Abstract
Erythropoietin (Epo) is known to control the erythroid developmental program through various biologic activities including maintenance of viability, cell proliferation, and/or cell maturation. In vitro experiments showed massive apoptosis in cultures of Epo-deprived colony-forming unit-erythroid (CFU-E) progenitors, demonstrating the Epo requirement of late-stage erythroid progenitors for survival. Based on these data, a model has been proposed whereby from CFU-E to proerythroblast stages, Epo acts rather as a survival factor than a proliferating factor. To investigate the relationship between Epo dependence and apoptotic mechanisms, we generated transgenic mice expressing the antiapoptotic human bcl-2 gene product in erythroid progenitors. Transgenic animals developed without any evidence of erythropoietic disorders. In vitro studies showed that overexpression of bcl-2 in erythroid progenitors delayed, but did not prevent the loss of CFU-E from Epo-deprivation. By measuring burst-forming unit-erythroid (BFU-E) and CFU-E–derived colonies, an enhanced sensitivity to low levels of Epo was demonstrated in adult bone marrow of transgenic mice with respect to nontransgenic animals. No spontaneous erythroid colonies were, however, observed in vitro in the absence of the cytokine, indicating that overexpression of bcl-2 is not sufficient to induce by itself a complete erythroid differentiation. Taken together, our data indicate that targeted erythroid overexpression of bcl-2 fails to alter the normal erythropoietic development in vivo and that erythroid progenitors remain strictly dependent on Epo for their survival.
APOPTOSIS PLAYS A central role in hematopoiesis in maintaining a cellular balance in terms of tissue development and control of cell numbers. Although Epo was initially characterized as a stimulator of both proliferation and differentiation of erythroid progenitors, it has been suggested that Epo mainly maintains the viability of progenitor cells. Dependence on Epo for survival was first demonstrated by in vitro studies using Friend virus-infected murine spleen cells.1 In this experimental model, withdrawal of Epo resulted in a pattern of morphological changes characteristic of an apoptotic process. Similar results were obtained when studying untransformed murine2,3 and human4 erythroid progenitors. As colony-forming unit-erythroid (CFU-E) undergo massive apoptosis when cultured in absence of Epo, the Epo-mediated promotion of cell survival appears as a major effect of this cytokine. These data led to the proposal of an erythropoiesis regulation model in which circulating Epo levels control the in vivo homeostatic balance of erythropoiesis by suppressing apoptosis and thereby regulating expansion of developing erythroid precursors.5,6 Among several observations supporting this hypothesis, one can note that only a minority of Epo-dependent progenitor cells give rise to mature cells at physiologic Epo concentrations.1 6
According to this model, suppression of programmed cell death should have important implications for both proliferation and maturation of these cells. We therefore examined whether an in vivo constitutive overexpression of an antiapoptotic protein may have the capacity to alter the growth behavior and Epo-dependence of late-stage erythroid progenitors. The protein Bcl-2 has been shown to prevent apoptosis triggered by various stimuli such as chemotherapeutic drugs, γ irradiation, viral infections, oxidant stress, and notably growth factor deprivation.7 Furthermore, Bcl-2 has recently been directly involved in the regulation of erythroid progenitor survival.8 Using the erythroid-specific regulatory sequences of the rat L-type pyruvate kinase gene,9 we generated transgenic mice expressing the human bcl-2 gene product in erythroid progenitors. We investigated whether the constitutive overexpression of bcl-2 in these cells might induce an erythroid proliferation in vivo, thereby leading to a polycythemic disorder. We also examined whether impairment of the apoptotic process would allow growth and differentiation of erythroid progenitors in vitro in the absence of Epo.
We show here that there is a delay in the apoptosis of transgenic CFU-E deprived of Epo. In addition, bcl-2-expressing CFU-E and burst-forming unit-erythroid (BFU-E) progenitors exhibit in vitro an enhanced sensitivity to Epo, compared with normal. No spontaneously differentiating colonies are however observed in in vitro cultures deprived of the cytokine. In vivo, transgenic animals develop without any erythropoietic disorder. These findings indicate that protection from apoptosis afforded by the constitutive overexpression of bcl-2 in erythroid progenitors does not lead to Epo independence and that bcl-2 transgenic erythroid progenitors remain dependent on Epo for their survival.
MATERIALS AND METHODS
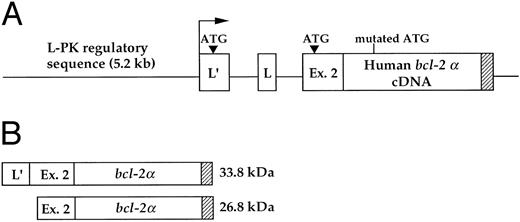
Generation of transgenic mice. A 0.9-kb fragment corresponding to the human bcl-2 α cDNA was linked to 5.2 kb of regulatory sequences of the rat L-type pyruvate kinase (L-PK ) gene. These regions encompass a distal erythroid-restricted enhancer, both the erythroid (L′)- and liver (L)-specific first exons of the gene, and part of the second exon of the L-PK gene9 (Fig 1A). Mutation of the bcl-2 α initiation codon (ATG into ACG) led to the synthesis of two hybrid PK-Bcl-2 proteins, whose translation starts in the L-PK gene first and second exon, respectively (Fig 1B). Transgenic mice (CBA/B6D2) were identified by Southern blot analysis by hybridization with the 0.9-kb fragment containing the bcl-2α coding sequence. All subsequent studies were performed on F1 mice. To generate 13-day old transgenic fetuses, male mice homozygous for the PK-bcl-2 transgene were bred with CBA/J nontransgenic females. Pregnancy was determined by the presence of a vaginal plug in spontaneously mated females; day 0 of gestation was the day of plug discovery.
Schematic representation of the transgenic construct. (A) The human bcl-2α cDNA was cloned in the second exon of the rat L-PK gene, maintaining the integrity of both the erythroid- (L′) and the hepatic- (L) alternative first exons of the gene. The arrow indicates the erythroid-specific transcriptional start site. (B) The transgene encodes two proteins that are translated from the indicated initiation ATG in the erythroid-specific first exon and second exon of the L-PK gene. The natural bcl-2α initiation codon was mutated by PCR. The bcl-2 signal/anchor motif, responsible for a cellular membrane location, is represented by hatched bars.
Schematic representation of the transgenic construct. (A) The human bcl-2α cDNA was cloned in the second exon of the rat L-PK gene, maintaining the integrity of both the erythroid- (L′) and the hepatic- (L) alternative first exons of the gene. The arrow indicates the erythroid-specific transcriptional start site. (B) The transgene encodes two proteins that are translated from the indicated initiation ATG in the erythroid-specific first exon and second exon of the L-PK gene. The natural bcl-2α initiation codon was mutated by PCR. The bcl-2 signal/anchor motif, responsible for a cellular membrane location, is represented by hatched bars.
Mice analyses. Blood collection was performed in EDTA-treated microtainer tubes (Becton Dickinson, Mountain View, CA). Hematocrits were determined by automated cell counter (Coulter Immunology, Hialeah, FL).
Northern blot. Total RNA was extracted from homogenized tissues using the guanidium thiocyanate procedure.10 Total RNA (10 μg) was electrophoresed through 1.5% (wt/vol) agarose-formaldehyde gels, transferred to Hybond-N+ nylon membranes, and probed with the 0.9-kb fragment containing the bcl-2α coding sequence. Membranes were washed in 2× SSC with 0.1% (wt/vol) sodium dodecyl sulfate (SDS) for 15 minutes at room temperature and twice in 1× SSC with 0.1% SDS for 15 minutes at 45°C.
Western blot. Tissues were mixed 1:1 with 2× Laemmli sample buffer, boiled for 3 minutes and spun for 10 minutes at 10,000g. Supernatants were resolved in 12% (wt/vol) SDS-polyacrylamide gel and transferred to a nitrocellulose filter. Membranes were blocked with 5% (wt/vol) milk powder in Tris-buffered saline with 0.05% (vol/vol) Tween 20 (Sigma, St Quentin Fallavier, France). Hybrid PK-Bcl–2 proteins were detected using an anti-Bcl–2 rabbit primary antibody (Dako, Glostrup, Denmark). Immunostaining was performed with peroxydase-coupled antirabbit IgG and enhanced chemiluminescence ECL (Amersham).
Subcellular fractionations. Erythroid progenitors from fetal liver (day 13) were selectively isolated by filtration on a 50 μ nylon membrane before being resuspended in 10 mmol/L Tris/HCl, pH 7.4, containing 10 mmol/L KCl, 5 mmol/L EDTA, 1 mmol/L Na3VO4 , 1 mmol/L phenylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 μmol/L pepstatin. Cells were lysed by three freeze/thawing cycles and 10 strokes of a tight-fitting Dounce pestle. Lysed cells were centrifuged at 2,500g for 10 minutes, pellets were discarded, and supernatants were centrifuged for 30 minutes at 150,000g. The resulting 150,000g supernatant was designated as the cytosol and the pellet as the crude membrane.
Erythroid progenitor cell assays. Cells were cultured using the methylcellulose method to evaluate BFU-E–derived colonies and the plasma clot technique to evaluate CFU-E–derived colonies. For BFU-E assays, a single cell suspension of bone marrow (5 × 105 cells) was plated in 1 mL of 1% methylcellulose in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Argy-Pontoise, France) supplemented with 20% fetal calf serum (FCS), 1% deionized bovine serum albumin (BSA; Sigma) and 10−4 mol/L β-mercaptoethanol in the presence of 100 U/mL murine recombinant interleukin-3 (IL-3; Pepro Tech, Rocky Hill, NJ). The plasma clot technique was adapted from McLeod et al11 with some modifications. Single suspensions of bone marrow and fetal liver cells (5 × 105/mL and 2 × 105/mL, respectively) were plated in IMDM, 20% FCS, 1% deionized BSA, 20 μg/mL L-asparagin, and 10% bovine citrate plasma (GIBCO, Paisley, Scotland). Various concentrations of recombinant human erythropoietin (generous gift from Dr M. Brandt, Boehringer Mannheim, Germany) were added to the cultures. Cultures were incubated at 37°C in a fully humidified atmosphere supplemented with 5% CO2 . Colonies were identified at day 2 (CFU-E–derived colonies) and day 7 (BFU-E–derived colonies) by morphologic criteria, direct microscopic observation of live cultures, or staining with benzidine and hematoxylin (fixed preparations of flattened plasma clots). For fetal liver CFU-E assays, cells were washed once in serum-free IMDM supplemented with 1% deionized BSA and 20 μg/mL iron-saturated transferrin. Cells were maintained for several hours at 37°C in the above medium until plating.
Amplification of PK-bcl–2 transcripts by reverse transcriptase-polymerase chain reaction (RT-PCR) on hematopoietic colonies. Granulocytic colonies and BFU-E–derived colonies were individually plucked from the methylcellulose under direct microscopic visualization 7 days after plating. Cells were washed once with phosphate buffered saline (PBS) and total RNA was prepared by the guanidium thiocyanate procedure.10 The sequence of synthetic oligonucleotides used in PCR is 5′-CACGCTTTGGAAGCATG-3′, which is complementary to a segment of the erythroid-specific first exon of the L-PK gene; 5′-ACTTCCCGGTTGTCGTACCC-3′, complementary to a segment of the bcl-2α coding sequence. Amplification products (260 bp) were run through 1.5% agarose gel.
RESULTS
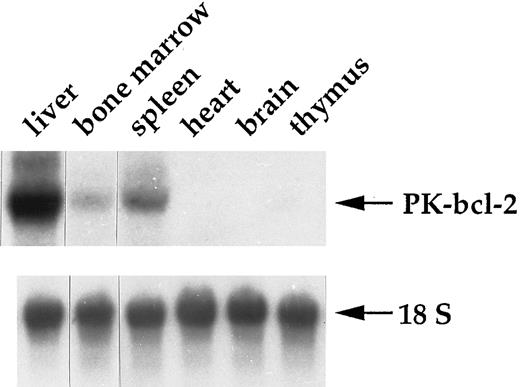
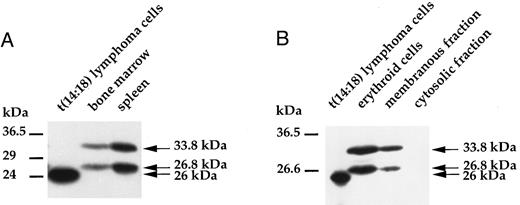
Targeted expression of bcl-2α cDNA in erythroid progenitors. Human bcl-2α cDNA expression was directed by the regulatory regions of the rat L-type pyruvate kinase gene that is normally transcribed from two alternative promoters and encodes a glycolytic enzyme. These two promoters direct the transcription of erythroid- (L′) and liver-specific (L) mRNAs, which differ only by their first exon. The L′ promoter is strictly specific to erythroid cells and is active in erythroid progenitors.12 The regulatory regions used in this study encompass a distal erythroid-specific enhancer required to stimulate the L′ promoter activity to a proper level.9 In the transgenic construct, most of the L-PK gene was replaced with the bcl-2α cDNA, but the integrity of both the erythroid- and liver-specific first exons of the gene was still maintained, as well as a part of exon 2, leading to a hybrid PK-bcl-2 gene (Fig 1A). Thus, this transgenic construct allowed the hybrid gene transcription from both alternative promoters. We have previously reported results concerning the bcl-2α cDNA expression in the liver.13 For the transgenic line studied in this work, which harbored 10 copies of the hybrid construct, the transgene expression was restricted to the liver and erythroid tissues, ie, spleen and bone marrow (Fig 2). The presence of the hybrid PK-bcl–2 gene product was further examined by Western analyses in erythroid tissues with a polyclonal antibody raised against a synthetic peptide of the human Bcl-2 protein. Two protein species were detected with apparent molecular weights of 33.8 kD and 26.8 kD in spleen and bone marrow of adult transgenic animals (Fig 3A) and in transgenic 13-day old fetal liver cells (Fig 3B). These proteins corresponded respectively to the expected hybrid protein and to a shorter form whose translation started from an alternate initiation codon present in the second exon of the L-PK gene (Fig 1B). Furthermore, both hybrid proteins were selectively integrated into cellular membranes, according to the presence of a signal/anchor motif in the bcl-2α coding sequence (Fig 3B).
Expression pattern of the PK-bcl-2 transgene. Hybrid PK-bcl-2 transcripts were selectively detected in the liver and erythropoietic tissues (spleen and bone marrow) by Northern blot analysis. A total of 10 μg of total RNAs were electrophoresed on a formaldehyde agarose-gel. After transfer, the membrane was hybridized with a human bcl-2 probe. Normalization of total RNA amounts was made by hybridizing the membrane with an 18S ribosomal RNA-specific probe.
Expression pattern of the PK-bcl-2 transgene. Hybrid PK-bcl-2 transcripts were selectively detected in the liver and erythropoietic tissues (spleen and bone marrow) by Northern blot analysis. A total of 10 μg of total RNAs were electrophoresed on a formaldehyde agarose-gel. After transfer, the membrane was hybridized with a human bcl-2 probe. Normalization of total RNA amounts was made by hybridizing the membrane with an 18S ribosomal RNA-specific probe.
Immunodetection of the transgene products. (A) Two protein species of similar abundance were observed in spleen and bone marrow of transgenic animals by Western blot, corresponding to the expected hybrid protein (33.8 kD) and to a shorter form (26.8 kD) whose translation started from an initiation codon present in the second exon of the L-PK gene. As a control, human Bcl-2 protein was detected in human follicular t(14:18) B-lymphoma cells at 26 kD. (B) A similar pattern was observed on analysis of transgenic erythroid cells isolated from 13-day old fetal livers. Both transgene products were selectively detected in the membranous fraction of these cells and were absent from the cytosolic fraction.
Immunodetection of the transgene products. (A) Two protein species of similar abundance were observed in spleen and bone marrow of transgenic animals by Western blot, corresponding to the expected hybrid protein (33.8 kD) and to a shorter form (26.8 kD) whose translation started from an initiation codon present in the second exon of the L-PK gene. As a control, human Bcl-2 protein was detected in human follicular t(14:18) B-lymphoma cells at 26 kD. (B) A similar pattern was observed on analysis of transgenic erythroid cells isolated from 13-day old fetal livers. Both transgene products were selectively detected in the membranous fraction of these cells and were absent from the cytosolic fraction.
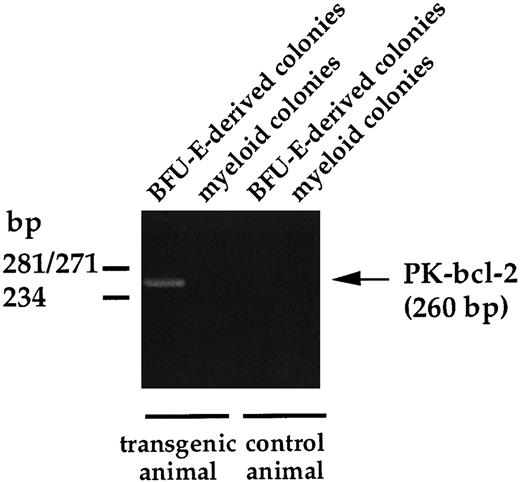
To ensure that the transgene expression was restricted to the erythroid compartment, we examined by RT-PCR the presence of hybrid PK-bcl–2 transcripts in erythroid and nonerythroid hematopoietic lineages. Bone marrow cells from adult PK-bcl–2 transgenic mice were plated in methylcellulose cultures, as reported in the Materials and Methods, and total RNA was extracted from sample colonies picked after 7 days of culture. As shown in Fig 4, only BFU-E–derived colonies allowed the amplification by RT-PCR of PK-bcl-2 mRNAs, whereas no transcripts were observed in granulocytic colonies. These results show that expression of bcl-2 is restricted to erythroid progenitors.
RT-PCR analysis of hybrid PK-bcl-2 transcripts on hematopoietic colonies. Granulocytic and BFU-E–derived colonies were individually plucked from the methylcellulose under direct microscopic visualization 7 days after plating, total RNA of each sample was extracted, and used for RT-PCR amplification. The PK-bcl–2-specific amplified fragment is 260 bp long.
RT-PCR analysis of hybrid PK-bcl-2 transcripts on hematopoietic colonies. Granulocytic and BFU-E–derived colonies were individually plucked from the methylcellulose under direct microscopic visualization 7 days after plating, total RNA of each sample was extracted, and used for RT-PCR amplification. The PK-bcl–2-specific amplified fragment is 260 bp long.
Overexpression of bcl-2 in erythroid progenitors did not result in any in vivo erythropoietic disorder. All PK-bcl–2 transgenic mice had normal development and body size. Neither abnormal mortality rate nor pathologic disorders were evidenced during 24 months of observation. Because overexpression of bcl-2 in erythroid progenitors may result in a disregulated production of mature red cells, blood collections were randomly performed from seven transgenic and seven control littermates aged 1 to 12 months. All transgenic and control mice tested had normal hematocrit values (Ht, 45% to 50%), indicating that targeted expression of bcl-2 in erythroid lineage did not induce polycythemia in these animals. Furthermore, histologic examination of hematopoietic organs, ie, spleen and bone marrow of transgenic mice between the age of 1 and 12 months did not show any obvious abnormality (data not shown).
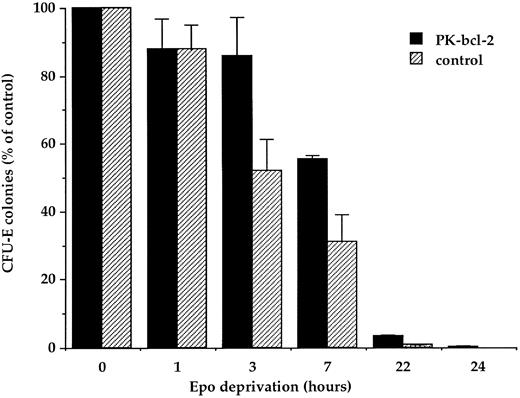
Effect of bcl-2 expression on survival of erythroid progenitors. The growth behavior of transgenic progenitors was examined by in vitro clonogenic progenitor cell assays. In an attempt to uncover a postulated Epo independence of transgenic erythroid progenitors, we first examined the effects of a total Epo deprivation. Previous in vitro studies have shown that apoptosis is a rapid consequence of Epo deprivation in murine fetal liver cells.2 Fetal liver cells from 13-day old transgenic fetuses were maintained for several hours in serum-free medium at 37°C before being plated in the presence of 2 U/mL of Epo. Survival and capacity to form erythroid colonies were compared for erythroid progenitors from either transgenic or normal 13-day-old fetal livers. Figure 5 reflects the mean of two representative experiments. The maximum number of day 13 fetal liver CFU-E–derived colonies, normally observed in the presence of 2 U/mL of Epo was taken as 100%. CFU-E cell survival to serum and Epo deprivation was significantly increased for cells from transgenic mice. Indeed, 86% ± 12% and 55% ± 0.5% of CFU-E from PK-bcl–2 mice survived to a 3- or 7-hour deprivation, respectively, compared with 52% ± 9% to 31% ± 9% of CFU-E cells from control mice. Deprivation of Epo for 24 hours resulted in the complete disappearance of control CFU-E–derived colonies, whereas few colonies, with a mature phenotype, could be observed for transgenic progenitors. In these experiments, no obvious morphologic changes were evidenced for transgenic terminally differentiated erythroid colonies compared with normal, as judged from cell morphologic analyses (data not shown). These results indicate that although overexpression of bcl-2 in erythroid progenitors cannot totally prevent the apoptotic process induced by Epo deprivation, it delays its onset. Therefore, in contrast to other bcl-2 transgenic model14 demonstrating an extended B-cell survival, the overexpression of bcl-2 in erythroid progenitors does not prevent the loss of CFU-E after longer than 24 hours of Epo deprivation.
Survival of transgenic and normal fetal liver CFU-E after Epo deprivation. Erythroid cells from 13-day old fetal livers were maintained in the absence of Epo for the indicated times and then plated in semisolid medium at a concentration of 2 × 105 cells/mL in the presence of 2 U/mL of Epo. CFU-E–derived colonies were scored at day 2. Results are expressed as the average number of colonies of duplicate cultures from two independent experiments.
Survival of transgenic and normal fetal liver CFU-E after Epo deprivation. Erythroid cells from 13-day old fetal livers were maintained in the absence of Epo for the indicated times and then plated in semisolid medium at a concentration of 2 × 105 cells/mL in the presence of 2 U/mL of Epo. CFU-E–derived colonies were scored at day 2. Results are expressed as the average number of colonies of duplicate cultures from two independent experiments.
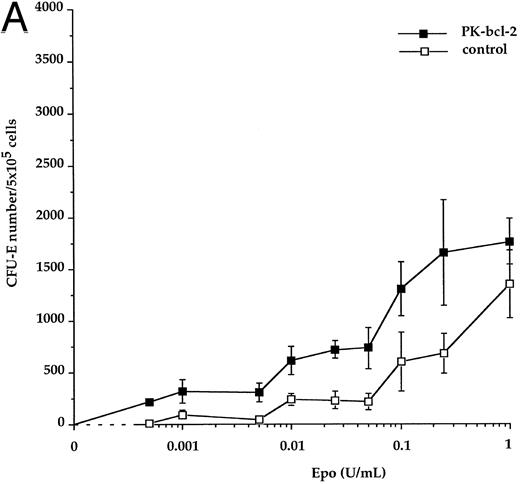
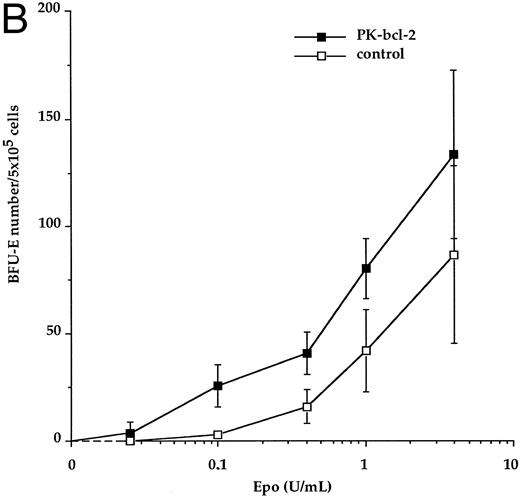
Enhanced erythropoietin sensitivity of bone marrow erythroid progenitors. We analyzed the sensitivity of erythroid progenitors to the cytokine by performing in vitro progenitor cell assays in the presence of various Epo concentrations. Adult bone marrow cells from both transgenic and nontransgenic (control) littermates were used in these experiments. The total number of transgenic CFU-E–derived colonies was not significantly different from control, after 2 days of culture, at the maximal doses of Epo. However, when transgenic CFU-E progenitors were cultured in the presence of 50 mU to 250 mU/mL of Epo, a twofold increase in the total number of CFU-E–derived erythroid colonies was observed, when compared with control. Using lower levels of Epo (1 mU to 50 mU/mL), the total number of CFU-E–derived colonies increased by up to threefold for transgenic mice, compared with normal (Fig 6A). At these Epo concentrations, transgenic CFU-E–derived colonies were observed at 42% ± 11% (50 mU/mL) and 18% ± 6% (1 mU/mL) of their maximal number (defined at 1 U/mL as a 100%), whereas normal CFU-E–derived colonies were present at 16% ± 6% and 7% ± 2%, respectively (see Table 1). Interestingly, transgenic CFU-E still responded to Epo at 0.5 mU/mL, whereas no CFU-E–derived colonies were evidenced for control bone marrow cells. Although decreased concentrations of Epo gave rise to decreased numbers of transgenic and control erythroid colonies in a similar dose-dependent manner, a significant difference between the response of bcl-2-expressing and control CFU-E was demonstrated (P < .001) at low concentrations of Epo tested. Similarly, the total number of transgenic BFU-E–derived colonies did not differ significantly from control. An increase of the overall number of BFU-E–derived colonies was observed at low Epo concentrations (below 0.4 U/mL) for transgenic mice with respect to control (Fig 6B). When Epo was supplied at 0.4 U/mL and 0.1 U/mL, transgenic BFU-E–derived colonies were present at 31% ± 7% and 19% ± 7%, respectively, of their maximal number, compared with 18% ± 9% and 3% ± 1%, respectively, for control (Table 1). However, these differences did not reach statistical significance at the BFU-E stage. No spontaneously differentiating erythroid colonies were however observed in CFU-E and BFU-E assays when Epo was omitted, neither for transgenic nor for control mice. These observations suggest that bcl-2-expressing erythroid progenitors are hypersensitive to Epo at concentrations that represent the range found in physiologic conditions.15
Epo-dose response curves from adult bone marrow transgenic CFU-E and BFU-E progenitors. Transgenic (closed symbols) and control erythroid progenitors (open symbols) were cultured in the presence of the indicated concentrations of recombinant Epo. CFU-E–derived colonies were scored at day 2 (A) and BFU-E–derived colonies were scored at day 7 (B). Results are expressed as the average number of colonies per 5 × 105 cells plated.
Epo-dose response curves from adult bone marrow transgenic CFU-E and BFU-E progenitors. Transgenic (closed symbols) and control erythroid progenitors (open symbols) were cultured in the presence of the indicated concentrations of recombinant Epo. CFU-E–derived colonies were scored at day 2 (A) and BFU-E–derived colonies were scored at day 7 (B). Results are expressed as the average number of colonies per 5 × 105 cells plated.
Maximal Response to Epo for Transgenic and Control Adult Bone Marrow Erythroid Progenitors
| CFU-E–derived colonies | |||||||||
| Epo U/mL | 1 | 0.25 | 0.1 | 0.05 | 0.025 | 0.01 | 0.005 | 0.001 | 0.0005 |
| Transgenic | 100 | 94 ± 28 | 74 ± 15 | 42 ± 11 | 41 ± 5 | 35 ± 8 | 17 ± 5 | 18 ± 6 | 12 ± 0.1 |
| Control | 100 | 50 ± 14 | 44 ± 21 | 16 ± 6 | 17 ± 6 | 17 ± 4 | 4 ± 1 | 7 ± 2 | 1 ± 0.1 |
| P | — | <.01 | <.02 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| BFU-E–derived colonies | |||||||||
| Epo U/mL | 4 | 1 | 0.4 | 0.1 | 0.025 | ||||
| Transgenic | 100 | 60 ± 10 | 31 ± 7 | 19 ± 7 | 3 ± 3 | ||||
| Control | 100 | 48 ± 22 | 18 ± 9 | 3 ± 1 | 0 | ||||
| P | — | NS | NS | <.05 | NS |
| CFU-E–derived colonies | |||||||||
| Epo U/mL | 1 | 0.25 | 0.1 | 0.05 | 0.025 | 0.01 | 0.005 | 0.001 | 0.0005 |
| Transgenic | 100 | 94 ± 28 | 74 ± 15 | 42 ± 11 | 41 ± 5 | 35 ± 8 | 17 ± 5 | 18 ± 6 | 12 ± 0.1 |
| Control | 100 | 50 ± 14 | 44 ± 21 | 16 ± 6 | 17 ± 6 | 17 ± 4 | 4 ± 1 | 7 ± 2 | 1 ± 0.1 |
| P | — | <.01 | <.02 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| BFU-E–derived colonies | |||||||||
| Epo U/mL | 4 | 1 | 0.4 | 0.1 | 0.025 | ||||
| Transgenic | 100 | 60 ± 10 | 31 ± 7 | 19 ± 7 | 3 ± 3 | ||||
| Control | 100 | 48 ± 22 | 18 ± 9 | 3 ± 1 | 0 | ||||
| P | — | NS | NS | <.05 | NS |
Results from Fig 6 are expressed as percentages of the maximal response, defined at 1 U/mL and 4 U/mL Epo for CFU-E and BFU-E, respectively. Data were obtained from duplicate cultures in seven (CFU-E) and three (BFU-E) independent experiments.
Abbreviation: NS, not significant.
DISCUSSION
A model of erythropoiesis regulation has been proposed whereby Epo levels control the in vivo expansion of erythroid progenitors compartment by sparing these cells from a natural apoptotic process.1 An essential feature of this model is that only a minority of Epo-dependent progenitors are responsible for the erythrocyte production, while the majority of these progenitors undergoes apoptosis at physiologic Epo concentrations. Thus, the variation of erythrocyte production in response to fluctuating Epo levels6 would mainly derive from Epo-mediated suppression of apoptosis. We developed a transgenic model allowing a constitutive expression of the antiapoptotic Bcl-2 protein in erythroid progenitors. Assuming that Epo may mainly act as a survival factor, we investigated whether the impairment of the apoptotic process may result in an Epo-independent erythroid proliferation in vivo, and would allow in vitro, growth and differentiation of erythroid progenitors in the absence of the cytokine.
In this transgenic model, the human bcl-2α cDNA is driven by the rat L-type pyruvate kinase gene (L-PK ) regulatory sequences allowing expression both in erythroid and hepatic tissues. Northern and RT-PCR experiments showed the erythroid specificity of the PK-bcl-2 transgene expression within the hematopoietic lineages (Figs 2 and 4). Western analyses confirmed the presence of the hybrid PK-bcl-2 gene products in erythroid tissues, ie, spleen, bone marrow, and 13-day old fetal liver cells and their subcellular localization into the membranes (Fig 3A and B). Two transgenic PK-bcl-2 proteins were detected that correspond to the expected hybrid protein (33.8 kD) and to an abundant form (26.8 kD) whose translation starts from a internal initiation site whithin the second exon of the L-PK gene.13 The functionality of the 26.8-kD PK-bcl–2 protein was assessed by the strong protective effect afforded by this hybrid PK-bcl–2 gene product in an acute and lethal hepatic apoptotic process. Indeed, we have previously shown that this protein protected mice from acute hepatic apoptosis induced by injection of an agonistic anti-Fas antibody.13
Overexpression of bcl-2 in erythroid progenitors did not result in any hematologic disorder in vivo, all transgenic animals developing without obvious expansion of the erythroblastic compartment as judged from blood smears and hematocrit values (Ht, 45% to 50%). The absence of erythroproliferative disease over a 24-month–long period of observation was unexpected in regard to numerous models of transgenic mice overexpressing bcl-2 in hematopoietic lineages.7 These experimental models have established that, in most cases, overexpression of bcl-2 can lead to hyperplasia by altering the normally tightly regulated process of development; the association with other genetic alterations16 furthermore favoring the progression to full malignancy.
The main point of our in vitro data is that bcl-2 transgenic erythroid progenitors remain strickly dependent on Epo for progression of their developmental programs and are unable to survive in vitro in the absence of the cytokine. In an important work, Fairbairn et al17 reported that IL-3–dependent FDCP-Mix myeloid progenitor cells transduced with a bcl-2 retrovirus underwent myelo-erythroid differentiation in the absence of added specific cytokines, leading to the hypothesis that growth factors may not be required for cell differentiation. Then, numerous in vitro experimental models have been developed to investigate whether cells receiving a survival stimulus (generally by overexpression of bcl-2 ) would be capable of spontaneous lineage commitment and development.18 The loss, albeit delayed, of transgenic erythroid progenitors in the absence of Epo indicates that overexpression of bcl-2, at least at the level attained in the L-PK-bcl–2 transgenic mice, cannot overcome the Epo-mediated survival signal, ie, Epo is still required for the final production of mature erythroid cells from their developmentally earlier progenitors. Similarly, transfection of a bcl-2 expression vector in the granulocyte colony-stimulating factor (G-CSF )–dependent 32D cell line was shown to be insufficient in inducing a complete granulocytic differentiation in the absence of G-CSF.19
Interestingly, in vitro clonogenic progenitor cell assays performed in the presence of low concentrations of Epo showed a statistically significant increase in the number of adult bone marrow transgenic CFU-E progenitors with respect to normal littermates (Table 1). These data indicate that transgenic erythroid progenitors manifest an enhanced response to Epo concentrations that represent the range normally found in physiologic conditions. The relationship between survival with Epo deprivation and increased Epo sensitivity in vitro has been previously noticed by Kelley et al20 who showed an heterogeneity in the onset of apoptosis of Friend virus-infected murine spleen cells resulting from differing sensitivities to Epo. Because the Bcl-2 protein is devoid of proliferation promoting activity,14,21 the increased number of terminally differentiated erythroid colonies could be related to an enhanced survival of transgenic progenitors due to the antiapoptotic properties of our transgene. Indeed, while a total deprivation of Epo induced a decrease in the number of CFU-E–derived colonies from control fetal livers, transgenic CFU-E remained, however, less sensitive to the lack of Epo in the medium (Fig 5). In agreement with other in vitro experimental models,17,22 23 our data show that overexpression of bcl-2 cannot suppress the cytokine deprivation-induced apoptotic process, but delays its onset.
Taken together, our in vitro data uncover a masked in vivo phenotype and indicate that targeted overexpression of bcl-2 affects the homeostasis of the erythroid compartment, without leading to an overt polycythemic state. The level of endogenous Bcl-2 in the erythroid lineage has not been extensively studied, but it seems to be expressed at low level in erythroblasts.24 It has been demonstrated for various interleukin-dependent progenitor cell lines,21,25 as for the Epo-dependent HCD-57 cells,8 that a downregulation of bcl-2 follows growth factors removal, preceeding the apoptotic process. However, some Epo-dependent cell lines either do not express bcl-226 or do not exhibit loss of bcl-2 expression when undergoing apoptosis in the absence of Epo, such as the pluripotent human cell line UT-7 (unpublished results). Furthermore, several inhibitors of apoptosis, especially the Bcl-2 family proteins, have been described to date,27 some of which may prove more effective in certain contexts than others. In this regard, the antiapoptotic Bcl-XL protein appears an important effector in primitive human hematopoietic progenitors, as judged from its high level of expression in those cells compared with Bcl-228 and its direct involvement in hematopoietic differentiation.29 Therefore, overexpression of bcl-2 could not be per se sufficient in our model to bypass the Epo-dependent developmental program of erythroid progenitors and, as reported in an in vitro experimental model,8 a cooperation with Bcl-XL would be required. Indeed, targeted disruption of the Bcl-X gene in mice is embryonic lethal, due to a massive cell death of immature hematopoietic cells,29 whereas erythropoiesis is normal in Bcl-2–deficient mice.30
In vivo, additional levels of erythroid homeostasis control are supposed to be effective. Because transgenic erythroid progenitors remain dependent on physiologic Epo titers, one cannot rule out the possiblity of a downregulation of circulating Epo titers in our transgenic mice. Our results nevertheless suggest that a mean twofold increase in erythroid progenitor numbers and enhanced sensitivity to Epo are not sufficient to disturb the regulatory network controlling in vivo the erythroid compartment. Consistent with this, Kirby et al15 recently reported that transgenic mice expressing a truncated form of the Epo receptor known to increase the sensitivity to this cytokine, did not exhibit an altered erythropoietic development in vivo nor a modified behavior of erythroid progenitors in vitro.
ACKNOWLEDGMENT
We thank Dr C. Beldjord for kindly providing the human follicular t(14:18) B-lymphoma cell line, Dr M. Tuilliez for histologic examination of transgenic mice, and Dr O. Bernard for critical reading of the manuscript.
Supported by grants from the Association contre le Cancer, the Ligue Nationale contre le Cancer, and from the Comité de Paris.
Address reprint request to C. Lacombe, MD, Unité 363 INSERM, 27 rue du Faubourg Saint Jacques, 75014 Paris, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal