Abstract
hCAP-18 is the only human member of the antibacterial and endotoxin-binding family of proteins known as cathelicidins. The antibacterial and endotoxin binding domains reside in the C-terminal 37 amino acids of the protein (LL-37) and this is believed to be unleashed from the neutralizing N-terminus by proteases from peroxidase positive granules. In human neutrophils, peroxidase positive and peroxidase negative granules can be subdivided into granule subsets that differ in protein content and ability to be exocytosed. To determine the localization of hCAP-18, we performed high-resolution immuno-electron microscopy and subcellular fractionation on Percoll density gradients. Biosynthesis of hCAP-18 was investigated in isolated human bone marrow cells. hCAP-18 was found to colocalize and comobilize with lactoferrin, but not with gelatinase in subcellular fractions. This was confirmed by electron microscopy. hCAP-18 is synthesized at the same stage of myeloid cell maturation as lactoferrin, and is efficiently targeted to granules. Like the peroxidase negative granule's matrix metalloproteinases, collagenase and gelatinase, hCAP-18 is also stored in unprocessed form. hCAP-18 is a major protein of specific granules where it is present in equimolar ratio with lactoferrin.
THE ANTIMICROBIAL efficacy of neutrophils relies mainly on proteins that are localized to granules.1 Several granule subsets have been identified that differ not only in their content of membrane and matrix proteins,2,3 but also in the extent to which the granules are mobilized and empty their matrix proteins either outside the cell or to the phagocytic vacuole.4,5 In the process of granule exocytosis, granule membrane is incorporated into the surface or the phagosomal membrane. Most bactericidal proteins are localized to peroxidase positive (also termed azurophil) granules1 (bactericidal permeability-increasing protein,6 CAP37,7,8 defensins9 ). Here they colocalize with proteases such as elastase, cathepsin G and proteinase 3,10 which are stored in granules as active enzymes.
Peroxidase negative granules are noted mainly for their membranes, which contain a variety of receptors for adhesion and phagocytosis in addition to the flavocytochrome b558 , an essential part of the superoxide generating electron transport system, known as the reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase.2 The matrix metalloproteases, collagenase, and gelatinase are stored in peroxidase negative granules as zymogens which may be activated by proteolytic or oxidative attack.11-15
Products from peroxidase positive granules and peroxidase negative granules meet in the phagocytic vacuole and may cooperate to enhance the function of the neutrophil. Myeloperoxidase from peroxidase positive granules transforms H2O2 , generated by the NADPH oxidase, to the more potent microbicidal agent, HOCl.16 Recently, a family of endotoxin-binding bactericidal proteins has been identified in peroxidase negative granules of neutrophils from a variety of mammalian species. Members of this family, termed cathelicidins,17 share a highly conserved N-terminal segment of 12 kD, which is homologous to cathelin, a protein purified from porcine neutrophils.18 In contrast, the C-terminus is highly divergent among the members of this family, ranging from 12 to 100 amino acids and from repeats of proline and arginine rich sequences to sequences capable of forming amphipatic α-helices. Several cathelicidins have been identified in neutrophils of ruminants, rodents, and pigs.17 In ruminants, the cathelicidins are localized to large granules,19 which is a granule subset specific for neutrophils of ruminants.20 In rabbits, p15 has been localized to specific granules,21 but this protein is only remotely related to cathelicidins.22 Recently, a cathelicidin was identified in human neutrophils and cloned.23-25 Genomic analysis strongly suggests that this is the only cathelicidin found in humans.26,27 This is in agreement with biochemical analysis of the content of human neutrophil granules.25 As for most other cathelicidins, the human cathelicidin, hCAP-18 has been shown to be stored intact as an 18-kD protein.25 The bactericidal and cytocidal activity of the protein is localized to the C-terminal 37 amino acids, which are capable of forming an amphipathic α-helix.23,26 In general, the cathelin-like segment must be removed from the C-terminal peptide of cathelicidins to unleash their microbicidal activity.28,29 This is assumed to take place during degranulation into the phagocytic vacuole, where cathelicidins are exposed to proteases from azurophil granules, in particular elastase.29
We decided to determine the subcellular localization of the human cathelicidin, hCAP-18, and to investigate whether the lack of multiple cathelicidins in human neutrophils is compensated by quantity of the cathelicidin present. Furthermore, we wished to determine which neutrophil precursors synthesize hCAP-18, to see, if the time of hCAP-18 biosynthesis agrees with its subcellular localization.
MATERIALS AND METHODS
Identification of hCAP-18.hCAP-18 was identified by specific rabbit antibodies raised against recombinant hCAP-18. hCAP-18 was quantitated by enzyme-linked immunosorbent assay (ELISA) with recombinant hCAP-18 as standard.30
Isolation of neutrophils and bone marrow (BM) cells.Neutrophils were isolated from peripheral blood by Dextran T-500 (Pharmacia, Uppsala, Sweden) induced sedimentation of erythrocytes and hypotonic lysis of residual erythrocytes present in the leukocyte rich supernatant, followed by centrifugation through Lymphoprep (Nygaard, Oslo, Norway) to separate polymorphonuclear cells from platelets and mononuclear cells.31 BM cells were obtained by aspiration from the posterior superior iliac crest from healthy volunteers. The BM was immediately anticoagulated in ACD (25 mmol/L sodium citrate, 126 mmol/L glucose) and an equal volume of 2% Dextran in saline was added. The leukocyte-rich supernatant was aspirated after sedimentation of red cells and centrifuged on top of a 2-layer Percoll (Pharmacia) gradient containing 9 mL Percoll, density 1.080, layered below 9 mL Percoll of density 1.065. This resulted in separation of BM cells into 3 bands that contain neutrophil precursors at different stages of maturation, as described previously.32
Biosynthesis of hCAP-18.Cells from the individual bands were pelleted and resuspended at 107 cells/mL in methionine and cysteine free medium (minimum essential medium Eagle [modified] with Earle's salts; GIBCO-BRL, Life Technologies Ltd, Paisley, Scotland) containing 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% (vol/vol) dialyzed fetal calf serum (FCS; GIBCO- BRL) and incubated for 30 minutes at 37°C. The cells were subsequently pelleted by centrifugation and pulsed by resuspending to a concentration of 3 × 107 cells/mL in medium as above to which [35S]-methionine/ [35S]-cysteine (Trans 35S-Label, 1211 Ci/mmol, ICN Pharmaceuticals Inc, Plainview, NY) had been added to a final concentration of 200 μCi/mL (hCAP-18 contains 2 methionines and 4 cysteines all located in the cathelin part25 ). The pulse was stopped after incubation for 60 minutes at 37°C by pelleting the cells, washing once by resuspension in saline and centrifugation, and finally resuspending the cells in RPMI-1640 (GIBCO-BRL) containing 10% dialyzed FCS at a concentration of 107 cells/mL. The cells were then incubated for 2 hours at 37°C. Then diisopropyl fluorophosphate (DFP; Sigma Chemical Co, St Louis, MO) was added to a concentration of 5 mmol/L. After 10 minutes, the cells were pelleted by centrifugation and resuspended to 107 cells/mL in radioimmuno precipitation (RIPA) buffer (150 mmol/L NaCl, 30 mmol/L HEPES, pH 7.3, 1% [vol/vol] Triton X-100 [Sigma], 1% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] sodium dodecyl sulfate [SDS], 1 mmol/L phenylmethylsulphonyl fluoride [Sigma], 200 kIE/mL aprotinin [Trasylol; Bayer, Leverkusen, Germany], 100 μg/mL leupeptin [Sigma], 1 mmol/L EGTA) and incubated for 60 minutes at 4°C. Undissolved material was pelleted by centrifugation at 100,000g for 20 minutes. The supernatants were immuno-precipitated with either antilactoferrin antibodies (DAKO A186; Dako A/S, Glostrup, Denmark); antielastase antibody (generous gift from Dr Inge Olsson, Lund University, Lund, Sweden) or with anti-hCAP-18 antibodies. The antibodies were coupled to Protein A-Sepharose gel (Pharmacia). Forty microliters of IgG coupled Protein A-Sepharose were added to 1 mL lysate or to medium from the pulse and chase, followed by incubation on ice for 2 hours. Incubation was terminated by centrifugation and the Sepharose pellets were washed four times in RIPA buffer and two times in phosphate-buffered saline (PBS). Immuno-precipitated proteins were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) by boiling the Sepharose particles in 100 μL SDS-sample buffer and electrophoresis on 12% polyacrylamide gels under reducing conditions.33 After fixation, the gels were stained by Coomassie blue and destained. Molecular weight (MW) markers were identified and marked with traces of [35S]-methionine and the gels submerged in Amplify (Amersham International, Amersham, UK) for 1 hour, dried, mounted on Kodak X-omat AR films (Eastman Kodak, Rochester, NY) with an intensifying screen, and exposed for 1 to 3 days at −80°C.
Immuno-cytochemistry.Cytospin preparations were fixed in 4% formaldehyde in 0.1 mol/L phosphate buffer pH 7.0 for 20 minutes at room temperature and permeabilized by incubation in 50 mmol/L Tris/HCl pH 7.6, 150 mmol/L NaCl (TBS) containing 1.0% Triton X-100 at room temperature for 30 min. Nonspecific binding was blocked by incubating for 10 minutes with TBS containing 1% bovine serum albumin (BSA; Sigma). Binding of primary antibody was performed during a 1-hour incubation at room temperature with hCAP-18 antibodies, diluted in TBS containing 0.25 % BSA. The slides were then washed 3 times in TBS and incubated for 1 hour with alkaline phosphatase-conjugated swine antirabbit antibodies (DAKO D 306) diluted 50-fold in TBS 0.25% BSA, washed twice in TBS and incubated in 100 mmol/L Tris/HCl pH 9.6 for 20 minutes with Fast-Red (Kem-En-Tec, Copenhagen, Denmark) as recommended by the manufacturer. After washing in running tap water, the slides were counter stained in Mayer's hematoxylin (Bie & Berntsen, Rødovre, Denmark) for 3 minutes, washed and mounted.
Release of granule proteins from intact cells.Isolated neutrophils were resuspended in Krebs Ringer phosphate buffer (KRP: 130 mmol/L NaCl, 5 mmol/L KCl, 1.27 mmol/L CaCl2 , 5 mmol/L glucose, 10 mmol/L NaH2HPO4 /Na2HPO4 , pH 7.4) to a concentration of 1.5 × 107 cells/ mL at 37°C, and stimulated with the one of the following agonists, fMLP (Sigma) at 10−8 mol/L, Ionomycin (Calbiochem, La Jolla, CA) at 1 μmol/L, or serum treated zymosan at 1 mg/mL. After 15 minutes, the stimulation was stopped by adding 2 volumes of ice-cold buffer (KRP), and the cells were pelleted by centrifugation. The supernatant was saved and the cells resuspended to an equal volume of buffer and lysed by 0.2% Triton X-100. Release of granule protein was calculated as amount present in supernatant divided by amount present in cells plus supernatant and given as a percent.
Subcellular fractionation of neutrophils.Isolated neutrophils were disrupted by nitrogen cavitation following treatment with 5 mmol/L DFP.31 The post-nuclear supernatant (10 mL), was loaded on a 3-layer Percoll density gradient (gradient volume 27 mL) and fractionated.3 Azurophil granules were identified by their content of myeloperoxidase,34 specific granules by lactoferrin,34 and gelatinase granules by gelatinase,3,35 all assayed by ELISA.3,36 Plasma membranes were identified by HLA, assayed by ELISA,37 and secretory vesicles by their content of latent alkaline phosphatase,38 assayed as described.39
Electron microscopy.All procedures were done at room temperature unless otherwise specified. The procedure has previously been described.40 Ten milliliters of heparinized blood was mixed with 2 mL of 6% Dextran in PBS and allowed to sediment for 60 minutes. The leukocyte-rich plasma was removed and centrifuged at 150g for 10 minutes. The pellet was washed once with PBS and allowed to rest for 15 minutes before fixation. The cells were fixed in 2% paraformaldehyde, 0.05% glutaraldehyde, 0.1 mmol/L sodium phosphate buffer, pH 7.4 for 1 hour. They were then either reacted first for peroxidase or directly cryoprotected, frozen, and stored in liquid nitrogen. The pellets were then cryo-sectioned and immunogold labeled as previously described.40 The ultrathin cryosections were labeled with biotinylated rabbit anti-hCAP-18 antibody, diluted 1:200, and incubated overnight at 4°C, followed by streptavidin G10 (Sigma, S-1139; Sigma) 1:50, blocked with cold protein A (P-6031; Sigma), then labeled with rabbit antilactoferrin (DAKO A-186) 1:1,000 followed by goat antirabbit G5 (Amersham RPN 420)1:50. Alternatively the following procedure was performed: The ultrathin cryosections were labeled with rabbit anti-hCAP-18 diluted 1:200 followed by goat antirabbit G15 1:50, blocked with cold protein A and avidin/biotin (SP-2001; Vector Laboratories, Burlingame, CA), and then labeled with biotinylated antilactoferrin antibody diluted 1:1,000 followed by streptavidin G10. Control experiments were also done simultaneously by omitting either the primary, secondary, or both antibodies, or replacing the antibodies with diluted rabbit serum.
RESULTS
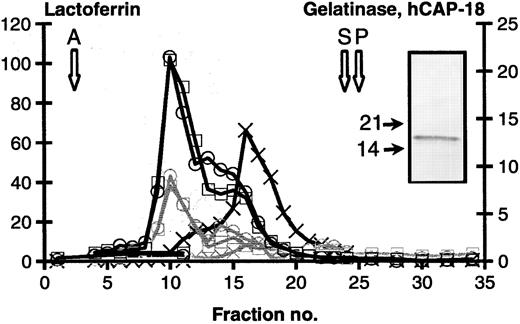
Subcellular fractionation.The localization of hCAP-18 in human neutrophils was determined by a specific and sensitive ELISA applied to fractions generated by subcellular fractionation on Percoll density gradients. These gradients can resolve most of the distinct mobilizable organelles that have been described, ie, azurophil granules identified by myeloperoxidase, specific granules identified by lactoferrin, gelatinase granules identified by a preponderance of gelatinase, and secretory vesicles identified by latent alkaline phosphatase. It should be noted that the peroxidase negative granules (specific granules and gelatinase granules) form a continuum from granules that are rich in lactoferrin and low in gelatinase to granules rich in gelatinase but low in lactoferrin, as previously demonstrated.3,40 Figure 1 shows that hCAP-18 colocalizes completely with lactoferrin, and does not show the same distribution profile as gelatinase. Another level can be added to the resolution by subcellular fractionation, if the dynamic changes in subcellular localization are investigated that occur when neutrophils are stimulated to exocytosis, since the different organelles are exocytosed in a strict hierarchy.4,41 42 When neutrophils are stimulated with 1.0 μmol/L Ionomycin, secretory vesicles and gelatinase granules are almost completely mobilized and exocytose their content whereas only 50% to 60% of specific granules, and less than 30% of azurophil granules exocytose their marker proteins. Figure 1 and Table 1 show that not only does hCAP-18 colocalize with lactoferrin, but it is also comobilized with lactoferrin. This strongly supports that hCAP-18 is localized in specific granules in human neutrophils.
Subcellular fractionation of neutrophils. Isolated neutrophils, either unstimulated (black lines) or stimulated by 1.0 μmol/L Ionomycin (gray lines) were cavitated and fractionated on a 3-layer Percoll density gradient. Fractions were collected from the bottom of the centrifuge tube and assayed for the following proteins: Myeloperoxidase (azurophil granules. Peak indicated by open arrow marked A). Lactoferrin (specific granules, □). Gelatinase (gelatinase granules, X). Latent alkaline phosphatase (secretory vesicles. Peak indicated by open arrow marked S). HLA (plasma membranes. Peak indicated by open arrow marked P) and hCAP-18 (o). Values are given as μg/mL. Insert: Immunoblot of control cells with anti-hCAP-18 antibody. MW marker indicated by solid arrows.
Subcellular fractionation of neutrophils. Isolated neutrophils, either unstimulated (black lines) or stimulated by 1.0 μmol/L Ionomycin (gray lines) were cavitated and fractionated on a 3-layer Percoll density gradient. Fractions were collected from the bottom of the centrifuge tube and assayed for the following proteins: Myeloperoxidase (azurophil granules. Peak indicated by open arrow marked A). Lactoferrin (specific granules, □). Gelatinase (gelatinase granules, X). Latent alkaline phosphatase (secretory vesicles. Peak indicated by open arrow marked S). HLA (plasma membranes. Peak indicated by open arrow marked P) and hCAP-18 (o). Values are given as μg/mL. Insert: Immunoblot of control cells with anti-hCAP-18 antibody. MW marker indicated by solid arrows.
Exocytosis of Granule Constituents in Response to Stimulation of Neutrophils
| . | Gelatinase (%) . | hCAP-18 (%) . | Lactoferrin (%) . | Myeloperoxidase (%) . |
|---|---|---|---|---|
| No addition | 4.7 | 2.3 | 2.5 | 1.5 |
| fMLP, 10−8 mol/L | 20.2 | 2.9 | 3.0 | 1.5 |
| Ionomycin, 1 μmol/L | 89.5 | 58.8 | 53.6 | 21.6 |
| STZ, 1 mg/mL | 22.9 | 7.6 | 8.8 | 3.6 |
| . | Gelatinase (%) . | hCAP-18 (%) . | Lactoferrin (%) . | Myeloperoxidase (%) . |
|---|---|---|---|---|
| No addition | 4.7 | 2.3 | 2.5 | 1.5 |
| fMLP, 10−8 mol/L | 20.2 | 2.9 | 3.0 | 1.5 |
| Ionomycin, 1 μmol/L | 89.5 | 58.8 | 53.6 | 21.6 |
| STZ, 1 mg/mL | 22.9 | 7.6 | 8.8 | 3.6 |
Exocytosis of granule constituents in response to stimulation of neutrophils. Isolated cells were incubated with or without stimulus. The amounts of granule proteins exocytosed into medium and amounts retained in cells was determined. Exocytosis is expressed as % of total amount present in cells and medium. Results are given as mean of four independent experiments except for Ionomycin stimulation where only 3 experiments were performed.
Localization of hCAP-18 in resting human neutrophils by immunogold labeling of ultrathin cryosections. (A) hCAP-18 (large gold particles) is shown to colocalize with lactoferrin (small gold particles) in many of the specific granules (arrows). Some lactoferrin positive granules did not label for hCAP-18 (arrowhead). The large dense peroxidase-positive granules (*) were rarely labeled with either antibody. Biotinylated hCAP-18 antibodies were labeled with streptavidin gold (10 nm) and lactoferrin antibodies were labeled with goat antirabbit gold (5 nm). The cells were reacted enzymatically for peroxidase before being frozen to identify the azurophil granules (*), while the specific granules were identified by immunogold labeled lactoferrin. Original magnification [OM] × 78,000. (B) Anti-hCAP-18 antibody was labeled with goat antirabbit gold (15 nm) and biotinylated antilactoferrin antibody was labeled with streptavidin gold (10 nm). No peroxidase reaction was performed before immunogold labeling. Arrows point to specific granules with both sizes of gold, and arrow heads to granules that label only for lactoferrin. OM × 77,500.
Localization of hCAP-18 in resting human neutrophils by immunogold labeling of ultrathin cryosections. (A) hCAP-18 (large gold particles) is shown to colocalize with lactoferrin (small gold particles) in many of the specific granules (arrows). Some lactoferrin positive granules did not label for hCAP-18 (arrowhead). The large dense peroxidase-positive granules (*) were rarely labeled with either antibody. Biotinylated hCAP-18 antibodies were labeled with streptavidin gold (10 nm) and lactoferrin antibodies were labeled with goat antirabbit gold (5 nm). The cells were reacted enzymatically for peroxidase before being frozen to identify the azurophil granules (*), while the specific granules were identified by immunogold labeled lactoferrin. Original magnification [OM] × 78,000. (B) Anti-hCAP-18 antibody was labeled with goat antirabbit gold (15 nm) and biotinylated antilactoferrin antibody was labeled with streptavidin gold (10 nm). No peroxidase reaction was performed before immunogold labeling. Arrows point to specific granules with both sizes of gold, and arrow heads to granules that label only for lactoferrin. OM × 77,500.
Immunoblot of whole cells showed that the protein is stored intact with an apparent MW of 18 kD. The MW calculated on basis of the deduced amino acid sequence of hCAP-18 is 16.0 kD. Thus, the molar concentration of hCAP-18 in neutrophils is equivalent to the molar concentration of lactoferrin (MW 82,000) (1.34 μmol/L hCAP-18 and 1.24 μmol/L lactoferrin in peak fraction in Fig 1).
Immuno-electron microscopy.To pinpoint the localization of hCAP-18 in neutrophils, immuno-electron microscopy was performed on frozen sections from intact cells. Triple labeling (Fig 2) shows that hCAP-18 colocalizes with lactoferrin in peroxidase negative granules. To evaluate whether the different sizes of gold particles affect the labeling of the proteins, both antigens (hCAP-18 and lactoferrin) were labeled with both sizes of gold (10 and 15 nm). Results of 25 micrographs with 10 nm gold for hCAP-18 and 15 nm gold for lactoferrin and 25 micrographs with reverse labeling are shown in Table 2. From this it can be concluded that hCAP-18 is virtually only present in granules that also contain lactoferrin, leaving no room for hCAP-18 in gelatinase granules, as also consistent with the subcellular fractionation data. It appears from the micrographs that some specific granules contain lactoferrin but no hCAP-18. It cannot be definitively concluded that this is a reflection of the true content of granules, since hCAP-18 is much smaller than lactoferrin and therefore more difficult to fix inside granules during preparation for immuno-electron microscopy, as previously noted.41 From our previous demonstration that hCAP-18 partitions into the hydrophobic phase in detergent phase separation,25 we would have expected hCAP-18 to be localized close to the membrane of granules. We found no evidence that this was the case when examined by immuno-electron microscopy (Fig 2).
Colocalization of hCAP-18 and Lactoferrin in Granules of Human Neutrophils Examined by Double Labeling Immuno-Electron Microscopy
| hCAP-18 10 nm Gold Lactoferrin 15 nm Gold . | h-CAP-18 Single Label . | Lactoferrin Single Label . | h-CAP-18 and Lactoferrin Double Label . |
|---|---|---|---|
| % of granules counted | 11.6 | 40.8 | 47.5 |
| % of gold particles counted | 6 | 21 | 73 |
| h-CAP-18 15 nm gold | |||
| Lactoferrin 10 nm gold | |||
| % of granules counted | 10.9 | 38.4 | 50.7 |
| % of gold particles counted | 3 | 34 | 63 |
| hCAP-18 10 nm Gold Lactoferrin 15 nm Gold . | h-CAP-18 Single Label . | Lactoferrin Single Label . | h-CAP-18 and Lactoferrin Double Label . |
|---|---|---|---|
| % of granules counted | 11.6 | 40.8 | 47.5 |
| % of gold particles counted | 6 | 21 | 73 |
| h-CAP-18 15 nm gold | |||
| Lactoferrin 10 nm gold | |||
| % of granules counted | 10.9 | 38.4 | 50.7 |
| % of gold particles counted | 3 | 34 | 63 |
Colocalization of hCAP-18 and lactoferrin in granules of human neutrophils examined by double labeling immuno-electron microscopy. Twenty-five micrographs of neutrophils double labeled with biotinylated hCAP-18 antibody, streptavidin gold 10 nm, followed by lactoferrin antibody and goat antirabbit gold 15 nm were examined along with 25 micrographs of neutrophils double labeled with hCAP-18 antibody, goat antirabbit gold 15 nm, followed by biotinylated lactoferrin antibody, streptavidin gold 10 nm. Only labeled granules were counted.
Biosynthesis of hCAP-18.We have previously shown that the proteins that serve as markers for the different granule subsets, azurophil granules, specific granules, and gelatinase granules, are synthesized at different stages of maturation of the neutrophil precursors.32 Based on this, we have proposed that there is no individual targeting of proteins to different granule subsets, and that granule proteins that are synthesized at the same time will localize to the same granule. The differences in content of the granule subsets are thus explained by differences in the timing of biosynthesis in relation to myeloid cell maturation. This was further substantiated by showing that NGAL, which is localized in specific granules in normal neutrophils became localized to azurophil granules when expressed in undifferentiated HL-60 cells.43
To determine the stage of maturation at which neutrophil precursors synthesize hCAP-18, we performed biosynthesis studies on cells isolated from normal human BM and separated on density gradients. We have previously shown that this will result in separation of myeloid cells at various stages of maturation.32 Band 1 contains exclusively band cells and segmented cells. Band 2 contains mainly myelocytes and metamyelocytes, while band 3 contains the immature myeloid cells, myeloblasts, and promyelocytes in addition to some of the more mature cells. The quality of separation was always checked by examination of a May-Grünewald-Giemsa stained cytospin preparation from each band. It is observed that hCAP-18 and lactoferrin are mainly synthesized in cells from band 2, while elastase, a constituent of azurophil granules, is largely synthesized in cells from band 3 (Fig 3). Furthermore, it is seen that hCAP-18 was synthesized as an 18-kD protein consistent with the molecular weight of the protein found in mature granules of peripheral blood neutrophils. No forms with a larger molecular weight could be observed in agreement with the rapid removal of signal peptides from newly synthesized proteins. If care was not taken to prevent proteolysis during immunoprecipitation, a major part of the hCAP-18 was found at a lower molecular weight corresponding to the cathelin-like part of hCAP-18. This proteolysis was prevented by treatment of cells with the serine protease inhibitor DFP after the biosynthetic pulse and chase, thus clearly showing that the degradation is an artifact due to free access of serine proteases to hCAP-18 during immunoprecipitation. The identity of the faint band immediately below hCAP-18 in Fig 3, lane 2 is unknown.
Biosynthesis of hCAP-18. BM cells were separated into myeloblasts and promyelocytes (band 3), myelocytes and metamyelocytes (band 2), and band cells and segmented cells (band 1), pulsed with [35S]-methionine/cysteine, washed, chased, and lyzed. The lysates were immuno-precipitated with antielastase, antilactoferrin, and anti-hCAP-18 antibodies. MW markers are indicated by solid arrows.
Biosynthesis of hCAP-18. BM cells were separated into myeloblasts and promyelocytes (band 3), myelocytes and metamyelocytes (band 2), and band cells and segmented cells (band 1), pulsed with [35S]-methionine/cysteine, washed, chased, and lyzed. The lysates were immuno-precipitated with antielastase, antilactoferrin, and anti-hCAP-18 antibodies. MW markers are indicated by solid arrows.
The efficiency of targeting to granules was assessed by precipitating hCAP-18 from the medium during pulse and chase. The amount of hCAP-18 that escaped targeting to granules and was recovered from medium was less than 5% of the amount that was recovered from cells, indicating that targeting to granules is efficient, although the mechanisms by which targeting occurs are unknown (data not shown).
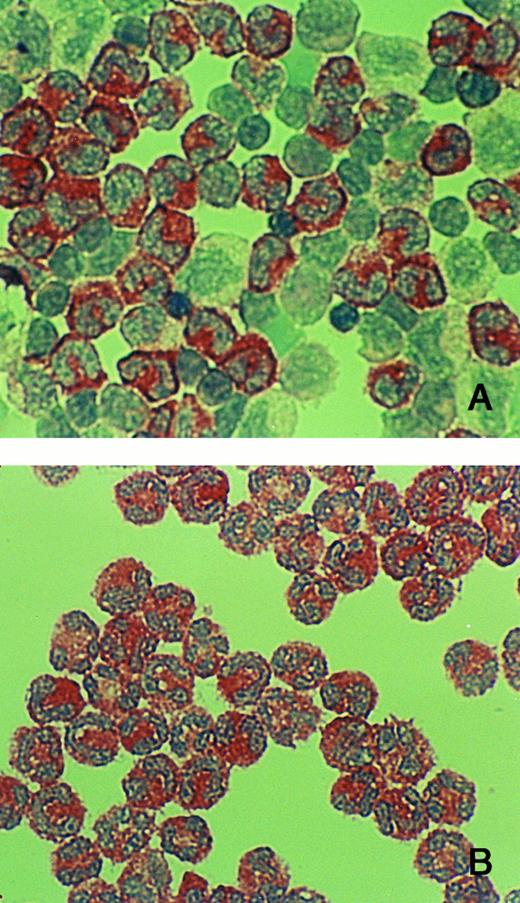
The biosynthesis studies agree with the immunohistochemistry on isolated BM cells, which show that hCAP-18 appears in metamyelocytes (Fig 4).
Immuno-cytochemistry of cells from BM. (A) Cells from band 2 (mainly myelocytes, and metamyelocytes, and some band cells). (B) Cells from band 1 (mainly band cells and segmented cells). Cytospin preparations were immuno-reacted with anti-hCAP-18 antibody. hCAP-18 starts to appear in metamyelocytes and is prominent in all more mature cells (band cells and segmented cells).
Immuno-cytochemistry of cells from BM. (A) Cells from band 2 (mainly myelocytes, and metamyelocytes, and some band cells). (B) Cells from band 1 (mainly band cells and segmented cells). Cytospin preparations were immuno-reacted with anti-hCAP-18 antibody. hCAP-18 starts to appear in metamyelocytes and is prominent in all more mature cells (band cells and segmented cells).
DISCUSSION
Cathelicidins are proantibacterial proteins of neutrophils from a variety of mammalian species. The antibacterial activity is confined to the C-terminal parts that differ greatly among the different cathelicidins, but share the characteristic features of being highly positively charged (cationic) and containing a high proportion of hydrophobic amino acids.17 These features are believed to be essential for the antibacterial activity of cathelicidins. For those that form amphipathic α-helices, such as hCAP-18,44 the positively charged side is believed to be essential for binding to bacterial phospholipids (and to the specialized phospholipids, endotoxins) while the hydrophobic part may be essential for insertion into the bacterial membrane. In accordance with this, the cathelin-like part that unites these proteins into one family, the cathelicidins, partly neutralizes the positive charge of the C-termini (pI of the cathelin moiety of hCAP 18 is 6.38 while the pI of the C-terminal 37 amino acids is 11.3, and the pI of the holoprotein is 9.63) and blocks their antimicrobial activity.17 It has recently been shown that the bactericidal peptides derived from cathelicidins may be toxic also to eukaryotic cells.45 Therefore, it is possible that the cathelin-like segments are needed for protection against damage to the granules in which the cathelicidins are stored. In addition, it is possible that the cathelin-part is important for trafficking through the synthetic apparatus (ER, Golgi complex and trans-Golgi network), since charge neutralizing segments are found in other cationic granule proteins (defensins) although these are removed after exit from the trans-Golgi network.46-48
Elastase has been shown to remove the cathelin-like part of some cathelicidins.29 It is possible that elastase may also be responsible for the proteolysis that we observed in the absence of effective serine protease inhibitors. We can show that proteases from azurophil granules are capable of digesting hCAP-18 (data not shown). Therefore, it is most likely that one major reason for targeting hCAP-18 to specific granules, as opposed to the azurophil granules where most other antibacterial proteins are located, is the need for separating hCAP-18 from the proteases that will release the antibacterial and possible cytocidal activity of the protein.
The localization of hCAP-18 in specific granules is explained by its time of biosynthesis according to our hypothesis of targeting-by-timing. In accordance with this, we found hCAP-18 and lactoferrin to be synthesized by cells at the same stage of maturation, as opposed to elastase which was synthesized in more immature cells. The gene for hCAP-18 has recently been sequenced.26,27 The region 5′-flanking the coding sequence contains a binding site for the transcription factor PU.1, a TATA-like sequence, and a CAAT− but no CCAAT sequence. PU.1 binding sites and CCAAT sequences are found in genes for other proteins localized to the same granules and synthesized at the same time as hCAP-18, eg, lactoferrin,49 CD18,50,51 gp91phox,52 and NGAL (Cowland J.B., and Borregaard N., EMBL database, acc. no. X99133). CCAAT-displacement protein is believed to be a coordinator of the expression of specific granule proteins.53 Overexpression of CCAAT-displacement protein has recently been shown to silence multiple specific granule proteins in a mouse model.54 It is possible that several of the proteins localized to specific granules are controlled by distinct transcription factors although transcription is coordinated during myelopoiesis. This may explain subtle differences in the distribution of these granule proteins within the population of peroxidase negative granules, and may furthermore explain a differential expression of these proteins in other tissues under conditions of stress, such as inflammation. NGAL has been shown to be synthesized by epithelial cells of the gastrointestinal tract during inflammation,55 and hCAP-18 was recently shown to be synthesized by keratinocytes in patients with psoriasis and contact dermatitis.56
hCAP-18 is present in specific granules of human neutrophils at a molar concentration as high as lactoferrin, the protein that dominates specific granules on an mg protein basis. This suggests that hCAP-18 plays an essential role, most probably in host defense, but possible also in wound repair as has been shown for another cathelicidin, PR39.57 The lack of a multitude of cathelicidins in human neutrophils, as opposed to neutrophils from other species, is most likely compensated by the high amount present in human neutrophils. Unfortunately, quantitative data on cathelicidins in other species is not available.
ACKNOWLEDGMENT
The expert technical assistance of Charlotte Horn and Ivy E. Hsieh is greatly appreciated.
Address reprint requests to Ole Sørensen, MD, The Granulocyte Research Laboratory, Rigshospitalet L-4042, 9 Blegdamsvej, DK-2100 Copenhagen Ø, Denmark.

![Fig. 2. Localization of hCAP-18 in resting human neutrophils by immunogold labeling of ultrathin cryosections. (A) hCAP-18 (large gold particles) is shown to colocalize with lactoferrin (small gold particles) in many of the specific granules (arrows). Some lactoferrin positive granules did not label for hCAP-18 (arrowhead). The large dense peroxidase-positive granules (*) were rarely labeled with either antibody. Biotinylated hCAP-18 antibodies were labeled with streptavidin gold (10 nm) and lactoferrin antibodies were labeled with goat antirabbit gold (5 nm). The cells were reacted enzymatically for peroxidase before being frozen to identify the azurophil granules (*), while the specific granules were identified by immunogold labeled lactoferrin. Original magnification [OM] × 78,000. (B) Anti-hCAP-18 antibody was labeled with goat antirabbit gold (15 nm) and biotinylated antilactoferrin antibody was labeled with streptavidin gold (10 nm). No peroxidase reaction was performed before immunogold labeling. Arrows point to specific granules with both sizes of gold, and arrow heads to granules that label only for lactoferrin. OM × 77,500.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/7/10.1182_blood.v90.7.2796/3/m_bl_0027f2.jpeg?Expires=1765993816&Signature=KfOyWG3phTuFHFJI~21u7AL18Ly6Ung5fNywLKE-rkoRa9XIWOvpxsSyn7HWkWMIgrEOJr6CNGOzIi3lyLwwRfD37H~QgD28lGaejJZI3iXiDlFcV0P-9k61jqzx7xs8pz~seg0v5AsxEM6uWtu0ppseWdTlFkhcaz1DJYfLohJkUv0SLlZaJkj~rR7br46wtKWkIU0sAVvU4icMJSRc~12MShKPcLrlz8OuQJ4prcU-ub3ynyeZdmeTAMYm3sraY2eakA8eE0JXHouaY3EIvggY~lPAOmMAjbljO~mkc9FS4M9yqM8kNm-Olr2wvgE4R06kg5qn9o8oYsXw5vi3Xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Biosynthesis of hCAP-18. BM cells were separated into myeloblasts and promyelocytes (band 3), myelocytes and metamyelocytes (band 2), and band cells and segmented cells (band 1), pulsed with [35S]-methionine/cysteine, washed, chased, and lyzed. The lysates were immuno-precipitated with antielastase, antilactoferrin, and anti-hCAP-18 antibodies. MW markers are indicated by solid arrows.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/7/10.1182_blood.v90.7.2796/3/m_bl_0027f3.jpeg?Expires=1765993816&Signature=ciH6AAZaAfCzGgIEGUF5~k6xh201dYxSkaeN~cmjUQ4LORsrpY09mewZtUZalO1N88fwZwzlvlyKmmBIgd~1uyY6UqURuxm2CrMorfjXAwX2fCdkD~xQJOtlbhyR9R2kMEqA0EX3lmmx5aydo99HT742KPXVl5TEOhK2Gb2e9GgPFX9neUr5wOgHpZacNPG~6zd72Mm2a2Db0yKE0MF4SjrqHRbo5MrfLS6QATc6r1dPIePqwD02P-qv3T0tPTSVeZyGegS4Rlnl4Oaz90vPsC8L9hLH0HK9hssUmp4~9tGIBe3~V6l4qZYK1xn~316~7R6oXxHqg4YbMtJdLEdN-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal