Abstract
Interleukin-15 (IL-15) is a recently characterized cytokine that shares many biological activities with IL-2 and interacts with the β and γ components of the IL-2 receptor. Unlike IL-2, which is secreted only by T cells, IL-15 is expressed preferentially by nonlymphoid tissues, epithelial, and fibroblast cell lines and by activated monocytes/macrophages. High concentrations of IL-15 have been shown in inflamed joints of rheumatoid arthritis patients, suggesting a role for IL-15 in inflammatory diseases where there is recruitment of leukocytes. Although monocytes have been shown to bind IL-15, its effects on these cells are not defined. In this report we show that supernatants of monocytes treated with IL-15–contained chemotactic activity for neutrophils and monocytes which was neutralized by anti-IL-8 or by anti-monocyte chemotactic protein 1 (MCP-1) antibodies, respectively. Secretion of IL-8 and MCP-1 proteins is detectable by enzyme-linked immunosorbent assay as early as 6 hours after stimulation with IL-15. Production of the two chemokines is correlated with induction by IL-15 of mRNA expression in monocytes. In addition, IL-8 and MCP-1 induction by IL-15 is differently regulated by interferon-γ (IFN-γ) and IL-4. IFN-γ inhibited IL-15–induced IL-8 secretion, but synergized with IL-15 in MCP-1 induction; whereas IL-4 inhibited both IL-8 and MCP-1 induction by IL-15. These results show that IL-15 can stimulate monocytes to produce chemokines that cause inflammatory cell accumulation. Thus, IL-15 locally produced at sites of inflammation may play a pivotal role in the regulation of the leukocyte infiltrate.
INTERLEUKIN-15 (IL-15), a novel cytokine identified on the basis of biological activities similar to IL-2, induces T-cell proliferation, generates cytotoxic effector cells and lymphokine-activated killer activity in natural killer cells, causes B-cell proliferation and differentiation, and stimulates chemotaxis of T cells.1-6 IL-15 also shares with IL-2 the β and γ subunits as common receptor components of their receptor complexes.1,7-10 However, the IL-15–receptor complex also includes an IL-15-R α-chain, distinct from the IL-2R α-chain, that allows high-affinity binding.8 10
IL-15 mRNA is expressed at high levels in lipopolysaccharide (LPS)-activated monocytes, in fibroblasts, epithelial cell-lines, and in several other nonimmune tissues such as placenta, skeletal muscle, heart, lung, liver, and kidney.1,11,12 However, in vivo, IL-15 has been detected only in chronic inflammatory diseases or autoimmune disorders. In synovial tissues from rheumatoid arthritis (RA) patients and in alveolar macrophages of sarcoidosis patients, IL-15 has been identified by immuno-cytochemistry; in RA synovial fluids, high levels of IL-15 (up to 1,200 ng/mL) have been detected by ELISA techniques.13 14
Although monocytes and polymorphonuclear cells (PMN) infiltrate the synovial cavities of RA patients, these cells do not migrate in response to IL-15 in vitro.6 In addition, subcutaneous injection of IL-15 in mice induces infiltration at the site of injection not only of lymphocytes but also of other mononuclear and of polymorphonuclear cells indicating that their recruitment might be mediated by induction of other proinflammatory cytokines such as chemokines.6,13 When activated by proinflammatory cytokines such as IL-1, tumor necrosis factor (TNF ), or leukemia inhibitory factor (LIF ), monocytes produce large amounts of chemotactic factors such as IL-8 and monocyte chemotactic protein 1 (MCP-1).15-18 These chemoattractants of 7 to 10 kD, that have heparin-binding properties and ability to bind and activate seven-transmembrane spanning receptors, induce adhesion to endothelial cells and chemotaxis of lymphocytes, monocytes, or PMN.15 19-22
Since monocytes have been reported to express binding sites for IL-15 but the functional significance of this interaction has not been defined, we investigated whether monocytes responded to IL-15 with production of chemotactic factors.7 23 Here we report that IL-15 stimulates monocytes to produce MCP-1 and IL-8, two chemokines that specifically attract monocytes and neutrophils, respectively.
MATERIALS AND METHODS
Cell culture.Peripheral blood mononuclear cells (PBMC) were obtained from normal volunteers who had provided informed consent. Monocytes were purified by Percoll gradient as described elsewhere.24 The purity of the monocyte preparations used in this study was 90% ± 4% as assessed by morphology on Giemsa-stained cytocentrifuge preparations and flow cytometry using the monocyte-specific monoclonal antibody, LeuM3. PMN were purified essentially as described elsewhere.24 The preparations contained at least 95% PMN as judged by morphological criteria; the remaining cells were mainly lymphocytes. Monocytes were cultured in RPMI 1640 (Advanced Biotechnology, Inc, Columbia, MD), containing 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, 20 mmol/L HEPES (GIBCO, Grand Island, NY), and 10% heat-inactivated fetal bovine serum (HyClone Lab, Logan, UT).
Cytokines.Human recombinant IL-15 was a kind gift of Dr Tony Troutt (Immunex, Seattle, WA), IL-4, IL-8, and MCP-1 were purchased from Peprotech (Rocky Hill, NJ). Human recombinant interferon-γ (IFN-γ; lot NN9027AX, specific activity 2.02 × 107 U/mg) was kindly provided by Dr H.M. Shephard (Genentech Labs, San Francisco, CA). All reagents and media, tested by the Endotoxin kit (Sigma, St Louis, MO), contained endotoxin at levels lower than 12 pg/mL.
Northern blot analysis.For RNA extraction, 20 × 106 monocytes were plated in 10 mL medium alone or supplemented with the indicated cytokines. Total RNA isolation and Northern blot analysis were performed as previously described.25 Briefly, 15 μg of total RNA from each sample were electrophoresed under denaturing conditions, blotted onto Nytran membranes (Schleicher & Schuell Inc, Keene, NH) and cross-linked by ultraviolet irradiation. Membranes were prehybridized at 42°C in Hybrisol (Oncor Inc, Gaithersburg, MD) and hybridized overnight with 2 × 106 counts per minute (cpm) of 32P-labeled probe. Membranes were then washed three times at room temperature for 10 minutes in 0.2× sodium saline citrate (SSC) 0.1% sodium dodecyl sulfate before being autoradiographed using Kodak XAR-5 films (Eastman Kodak, Rochester, NY) and intensifying screens at 80°C. Probes were labeled by random priming reaction using a commercial kit (Boehringer Mannheim Biochemicals, Indianapolis, IN) and α32P-deoxycytidine triphosphate (3,000 Ci/mmol; Amersham, Arlington Heights, IL). The specific activity was always higher than 109 cpm/μg. IL-8 and MCP-1 full-length cDNA, kindly provided by Dr K. Matsushima (Kanazawa University Cancer Institute, Kanazawa, Ishikawa-ken, Japan) were used.
Cytokine determination.IL-8, TNF-α, and IL-1β content of supernatants were determined by using commercial enzyme-linked immunosorbent assay (ELISA) kits obtained from Amersham while MCP-1 concentration was assessed by an ELISA kit purchased from R&D (Minneapolis, MN), following the manufacturer's instructions (sensitivity of assays: 3 pg/mL).
Migration assays.Monocyte-derived supernatants were diluted 1:2 with RPMI 1640 containing 1% bovine serum albumin (BSA), and incubated at 37°C for 20 minutes with irrelevant antibody (goat IgG; Sigma), anti-IL-8 or anti-MCP-1 neutralizing antibodies (R&D) at a concentration of 10 μg/mL, and assayed for chemotactic activity for monocytes and PMN. Migration of monocytes and PMN (1.5 × 106 cells/mL in RPMI 1640 + 1% BSA) was evaluated by a microchamber technique as described elsewhere.24 For monocytes, 5-μm pore-size polycarbonate filters were employed. Under the assay conditions employed, only monocytes in PBMC preparations migrated across the filter. Polyvinylpyrrolidone-free polycarbonate filters were used for PMN. At the end of the incubation (90 minutes for monocytes and 30 minutes for PMN), filters were removed, fixed, and stained by Diff-Quik (Harleco, Gibbstown, NJ), and three oil immersion fields were counted after coding samples. In each assay, N-formylmethionyl-leucyl-phenylalanine (fMLP; Sigma) at a concentration of 10 nmol/L was used as a standard chemoattractant for monocytes or PMN. Statistical analysis of the results was performed by using analysis of variance (ANOVA) test for repeated measures.
RESULTS
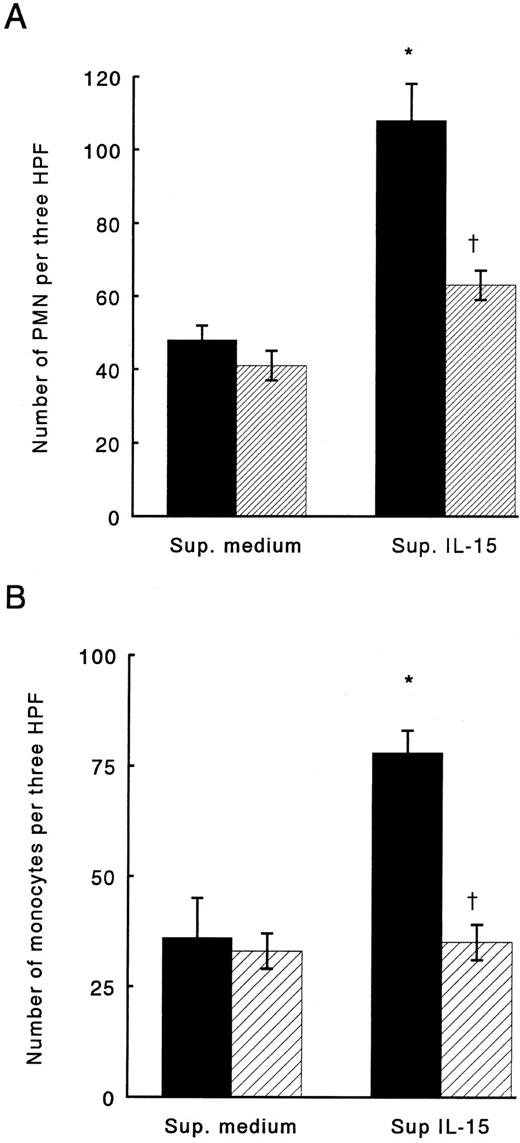
To assess whether IL-15 might induce chemokine production in monocytes, cells were stimulated with IL-15 at concentrations up to 1,000 ng/mL. After 24 hours of incubation, supernatants derived from the culture were examined for PMN and monocyte chemotactic activity. IL-15–stimulated monocytes released chemoattractants for both monocytes and PMN into supernatants; in contrast, boiled-IL-15 failed to induce secretion of chemotactic factors (data not shown). To identify which chemokines were produced by IL-15–stimulated monocytes, we used neutralizing antibodies against IL-8 and MCP-1, two chemokines produced in large amounts by monocytes stimulated with other cytokines, such as IL-1, TNF, or LIF16-18. Anti-IL-8 antibodies neutralized up to 65% of the PMN chemotactic activity in supernatants derived from IL-15–stimulated monocytes, while IgG, used as control, did not affect PMN chemotaxis in response to the same supernatants (Fig 1A). Monocyte chemotactic activity of supernatants from IL-15 stimulated monocyte was reduced up to 90% by preincubation with anti-MCP-1 antibodies; normal IgG did not change monocyte response to the medium (Fig 1B).
Chemotactic activity of supernatants derived from monocytes stimulated with IL-15 for PMN (A) or monocytes (B). (▪) Supernatants treated with control IgG. (▨) Supernatants treated with anti-IL-8 or anti-MCP-1 neutralizing antibodies. Results represent mean ± SE of three independent experiments. *Significant chemotactic response in comparison to medium (P < .05). †Significant inhibition by treatment with neutralizing antibodies in comparison to treatment with control IgG (P < .05).
Chemotactic activity of supernatants derived from monocytes stimulated with IL-15 for PMN (A) or monocytes (B). (▪) Supernatants treated with control IgG. (▨) Supernatants treated with anti-IL-8 or anti-MCP-1 neutralizing antibodies. Results represent mean ± SE of three independent experiments. *Significant chemotactic response in comparison to medium (P < .05). †Significant inhibition by treatment with neutralizing antibodies in comparison to treatment with control IgG (P < .05).
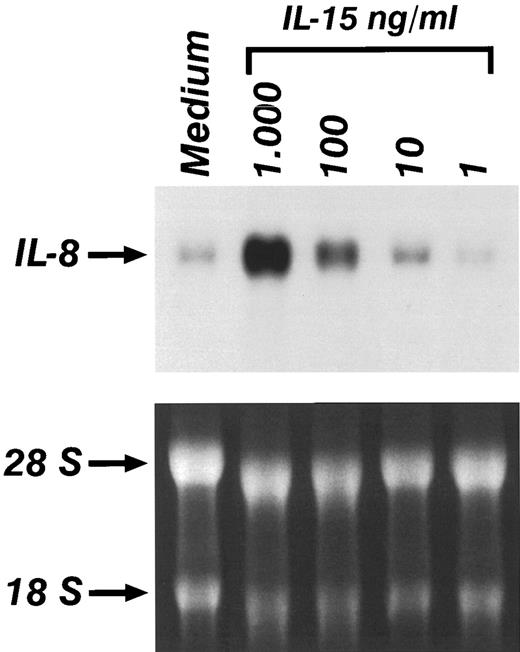
Experiments were performed to determine whether the production of IL-8 and MCP-1 in monocytes stimulated by IL-l5 was associated with an increased expression of mRNA for these chemokines. Total RNA was obtained from monocytes treated with increasing concentrations of IL-15 for 6 hours. Figure 2 shows that a low basal level of IL-8 mRNA was detected in untreated monocytes. IL-15 caused a dose dependent increase of IL-8 mRNA. Time course experiments indicated that IL-8 mRNA expression was observed as early as 1 hour after stimulation and was maximal after 6 hours (data not shown). Induction of MCP-1 mRNA was also detected after IL-15 stimulation; however, the peak of MCP-1 mRNA expression was reached 18 hours after stimulation. Donor to donor variation was obtained in the constitutive expression of IL-8 and MCP-1 mRNA expression in unstimulated cells; however, in 6 out of 6 donors, an increased expression of IL-8 and MCP-1 mRNA was consistently observed after treatment with IL-15.
Northern blot analysis of the expression of IL-8 in the presence of increasing concentrations of IL-15. Monocytes were incubated for 4 hours in medium alone or supplemented with IL-15. Lower panel shows ethidium-bromide staining of total RNA.
Northern blot analysis of the expression of IL-8 in the presence of increasing concentrations of IL-15. Monocytes were incubated for 4 hours in medium alone or supplemented with IL-15. Lower panel shows ethidium-bromide staining of total RNA.
To confirm that the induction of IL-8 and MCP-1 mRNA was associated with protein secretion, supernatants from monocytes were harvested at various times after stimulation with IL-l5, and IL-8 and MCP-1 protein levels were quantitated by ELISA. As shown in Table 1, IL-8 production by monocytes was increased by stimulation with IL-15 at concentrations as low as 100 ng/mL, as early as 6 hours. After 24 hours of stimulation, the production of IL-8 reached its maximal level. No further increase could be detected at subsequent time points (data not shown). MCP-1 was detectable 6 hours after stimulation of the cells with 100 ng/mL of IL-15, but decreased with time (Table 2). The lack of accumulation of MCP-1 in the culture medium might be caused by increased utilization and degradation of the chemokine via MCP-1 receptors expressed on monocytes. Significant amounts of both IL-8 and MCP-1 were consistently detected in supernatants derived from monocytes stimulated with IL-15 at concentrations of 100 ng/mL in three out of three subjects studied (P < .05).
Production of IL-8 by Human Monocytes Upon Stimulation by IL-15
| Treatment* . | IL-8 (ng/mL) Mean (SD) . | |
|---|---|---|
| . | 6 h . | 24 h . |
| Medium | 14 (3.3) | 27 (4.1) |
| IL-15 (10 ng/mL) | 25 (1.8) | 42 (3.4) |
| (100 ng/mL) | 34† (2.6) | 70† (6.2) |
| (1,000 ng/mL) | 141† (6.4) | 176† (8.7) |
| Treatment* . | IL-8 (ng/mL) Mean (SD) . | |
|---|---|---|
| . | 6 h . | 24 h . |
| Medium | 14 (3.3) | 27 (4.1) |
| IL-15 (10 ng/mL) | 25 (1.8) | 42 (3.4) |
| (100 ng/mL) | 34† (2.6) | 70† (6.2) |
| (1,000 ng/mL) | 141† (6.4) | 176† (8.7) |
Supernatants derived from monocytes cultured with increasing concentrations of IL-15 (from 10 to 1,000 ng/mL), or medium alone for 6 or 24 hours were analyzed in duplicate for their concentration of IL-8 by ELISA. Results shown are representative of a single experiment out of three performed.
Significant production in comparison to medium (P < .05).
Production of MCP-1 by Human Monocytes Upon Stimulation by IL-15
| Treatment* . | MCP-1 (ng/mL) . | |
|---|---|---|
| . | Mean (SD) . | |
| . | 6 h . | 24 h . |
| Medium | 0.16 (0.1) | <0.014 (0.01) |
| IL-15 (10 ng/mL) | 0.44 (0.2) | 0.05 (0.09) |
| (100 ng/mL) | 0.78† (0.16) | 0.14 (0.12) |
| (1,000 ng/mL) | 1.85† (0.31) | 1.03† (0.2) |
| Treatment* . | MCP-1 (ng/mL) . | |
|---|---|---|
| . | Mean (SD) . | |
| . | 6 h . | 24 h . |
| Medium | 0.16 (0.1) | <0.014 (0.01) |
| IL-15 (10 ng/mL) | 0.44 (0.2) | 0.05 (0.09) |
| (100 ng/mL) | 0.78† (0.16) | 0.14 (0.12) |
| (1,000 ng/mL) | 1.85† (0.31) | 1.03† (0.2) |
Supernatants derived from monocytes cultured with increasing concentrations of IL-15 (from 10 to 1,000 ng/mL), or medium alone for 6 or 24 hours were analyzed in duplicate for their concentration of MCP-1 by ELISA. Results shown are representative of a single experiment out of three performed.
Significant production in comparison to medium (P < .05).
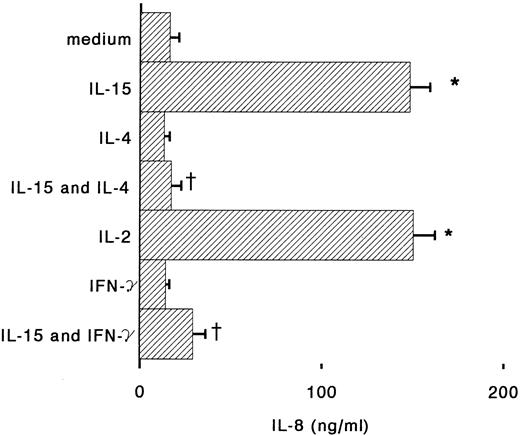
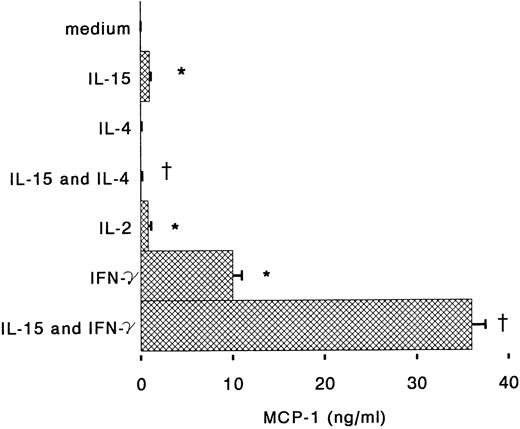
Antigen-dependent activation of T cells induces proliferation but also cytokine-production; IL-2, IL-4, and IFN-γ, secreted by T cells, can also regulate many monocyte functions including the production of chemokines.26 IFN-γ is known to stimulate cytotoxic and antibacterial activities of monocytes, but also to inhibit IL-8 expression and to induce MCP-1 and IP-10 secretion.18,27,28 When monocytes were incubated for 6 hours with IL-15 and IFN-γ, we observed that IL-8 release in the supernatants was reduced almost to the basal level (Fig 3). IFN-γ alone was about 10 times more potent than IL-15 for the induction of MCP-1, but when the two cytokines were used together, a synergistic upregulation of MCP-1 production was observed (Fig 4). IL-4 may display both stimulatory and inhibitory effects on monocytes: it induces CD23 expression,29 but inhibits IL-1β and TNF-α production by LPS-activated monocytes.30 31 We found that IL-4, used at concentrations of 100 U/mL, is a potent inhibitor of IL-8 and MCP-1 production by human monocytes stimulated with IL-15 (Figs 3 and 4).
Regulation by IL-4 and IFN-γ of IL-8 production in IL-15–stimulated monocytes. Monocytes were incubated with medium alone or supplemented with IL-15 (1,000 ng/m), IL-4 (100 U/mL), IFN-γ (500 U/mL), IL-15 plus IL-4, or IL-15 plus IFN-γ. After 6 hours supernatants were assayed for IL-8 content by ELISA. Results represent mean ± SE of IL-8 concentrations of three independent experiments. *Significant difference of IL-8 concentration (P < .05). †Significant inhibition by treatment (P < .05).
Regulation by IL-4 and IFN-γ of IL-8 production in IL-15–stimulated monocytes. Monocytes were incubated with medium alone or supplemented with IL-15 (1,000 ng/m), IL-4 (100 U/mL), IFN-γ (500 U/mL), IL-15 plus IL-4, or IL-15 plus IFN-γ. After 6 hours supernatants were assayed for IL-8 content by ELISA. Results represent mean ± SE of IL-8 concentrations of three independent experiments. *Significant difference of IL-8 concentration (P < .05). †Significant inhibition by treatment (P < .05).
Regulation by IL-4 and IFN-γ of MCP-1 production by IL-15–stimulated monocytes. Monocytes were incubated under the conditions described in the Fig 3 legend. After 6 hours supernatants were assayed for IL-8 content by ELISA. Results represent mean ± SE of MCP-1 concentrations of three independent experiments. *Significant difference of MCP-1 concentrations (P < .05). †Significant inhibition by treatment (P < .05).
Regulation by IL-4 and IFN-γ of MCP-1 production by IL-15–stimulated monocytes. Monocytes were incubated under the conditions described in the Fig 3 legend. After 6 hours supernatants were assayed for IL-8 content by ELISA. Results represent mean ± SE of MCP-1 concentrations of three independent experiments. *Significant difference of MCP-1 concentrations (P < .05). †Significant inhibition by treatment (P < .05).
DISCUSSION
We have shown that IL-15, in addition to its effect on T cells, also exhibits effects on monocytes, and may act as a proinflammatory cytokine inducing monocytes to secrete both PMN and monocyte chemotactic factors. IL-8 and MCP-1 are chemokines identified in supernatants derived from IL-15–stimulated monocytes as shown by experiments of neutralization of chemotactic activities of supernatants with anti-IL-8 or anti-MCP-1 as well as with immunoenzymatic assays (Figs 1 and 2, Tables 1 and 2). IL-8 and MCP-1, as well as IL-15, are also present in inflamed joints of rheumatoid arthritis patients,13,21,32 suggesting that IL-15 stimulation of synovial macrophages might contribute to the induction of IL-8 and MCP-1 production in synovial fluids. High concentrations of the two chemokines are detected within 6 hours of stimulation of monocytes with IL-15 at concentrations as low as 100 ng/mL (Tables 1 and 2). Activated monocytes may secrete other cytokines such as IL-1α and β, and TNF-α, that in turn can induce production of IL-8 and MCP-1.16,18 Thus, IL-15 induction of chemokines might be mediated through release of these proinflammatory cytokines. However, monocyte supernatants assayed for IL-1β, and TNF-α content by ELISA, showed that IL-15 at concentrations of 10 or 100 ng/mL failed to induce detectable amounts of IL-1β, and TNF-α (data not shown), as previously reported by McInnes et al.33 Instead, we saw that higher concentrations of IL-15 (1,000 ng/mL), consistently induced secretion of both IL-1β and TNF-α (data not shown). As a whole, our results indicate that IL-15, at concentrations up to 100 ng/mL, may directly induce IL-8 and MCP-1 at both mRNA and protein level without involving TNF-α or IL-1β; but higher concentrations of IL-15 (1,000 ng/mL) also induce a concomitant release of TNF-α, and IL-1β that may enhance the level of chemokine production.
IL-15, originally identified for its T-cell proliferative activity in supernatants derived from a monkey-kidney epithelial cell line (CVI/EBNA) and from a human adult T-cell leukemia cell line (HuT-102), is expressed at the mRNA level ubiquitously in organs and tissues.1,2 Although IL-15 mRNA is induced in vitro on a variety of stimuli, IL-15 protein is usually not detectable in supernatants, probably because its secretion is tightly regulated at a posttranslational level.12,34 However, IL-15 protein is secreted in large amounts in synovial fluid derived from RA patients (up to 1,200 ng/m), and expressed in macrophages derived from patients affected by pulmonary sarcoidosis, and in fibroblasts and keratinocytes of normal skin, suggesting that IL-15 protein may be secreted in vivo during inflammatory responses.13 14 We found that production of IL-8 and MCP-1 is induced by IL-15 at concentrations (100 ng/m) that are reached in pathological conditions such as RA, that are characterized by an important inflammatory response. In these diseases, IL-15 may contribute to leukocyte recruitment to tissues not only by direct chemotaxis of T cells, but also by induction of other chemoattractants such as IL-8 and MCP-1.
T cells recruited to the site of inflammation, if activated by T-cell receptor triggering may differentiate into a Th1 or Th2 phenotype, depending on the pattern of cytokine production. Secretion of IFN-γ will activate leukocyte cytotoxicity, while predominance of IL-4 will divert the immune system to mount an antibody-mediated response. IL-4 is known to act on monocytes through the common chain pathway exerting both activating or inhibiting effects. However, in monocytes IL-4 is a potent inhibitor of cytokine release induced by bacterial components or by proinflammatory cytokines.30 35 We observed that IL-4 prevents IL-15 induction of the chemokines IL-8 and MCP-1 in human monocytes. The effect of IL-4 was obtained at concentrations as low as 100 U/mL and could not be overcome by increasing IL-15 concentrations up to 1,000 ng/mL (data not shown).
In contrast to IL-4, IFN-γ activates many functions of monocytes including antibacterial activity, cytotoxicity, and cytokine production.26 We observed that production of IL-8 and MCP-1 induced by IL-15 can be modulated by IFN-γ. IL-8 secretion by IL-15–stimulated monocytes is inhibited upon treatment with IFN-γ. Conversely, MCP-1 secretion, induced by IL-15, is strongly synergized by IFN-γ. We speculate that Th1 and/or Th2 cytokines produced by T cells may fine-tune chemokine expression in monocytes upon IL-15 stimulation in vivo. IL-4, preventing IL-8 and MCP-1 production, will exert anti-inflammatory properties by reduction of PMN and monocyte infiltration in inflamed tissue. Secretion of IFN-γ may act in vivo as a switch that, by differentially regulating IL-8 and MCP-1 production, diverts the leukocyte infiltrate from neutrophilic to monocytic.
The inhibitory effect of IFN-γ on IL-8 expression was previously observed in monocytes stimulated with IL-1, IL-2, or LIF18,27; IFN-γ inhibits transcriptional activation of IL-8 gene by decreasing NF-kB transactivating activity.36 It is unclear, however, how IFN-γ might enhance IL-15–induced secretion of MCP-1. We have previously reported that IFN-γ does not affect LIF-induced production of MCP-1,18 indicating that the combined effect of IL-15 and IFN-γ might involve other mechanisms such as the upregulation of IL-15–receptor expression or of other components involved in the signaling pathways of these two cytokines. Monocytes, constitutively express IL-2Rβ chain, the common γ chain, and the tyrosine kinase Jak-3 that mediates, in other cell types, the signaling response to IL-15.37-40 It has been reported that IFN-γ upregulates the expression of these IL-15–receptor subunits, suggesting a possible mechanism for the observed synergism between IL-15 and IFN-γ.37-39 Nevertheless, the signaling pathway of IL-15 in human monocytes has not been defined. It is unclear whether unstimulated monocytes express IL-15R α chain or other IL-15–receptor subunits identified on mast-cells. Although lacking the IL-2R γ chain, mast-cells still bind IL-15, and upon stimulation with the cytokine, tyrosine-phosphorylation of Jak-2 but not of Jak-3 and Jak-1 was observed.34 41 We are currently studying the expression and regulation of IL-15 receptor α chain in monocytes to characterize the signaling pathway of IL-15 in these cells and to clarify whether the effects of IL-15 described in this study might be mediated through these additional IL-15 receptor subunits.
ACKNOWLEDGMENT
The authors thank Drs Ji Ming Wang and Michael Grimm for kindly reviewing the manuscript, and Dr Antonio Sica for helpful suggestions.
Supported by a fellowship of “Comitato Promotore TeleThon,” Rome, Italy (to R.B). Partly supported by a grant from “First National Project on Tuberculosis, Istituto Superiore di Sanita,” Rome, Italy; and by TeleThon, Rome, Italy, Grant A.42 (to L.D.N).
Address reprint requests to Tiziana Musso, PhD, Dipartimento di Sanita' Pubblica, Universita' di Torino, Via Santena 9, 10126 Torino, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal