receptor function in host defense has previously been shown by reports that impaired phagocyte Fcγ-receptor function in patients with alcoholic cirrhosis and end-stage renal disease is associated with an increased incidence of pyogenic infections in these patients.34 35
It is known that infection is associated with the release of various cytokines and inflammatory mediators. For example, streptococcal antigens are potent inducers of IFN-γ production.36,37 Not surprisingly, phagocytes isolated from patients with group A β-hemolytic streptococcal pharyngitis have increased expression of FcγRI.33 Simms et al32 showed that patients with acute bacterial infection had an increased capacity to perform FcγR-mediated phagocytosis. This effect was correlated with FcγRI upregulation. Presumably, this upregulation in phagocytic function represents a physiologic response to infection. As shown in this study, in vivo rhIFN-γ treatment mimics this physiologic response to infection.
Capsoni et al38 looked at the effect of in vitro IFN-γ treatment on human neutrophil FcγR-mediated phagocytosis and found that doses up to 500 U/mL (1.7 ng/mL) had no effect in their experimental model. This in vitro dose was comparable to plasma levels observed in cancer patients treated with IFN-γ.39 It is possible that their results conflict with the results of this study due to the short duration of IFN-γ treatment (30 minutes at 37°C) used in their model.38 In this study, FcγRI expression by neutrophils was upregulated by 24 hours (Fig 2) but not 4 hours after the initiation of therapy (data not shown).
The washout kinetics of IFN-γ–related effects seen in this study were also informative. Neutrophil and monocyte expression of FcγRI (as well as monocyte expression of FcγRII, FcγRIII, CD11a, CD11b, and CD18) all substantially decreased within 48 hours of cessation of rhIFN-γ and were back to normal baseline levels by day 7. Furthermore, other markers of IFN-γ treatment (serum neopterin, β2 -m, and LBP) also returned to baseline by day 7. The kinetics of these IFN-γ effects are similar to the in vitro effects of IFN-γ on monocyte and monocyte-like cell line U937 expression of FcγRI and FcγRII mRNA and protein as reported by Comber et al.12 13 These kinetics of rhIFN-γ–related effects have potentially important implications concerning the dosing frequency of rhIFN-γ prophylaxis in CGD.
IFN-γ has been shown to be a potent immunomodulator, with in vitro effects on the monocyte/macrophage defense system.17,40-43 In this study, the effect of in vivo rhIFN-γ on the kinetics and magnitude of monocyte surface molecule expression were examined. As others have previously reported, increased expression of β2 -integrins and HLA-DR by monocytes after rhIFN-γ treatment was observed.30,44-46 Contrary to other reports, no significant change in expression of the LPS/LBP receptor CD14 was seen.9,47 In addition to the expected increase in FcγRI, an unexpected increase in FcγRIII expression by monocytes was seen. Monocyte FcγRII expression also significantly increased, although, as others have reported,12 13 the magnitude of this increase (1.2-fold) was much less striking than that seen with FcγRI (3.3-fold).
As an immunomodulatory cytokine, IFN-γ plays a role in the induction of serum factors and acute-phase proteins. Two such proteins, neopterin and β2 -m, are known to be induced by IFN-γ treatment.28-30 As expected, in vivo rhIFN-γ treatment did induce a significant increase in serum neopterin and β2 -m levels in normal subjects. Conflicting reports exist in the literature regarding IFN-γ and complement synthesis.48,49 In this study, in vivo IFN-γ therapy had no significant effect on either C3 or C4 levels. Contrary to earlier reports, sCD14 did not significantly increase after in vivo IFN-γ treatment.30 However, these results were consistent with the failure to find a significant change in membrane CD14 expression by monocytes and neutrophils after in vivo IFN-γ. In vivo rhIFN-γ treatment was associated with a significant increase in serum LBP levels noted 24 hours after the initiation of treatment. LBP is known to be an acute-phase reactant produced predominantly in the liver.50-53 This is the first report that IFN-γ induces LBP as part of an acute-phase response.
In this study, rhIFN-γ treatment was well tolerated at a dose of 0.05 mg/m2 SC daily for 2 doses. Many of the study subjects received acetaminophen to minimize the expected fever, headache, and myalgia. An acetaminophen-related effect on the parameters studied cannot be excluded. However, an in vitro study using therapeutic concentrations of acetaminophen showed inhibition of phagocytosis rather than the improvement in phagocytosis seen in this study.54 Laboratory results were remarkable for a significant decrease in total leukocyte and neutrophil counts after rhIFN-γ treatment. This effect was not reported in the multicenter, phase III study of IFN-γ in patients with CGD in which longer interval time points were evaluated,1 although it was seen in studies in which IFN-γ was administered over a short term, as in this study.46 Landmann et al30 reported tachyphylaxis of this and other IFN-γ–related effects in their study of prolonged SC IFN-γ administration. The rapid onset of decreased leukocyte and neutrophil counts in our study makes it unlikely that this effect is secondary to decreased leukocyte production in the bone marrow.
In conclusion, the protective effect of rhIFN-γ in patients with CGD may be related to upregulation of Fcγ-receptor expression by phagocytes, increased serum levels of opsonin LBP, and a subsequent improvement in the ability of phagocytes to ingest microbes. In addition, improved phagocyte trafficking may occur secondary to increased expression of monocyte β2 -integrins. Because these improvements in host defense were seen in normal hosts, rhIFN-γ may have broader applications as adjuvant treatment in disorders of host defense including but not limited to CGD.
ACKNOWLEDGMENT
The authors thank Valerie Moreau for assistance with preparation of the manuscript, the nurses and laboratory personnel of the Scripps Clinic GCRC for invaluable help, John Ruzinski from the Seattle VA for running the serum sCD14 and LBP assays, and Brian Paasch and the department of Assay Services at Genentech for running the neopterin, β2 -m, and sICAM assays.
Supported by National Institutes of Health Grants No. AI-24838 (J.T.C.), GM-37696 (T.R.M.), and RR-00833 (Scripps Clinic GCRC); Public Health Services Grant No. DK07022 (D.E.S.); the Medical Research Service of the Department of Veterans Affairs (T.R.M.); and Genentech, Inc.
Address reprint requests to Deborah E. Schiff, MD, Department of Molecular and Experimental Medicine, CAL-1, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037.


![Fig. 6. The effects of FcγRI antagonists on rhIFN-γ–enhanced bacterial ingestion by neutrophils. The graph shows the effect of anti-FcγRI MoAb (197) and F(ab′)2 (32.2), and control murine IgG2a (mIgG2a) and murine F(ab′)2 [mF(ab′)2 ] on phagocytosis of HI-PHS–opsonized S aureus-BODIPY BioParticles by neutrophils. The mean MFI of neutrophils obtained from two different study subjects (□) before in vivo rhIFN-γ administration (0.05 mg/m2/dose SC daily for 2 days) and (▪) 48 hours after the initiation of in vivo rhIFN-γ treatment are depicted. Asterisks indicate statistically significant inhibition by the MoAb or F(ab′)2 when compared with the corresponding 48-hour control in which no MoAb or F(ab′)2 was present (Student's t-test, P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3187/4/m_bl_0003f6.jpeg?Expires=1767727843&Signature=hQLm5Hp1IKlYQAbZhkMMFixUJA8gBwyxk~sUT57~VXWOqc9UP5j7lGUEcu~KAELHmXIHqxFjjyd9cSBhOiyb3nGldLeWd-UiGagIhUh2vASD4U9swAkPJip9rlmyAkRgOGYl8bi-Ig1V8luE~LR6WJfePyaXCLPfgucAo6FFzTbkU3rVnCFeqmFS0jXwAfN8qm4~8jR7ByRJwrmVxhIg3E6RROdJiIlferyT4Oc47Mq~nnmpgkpLwqLE9aI8LmJKbB14dQ7l0teIpykvmlk-2XQittyttnUFSQ5aATP~-H9cMxTqjUGhyk5mljCcy3oSlxvWSBIm6C4u08I0JfRz9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal