Abstract
As an important determinant of the response to chemotherapy, measurements of cellular drug resistance may provide prognostically significant information, which could be useful for optimal risk-group stratification. The objective of this report is to determine the relation between in vitro resistance to 12 drugs, measured with the colorimetric methyl-thiazol-tetrazolium (MTT) assay, and long-term clinical response to chemotherapy in 152 children with newly diagnosed acute lymphoblastic leukemia. At risk-group stratified analyses, in vitro resistance to prednisolone, L-asparaginase, and vincristine were each significantly (P < .01) related to the probability of disease-free survival (pDFS) after combination chemotherapy. The combination of data for prednisolone, L-asparaginase, and vincristine provided a drug-resistance profile with prognostic independent significance superior to that of any single drug or any other factor. The 3-years pDFS was 100% for the group with the most sensitive profile, 20% of all patients, 84% (SE 6%) for the group with an intermediately sensitive profile, 40% of all patients, and 43% (SE 8%) for the remaining group with the most resistant profile (P < .001). In conclusion, the extent of in vitro cellular resistance to prednisolone, L-asparaginase, and vincristine, measured using the MTT assay, was significantly related to the clinical response to combination chemotherapy. Treatment failure in newly diagnosed childhood ALL can be predicted based on cellular drug resistance data.
IN VITRO cell culture drug resistance assays have a number of potentially valuable applications in leukemias, as well as in other malignancies. Although they are already being used for drug screening, there is a reluctance to use these assays for selection of patients for phase II trials or adjuvant chemotherapy, for rational improvements in current, mostly empirically derived, chemotherapeutic regimens, for risk-group stratification, and for individualized tailored therapy.1 This may be explained by the fact that very few studies have been reported in which the feasibility and successfulness of assay-selected chemotherapy were shown. However, the studies in which assay-selected and clinician-selected therapy were compared did show a difference in treatment outcome in favor of assay-selected therapy for patients with solid tumors2-6 and leukemias.7
Other studies have shown the clinical relevance of in vitro drug resistance assays. Firstly, in vitro drug resistance profiles of different leukemia types reflect the empirically known clinical effectiveness of the different drugs in these subtypes.8-11 Secondly, relapsed leukemia samples are more drug resistant in vitro than untreated samples, in agreement with clinical experience.9,11-13 Finally, a large number of studies have reported significant correlations between results of in vitro drug resistance testing and the clinical outcome in childhood leukemia7,12,14-17 and adult leukemia.11 18-24 However, these studies were retrospective, often concerned small and heterogeneous patient-groups, and tested few drugs.
The purpose of the present study was to determine the relation between in vitro resistance to each of 12 single drugs and the clinical outcome after combination chemotherapy in a large number of consecutively diagnosed children with acute lymphoblastic leukemia (ALL). In vitro drug resistance was assessed using the colorimetric methyl-thiazol-tetrazolium (MTT) assay.25 26
MATERIALS AND METHODS
Patients and patient samples.Eligible patients were children (age, 0 to 18 years) with non-B ALL, newly diagnosed between 1989 and 1994. Bone marrow (BM) and peripheral blood (PB) samples and smears of 306 consecutive patients were sent by local institutions to the Dutch Childhood Leukemia Study Group (DCLSG) laboratory for confirmation of the diagnosis ALL, French-American-British (FAB) classification,27 and immunophenotyping as recently described.28 Precursor-B ALL (HLA-DR+, CD19+) was divided in pro-B (CD10−, cytoplasmic μ [cμ]−), common (CD10+, cμ−), and pre-B (CD10+ or CD10−, cμ+). Fresh samples with sufficient cells from 190 children were sent by the DCLSG laboratory to the research laboratory for pediatric hemato-onco-immunology of the Free University Hospital in Amsterdam for in vitro drug-resistance testing. In the remaining 116 cases material was not available for experimental studies, or the DCLSG decided to use it for other studies which had a higher priority at that time. Results for prednisolone from 93 of these 190 samples have been presented in a previous preliminary meeting report limited to the short-term response to prednisolone only.29 Based on previous DCLSG studies, hyperdiploidy was defined as a DNA-index of 1.16 to 1.35 (to define a group with a good prognosis), and nonhyperdiploidy as a DNA-index of <1.16 or >1.35, as determined by flow cytometry.30
In vitro drug resistance.This was measured with the cell culture MTT assay.25 BM and PB ALL samples were evaluated together, because they do not differ in drug resistance.31 Fresh (noncryopreserved) leukemic cells were cultured in RPMI 1640 (Dutch modification; GIBCO, Uxbridge, UK), containing fetal calf serum and other supplements.25
Twelve drugs were tested, each at six concentrations as described previously.8 Methotrexate was not included in the panel because this drug is not cytotoxic to human leukemia samples in nonclonogenic assays.25,31 Leukemic cells were incubated with each drug at each concentration in duplicate in wells of microculture plates at 37°C in humidified air with 5% CO2 . Six wells contained leukemic cells in drug-free medium to determine the control cell survival and the percentage of leukemic cells after culture. Six wells contained medium only to blank the spectrophotometer. After 4 days, 10 μL (5 mg/mL) MTT salt (Sigma Chemical Corp, St Louis, MO) was added for 6 hours. MTT is reduced to colored formazan crystals by living cells only. The crystals were dissolved with 100 μL of acidified isopropanol, and formazan production was quantitated using a spectrophotometer at 562 nm. The optical density (OD) is linearily related to the cell number.32 Leukemic cell survival (LCS) was calculated at each drug concentration by the equation LCS = (OD treated well/mean OD control wells) × 100%. The drug concentration lethal to 50% of the ALL cells (the LC50) was used as the measure of resistance. Samples were considered evaluable if the drug-free control wells contained ≥80% leukemic cells before and ≥70% leukemic cells after 4 days of culture and if the control optical density at day 4 exceeded 0.050. The MTT assay gives reliable results under these conditions.25,33,34 The percentage of leukemic cells was determined by May-Grünwald-Giemsa staining and light microscopy. The coefficient of variation of the optical density of the control wells in the successful assays is a median of 5.2% (range, 0.9% to 15.3%). The intra-assay variation (duplicates) and interassay variation (repeated testing of frozen sample) in LC50 values for all drugs are well within one dilution step.8
Treatment.Patients were treated according to DCLSG protocols (ALL-VII and ALL-VIII), which are based on treatment principles of the International Berlin-Frankfurt-Münster (BFM) Study Group, but without cranial irradiation. All patients first received a 1-week systemic monotherapy with prednisolone (60 mg/m2/d), and 1 injection with methotrexate intrathecally at day 1 of the prednisolone window. Patients were then stratified into one of three risk groups (and received risk-adapted treatment): (1) low risk in case of a BFM risk factor (based on peripheral leukemic cell count, and liver and spleen size)35 of <0.8, excluding patients with mediastinal enlargement, T-cell lineage ALL, extramedullary disease (including central nervous system [CNS] leukemia), and/or features as described for high-risk patients; (2) medium risk in case of a BFM risk factor of >0.8, and/or patients with mediastinal enlargement, T-cell lineage ALL, and/or extramedullary disease, but excluding patients with features as described for the high-risk group; (3) high risk, independent of BFM risk factor, in case of immunophenotypically acute undifferentiated leukemia, poor clinical response to the systemic prednisolone monotherapy (≥1,000 leukemic cells/μL PB at day 8), no complete remission (CR) after 33 days of induction chemotherapy, and/or presence of t(4; 11), t(9; 22) or bcr-abl rearrangement. All drugs included in the in vitro panel were used in the treatment of at least some of the patients. The following drugs of our panel were used in all patients: prednisolone, vincristine, L-asparaginase, daunorubicin, cytarabine, and mercaptopurine.
Treatment outcome.The results of treatment were evaluated at the DCLSG operations office, using 3-monthly progress reports from the treating clinicians. Routine BM, PB, and cerebrospinal fluid examinations were performed at the central laboratory of the DCLSG during chemotherapy and up to 3 years after cessation of therapy.
CR was defined as less than 5% leukemic blasts in representative BM containing megakaryocytes and granulocytic precursors with some degree of maturation, and no manifestation of leukemia elsewhere. Failure to achieve CR after 33 days of induction chemotherapy (induction failure) was considered an event at day 0. Early death was defined as death before completion of induction therapy. Event-free survival (EFS) was defined as the time from diagnosis to induction failure, relapse, second malignancy, or death. Disease-free survival (DFS) was defined as the time from diagnosis to induction failure or relapse (leukemia-related events). For estimation of DFS, toxic deaths in remission were censored at the time of occurrence and early deaths at day 0. For analyses of both EFS and DFS, patients who were event- and disease-free, respectively, were censored at the time of latest follow-up as evaluated at this planned analysis.
Statistics.Differences in distribution of variables were tested with the Mann-Whitney U (MWU) test or the chi-squared test. Estimates of the probability (P ) of EFS and DFS (with standard errors, SE) were calculated according to the Kaplan-Meier product limit analysis.36 Because toxic and early deaths presumably are unrelated to cellular drug resistance, in contrast to induction failures and relapses, results of DFS analysis will be emphasized. Univariate and multivariate statistical comparisons of outcome were conducted by proportional hazard Cox regression analysis, after stratification for risk group.37 The model for multivariate analysis included the conventional prognostic factors age, BFM risk factor, immunophenotype and DNA ploidy, and in vitro drug resistance. Information on karyotype was not available for the majority of patients and therefore was not included in this analysis. The analyses were two-tailed at a significance level of 1%.
RESULTS
Material was available for cellular drug-resistance testing from 190 out of 306 patients consecutively treated according to DCLSG protocols. These 190 patients were more likely to have high leukemic cell burden (white blood cell count [WBC], BFM risk factor), T-cell ALL, and a lower pDFS compared with 116 patients from whom material was not received (Table 1). In 152 (80%) of 190 patient samples, at least one drug (depending on the amount of material available) was successfully tested. Table 2 shows the characteristics of these 152 patients, and the events that occurred so far, at a median follow-up of 46 months for patients at risk (range, 17 to 81 months). In the remaining 38 patients (Table 2) information on in vitro drug resistance was not obtained, because samples were not suitable for testing because of a lack of cells or a low percentage of ALL cells (n = 9), or because of assay failures: infection (n = 1), too low of an OD signal in drug-free control cultures (n = 12), or too low (<70%) of a percentage of ALL cells in drug-free control cultures after 4 days (n = 16). These 38 patients were more likely to have DNA hyperdiploidy, a low leukemic cell burden, and a higher pDFS than the 152 successfully tested cases (Table 2). For the latter samples, the percentage of ALL cells of the total number of viable cells was median 93% (range, 80% to 100%) at the start of the MTT assay, and median 89% (range, 70% to 99%) after 4 days of culture in drug-free control wells. Survival of the ALL cells in drug-free cultures at day 4 compared to day 0 was median 62%. Control cell survival was not related to the clinical outcome to combination chemotherapy. Patients who did not obtain CR or who suffered a relapse had a median control cell survival of 63% (range, 29% to 135%), the complementary group had a median of 60% (range, 13% to 154%) (P = .32). Dividing patients into four equally large groups based on control cell survival (from low to high) also did not identify a subgroup of patients with a significantly different pDFS.
Characteristics of 306 Children With Newly Diagnosed ALL Treated According to DCLSG Protocols VII or VIII, Divided in Patients of Whom We Did (n = 190) or Did Not (n = 116) Receive Material for In Vitro Cellular Drug-Resistance Testing
| . | Material Received . | Material Not Received . | P Value . |
|---|---|---|---|
| No. of patients | 190 | 116 | |
| Age in mo, median (range) | 58 (2-190) | 49 (1-215) | .13 |
| Female/male ratio | 79:111 | 60:56 | .08 |
| WBC × 109/L, median (range) | 19.5 (0.5-746) | 6.5 (0.3-481) | <.0001 |
| BFM risk factor,* median (range) | 0.99 (0-2.05) | 0.78 (0-1.97) | <.0001 |
| FAB type | .07 | ||
| L1 | 155 | 102 | |
| L2 | 33 | 12 | |
| AUL† | 2 | 0 | |
| Unknown | 0 | 2 | |
| Immunophenotype | .002 | ||
| Pro-B | 9 | 4 | |
| Common | 99 | 70 | |
| Pre-B | 45 | 25 | |
| T | 36 | 9 | |
| Unknown | 1 | 8 | |
| DNA ploidy | .34‡ | ||
| Nonhyperdiploid | 137 | 40 | |
| Hyperdiploid | 34 | 14 | |
| Unknown | 19 | 62 | |
| DCLSG protocol VII/VIII | 100/90 | 62/54 | .89 |
| Risk group | <.001 | ||
| Low | 54 | 58 | |
| Medium | 115 | 53 | |
| High | 21 | 5 | |
| Follow-up, median (range) (in mo) | 47 (17-81) | 51 (31-77) | .32 |
| Events | .09 | ||
| No CR | 1 | 1 | |
| Relapse | 49 | 20 | |
| Early death | 2 | 0 | |
| Toxic death | 4 | 3 | |
| No event | 134 | 92 | |
| pDFS (%) | .05 | ||
| 3 yr (SE) | 76 (4) | 84 (4) | |
| 4 yr (SE) | 71 (5) | 80 (5) |
| . | Material Received . | Material Not Received . | P Value . |
|---|---|---|---|
| No. of patients | 190 | 116 | |
| Age in mo, median (range) | 58 (2-190) | 49 (1-215) | .13 |
| Female/male ratio | 79:111 | 60:56 | .08 |
| WBC × 109/L, median (range) | 19.5 (0.5-746) | 6.5 (0.3-481) | <.0001 |
| BFM risk factor,* median (range) | 0.99 (0-2.05) | 0.78 (0-1.97) | <.0001 |
| FAB type | .07 | ||
| L1 | 155 | 102 | |
| L2 | 33 | 12 | |
| AUL† | 2 | 0 | |
| Unknown | 0 | 2 | |
| Immunophenotype | .002 | ||
| Pro-B | 9 | 4 | |
| Common | 99 | 70 | |
| Pre-B | 45 | 25 | |
| T | 36 | 9 | |
| Unknown | 1 | 8 | |
| DNA ploidy | .34‡ | ||
| Nonhyperdiploid | 137 | 40 | |
| Hyperdiploid | 34 | 14 | |
| Unknown | 19 | 62 | |
| DCLSG protocol VII/VIII | 100/90 | 62/54 | .89 |
| Risk group | <.001 | ||
| Low | 54 | 58 | |
| Medium | 115 | 53 | |
| High | 21 | 5 | |
| Follow-up, median (range) (in mo) | 47 (17-81) | 51 (31-77) | .32 |
| Events | .09 | ||
| No CR | 1 | 1 | |
| Relapse | 49 | 20 | |
| Early death | 2 | 0 | |
| Toxic death | 4 | 3 | |
| No event | 134 | 92 | |
| pDFS (%) | .05 | ||
| 3 yr (SE) | 76 (4) | 84 (4) | |
| 4 yr (SE) | 71 (5) | 80 (5) |
A measure for the leukemic cell burden, based on peripheral leukemic cell count, and liver and spleen size.35
Acute undifferentiated leukemia.
Excluding cases with unknown DNA ploidy.
Characteristics of 190 Children With Newly Diagnosed ALL of Whom Material Was Received for In Vitro Cellular Drug-Resistance Testing, Divided in Patients in Whom Such Testing Was Successful (n = 152) and in Whom It Was Not (n = 38)
| . | Assay Successful . | Assay Unsuccessful . | P Value . |
|---|---|---|---|
| No. of patients | 152 | 38 | |
| Age in mo, median (range) | 61 (2-190) | 48 (15-178) | .17 |
| Female/male ratio | 65:87 | 14:24 | .51 |
| WBC × 109/L, median (range) | 22.9 (0.5-746) | 8.5 (1.3-178) | .007 |
| BFM risk factor,* median (range) | 1.06 (0.31-2.03) | 0.85 (0-2.05) | .001 |
| FAB type | .44 | ||
| L1 | 123 | 32 | |
| L2 | 28 | 5 | |
| AUL† | 1 | 1 | |
| Immunophenotype | .40 | ||
| Pro-B | 6 | 3 | |
| Common | 77 | 23 | |
| Pre-B | 38 | 7 | |
| T | 31 | 5 | |
| DNA ploidy | <.0001‡ | ||
| Nonhyperdiploid | 119 | 18 | |
| Hyperdiploid | 18 | 16 | |
| Unknown | 15 | 4 | |
| DCLSG protocol VII/VIII | 80/72 | 20/18 | 1.0 |
| Risk group | .03 | ||
| Low | 38 | 16 | |
| Medium | 99 | 16 | |
| High | 15 | 6 | |
| Follow-up, median (range) (in mo) | 46 (17-81) | 51 (29-81) | .77 |
| Events | .04 | ||
| No CR | 1 | 0 | |
| Relapse | 44 | 5 | |
| Early death | 2 | 0 | |
| Toxic death | 3 | 1 | |
| No event | 102 | 32 | |
| pDFS (%) | .04 | ||
| 3 yr (SE) | 73 (4) | 89 (6) | |
| 4 yr (SE) | 68 (6) | 85 (8) |
| . | Assay Successful . | Assay Unsuccessful . | P Value . |
|---|---|---|---|
| No. of patients | 152 | 38 | |
| Age in mo, median (range) | 61 (2-190) | 48 (15-178) | .17 |
| Female/male ratio | 65:87 | 14:24 | .51 |
| WBC × 109/L, median (range) | 22.9 (0.5-746) | 8.5 (1.3-178) | .007 |
| BFM risk factor,* median (range) | 1.06 (0.31-2.03) | 0.85 (0-2.05) | .001 |
| FAB type | .44 | ||
| L1 | 123 | 32 | |
| L2 | 28 | 5 | |
| AUL† | 1 | 1 | |
| Immunophenotype | .40 | ||
| Pro-B | 6 | 3 | |
| Common | 77 | 23 | |
| Pre-B | 38 | 7 | |
| T | 31 | 5 | |
| DNA ploidy | <.0001‡ | ||
| Nonhyperdiploid | 119 | 18 | |
| Hyperdiploid | 18 | 16 | |
| Unknown | 15 | 4 | |
| DCLSG protocol VII/VIII | 80/72 | 20/18 | 1.0 |
| Risk group | .03 | ||
| Low | 38 | 16 | |
| Medium | 99 | 16 | |
| High | 15 | 6 | |
| Follow-up, median (range) (in mo) | 46 (17-81) | 51 (29-81) | .77 |
| Events | .04 | ||
| No CR | 1 | 0 | |
| Relapse | 44 | 5 | |
| Early death | 2 | 0 | |
| Toxic death | 3 | 1 | |
| No event | 102 | 32 | |
| pDFS (%) | .04 | ||
| 3 yr (SE) | 73 (4) | 89 (6) | |
| 4 yr (SE) | 68 (6) | 85 (8) |
A measure for the leukemic cell burden, based on peripheral leukemic cell count, and liver and spleen size.35
Acute undifferentiated leukemia.
Excluding cases with unknown DNA ploidy.
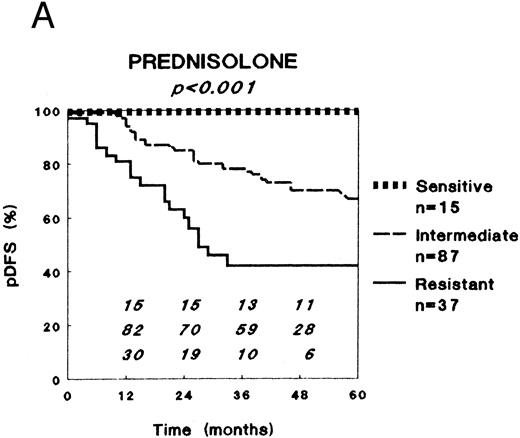
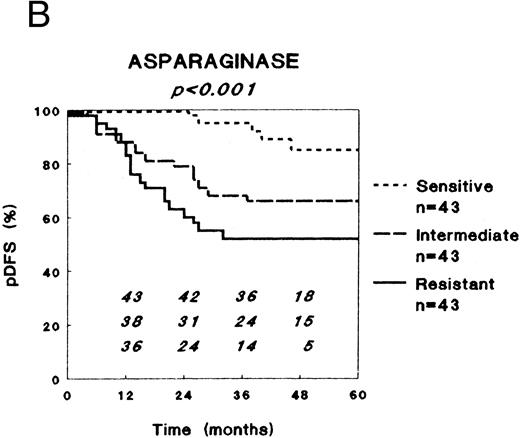
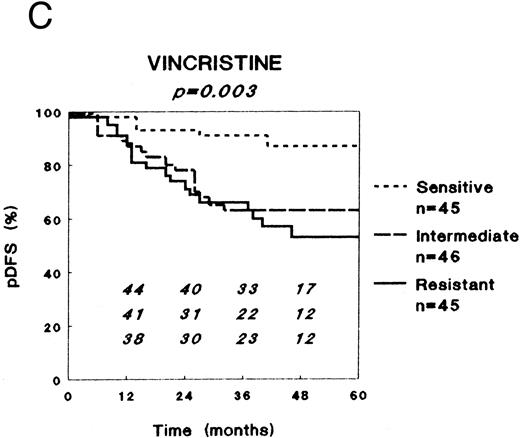
In vitro drug resistance versus clinical outcome after combination chemotherapy.LC50 values varied markedly between the patient samples for all drugs. For each single drug, except prednisolone, patients were classified in three equally large groups (to avoid response-driven cut-off points) as sensitive (33% lowest LC50 values), intermediately sensitive (33% intermediate LC50 values), or resistant (33% highest LC50 values). For prednisolone these three groups were defined using the cut-off values obtained in our retrospective study (0.1 and 150 μg/mL) to prospectively confirm those results, and because the LC50 values for prednisolone show a rather skewed distribution.16
For all drugs DFS Kaplan-Meier curves were plotted, as shown for prednisolone, L-asparaginase, and vincristine in Fig 1. Table 3 shows the pDFS at 3 years for sensitive, intermediately sensitive, and resistant patients for each drug. At univariate analysis (stratified for risk group), in vitro resistance to prednisolone, L-asparaginase, and vincristine were of prognostic significance regarding the pDFS. The extent of in vitro resistance to dexamethasone, vindesine, daunorubicin, doxorubicin, mitoxantrone, mercaptopurine, thioguanine, cytarabine, and teniposide was not of prognostic significance. Of the other factors studied, only age and leukemic cell burden as expressed by the BFM risk factor had (borderline) prognostic significance at univariate analyses, whereas sex, WBC, FAB type, immunophenotype, and DNA ploidy had not. With respect to EFS, very similar results were found (data not shown), as was the case for analyses stratified for treatment protocol.
Relation between in vitro drug resistance and probability of DFS (pDFS) in newly diagnosed childhood ALL, for (A) prednisolone, (B) L-asparaginase, and (C) vincristine. The numbers in the figures along the X-axis indicate the patients at risk at the different time-points.
Relation between in vitro drug resistance and probability of DFS (pDFS) in newly diagnosed childhood ALL, for (A) prednisolone, (B) L-asparaginase, and (C) vincristine. The numbers in the figures along the X-axis indicate the patients at risk at the different time-points.
The Relation Between In Vitro Drug Resistance and Probability of DFS (pDFS) 3 Years From Diagnosis, in 152 Children With Newly Diagnosed ALL
| Drug . | 3-yr pDFS (%) With SE in Parentheses . | P Value3-150 . | ||
|---|---|---|---|---|
| . | Sensitive . | Intermediate . | Resistant . | . |
| Prednisolone | 100 | 78 (5) | 42 (10) | <.001 |
| Dexamethasone | 88 (5) | 70 (8) | 60 (9) | .012 |
| L-asparaginase | 95 (4) | 68 (8) | 52 (10) | <.001 |
| Vincristine | 91 (5) | 63 (8) | 66 (8) | .003 |
| Vindesine | 82 (7) | 67 (9) | 72 (8) | .096 |
| Daunorubicin | 79 (7) | 74 (8) | 64 (9) | .66 |
| Doxorubicin | 79 (8) | 79 (9) | 61 (9) | .052 |
| Mitoxantrone | 77 (8) | 78 (8) | 63 (9) | .12 |
| Mercaptopurine | 81 (7) | 78 (8) | 63 (9) | .17 |
| Thioguanine | 84 (7) | 71 (8) | 68 (8) | .58 |
| Cytarabine | 72 (8) | 82 (7) | 63 (9) | .32 |
| Teniposide | 79 (8) | 72 (9) | 69 (9) | .48 |
| Resistance profile3-151 | 100 | 84 (6) | 43 (8) | <.001 |
| Drug . | 3-yr pDFS (%) With SE in Parentheses . | P Value3-150 . | ||
|---|---|---|---|---|
| . | Sensitive . | Intermediate . | Resistant . | . |
| Prednisolone | 100 | 78 (5) | 42 (10) | <.001 |
| Dexamethasone | 88 (5) | 70 (8) | 60 (9) | .012 |
| L-asparaginase | 95 (4) | 68 (8) | 52 (10) | <.001 |
| Vincristine | 91 (5) | 63 (8) | 66 (8) | .003 |
| Vindesine | 82 (7) | 67 (9) | 72 (8) | .096 |
| Daunorubicin | 79 (7) | 74 (8) | 64 (9) | .66 |
| Doxorubicin | 79 (8) | 79 (9) | 61 (9) | .052 |
| Mitoxantrone | 77 (8) | 78 (8) | 63 (9) | .12 |
| Mercaptopurine | 81 (7) | 78 (8) | 63 (9) | .17 |
| Thioguanine | 84 (7) | 71 (8) | 68 (8) | .58 |
| Cytarabine | 72 (8) | 82 (7) | 63 (9) | .32 |
| Teniposide | 79 (8) | 72 (9) | 69 (9) | .48 |
| Resistance profile3-151 | 100 | 84 (6) | 43 (8) | <.001 |
Classified as defined in the text.
Univariate analysis stratified for risk group.
Combined results for prednisolone, L-asparaginase, and vincristine.
In vitro drug resistance profile as prognostic factor.We next combined the data of the drugs that all patients had received during induction treatment and that were each significantly correlated with the long-term clinical outcome: prednisolone, L-asparaginase, and vincristine. For each of these three drugs patients were classified according to the definitions mentioned above, and a sensitive result was counted as 1, an intermediate result as 2, and a resistant result as 3. For each patient a score was calculated by adding up these counts. Thus, the score ranged from 3 (sensitive to all three drugs) to 9 (resistant to all three drugs). Table 4 shows that an increase of this “drug resistance profile score” is associated with an increased event-rate (χ2 = 25.7, P < .001). Three patient groups were defined arbitrarily (Fig 2). The group with the most sensitive profile (score 3 or 4), 20% of all patients, had a 3-year pDFS of 100%; the group with an intermediately sensitive profile (score 5 or 6), 40% of all patients, had a 3-year pDFS of 84% (SE 5.7%); and the group with the most resistant profile (score 7, 8, or 9), 41% of all patients, had a 3-year pDFS of 43% (SE 8.1%) (P < .001). Multivariate analysis showed the independent prognostic significance of this drug-resistance profile, which was clearly superior to any factor including age and leukemic cell burden, the only other single factors with (borderline) prognostic significance at univariate analysis (Table 5). All the analyses were done with stratification for risk group.
Drug-Resistance Profile (combined data for prednisolone, L-asparaginase, and vincristine) and the Occurrence of Leukemia-Related Events: No CR, Relapse
| . | Most Sensitive ↔ Most Resistant . | ||||||
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . |
| Score* . | |||||||
| . | . | ||||||
| . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . |
| No. of patients | 5 | 18 | 20 | 27 | 23 | 13 | 12 |
| Event | 0 | 0 | 4 | 6 | 14 | 6 | 6 |
| Event-rate (%) | 0 | 0 | 20 | 22 | 61 | 46 | 50 |
| . | Most Sensitive ↔ Most Resistant . | ||||||
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . |
| Score* . | |||||||
| . | . | ||||||
| . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . |
| No. of patients | 5 | 18 | 20 | 27 | 23 | 13 | 12 |
| Event | 0 | 0 | 4 | 6 | 14 | 6 | 6 |
| Event-rate (%) | 0 | 0 | 20 | 22 | 61 | 46 | 50 |
For each drug a ‘sensitive’ LC50 was counted as 1, an ‘intermediate’ LC50 as 2, and a ‘resistant’ LC50 as 3. Combining these counts results in a total score from 3 to 9.
Relation between in vitro drug resistance profile, obtained by combining results for prednisolone, L-asparaginase, and vincristine (see text), and probability of DFS in newly diagnosed childhood ALL. The numbers in the figure along the X-axis indicate the patients at risk at the different time-points.
Relation between in vitro drug resistance profile, obtained by combining results for prednisolone, L-asparaginase, and vincristine (see text), and probability of DFS in newly diagnosed childhood ALL. The numbers in the figure along the X-axis indicate the patients at risk at the different time-points.
Multivariate Risk Group Stratified Analysis of the Predictive Value of Potentially Prognostic Factors of Leukemia-Related Events in 152 Children With Initial ALL
| Factor . | Relative Risk . | P Value Multivariate5-151 . |
|---|---|---|
| . | (95% C.I.)5-150 . | . |
| Age | ||
| <2 or ≥10 yr v 2-9 yr | 0.7 (0.3-1.5) | .33 |
| BFM risk factor5-152 | ||
| ≥1.20 v <1.20 | 1.8 (0.8-4.0) | .16 |
| Immunophenotype | ||
| Pro-B or T-cell v common or pre-B | 2.5 (0.9-7.0) | .09 |
| DNA ploidy | ||
| Nonhyperdiploid v hyperdiploid | 1.0 (0.2-4.8) | .97 |
| Drug-resistance profile | ||
| Resistant (score ≥7) v sensitive (score <7) | 7.0 (2.9-16.5) | <.001 |
| Factor . | Relative Risk . | P Value Multivariate5-151 . |
|---|---|---|
| . | (95% C.I.)5-150 . | . |
| Age | ||
| <2 or ≥10 yr v 2-9 yr | 0.7 (0.3-1.5) | .33 |
| BFM risk factor5-152 | ||
| ≥1.20 v <1.20 | 1.8 (0.8-4.0) | .16 |
| Immunophenotype | ||
| Pro-B or T-cell v common or pre-B | 2.5 (0.9-7.0) | .09 |
| DNA ploidy | ||
| Nonhyperdiploid v hyperdiploid | 1.0 (0.2-4.8) | .97 |
| Drug-resistance profile | ||
| Resistant (score ≥7) v sensitive (score <7) | 7.0 (2.9-16.5) | <.001 |
95%-Confidence interval.
Using a model including all factors mentioned.
Based on peripheral leukemic cell count, and liver and spleen size.35
DISCUSSION
In the present study of 152 children with newly diagnosed ALL, increased in vitro resistance to each of the single drugs prednisolone, L-asparaginase, and vincristine was significantly associated with a worse clinical outcome after combination chemotherapy. Combining the results for these drugs created a drug resistance profile with independent prognostic significance superior to that of any other factor studied (karyotype not being included). There was a progressive decrease in pDFS from highest in those patients whose leukemia cells were most sensitive to lowest in those whose cells were most resistant (Fig 2). Although the analyses were multivariate and stratified for risk group, our results may not be representative of all children with ALL because we tested a selected group of patients (Tables 1 and 2).
The present study is the first to show in a large group of children with ALL treated with contemporary chemotherapy that in vitro cellular drug resistance testing provides significant information about the clinical outcome after combination chemotherapy. This confirms the results of a large number of retrospective studies which showed significant in vitro–in vivo correlations in smaller groups of childhood and adult leukemia patients.7,11,12,14-24 Using similar cut-off points for sensitivity and resistance as in our retrospective study on cryopreserved leukemic cells,16 we confirm the relation between in vitro resistance to prednisolone and clinical outcome in childhood ALL. However, with respect to the other drugs tested the present results are different from those of the retrospective study. This may be explained by differences in treatment intensity, with much better overall treatment results in the current study. The DCLSG will prospectively study the prognostic significance of the drug-resistance profile described here in its current protocol IX. The German CoALL Group will change therapy for part of their ALL patients based on our in vitro cellular drug resistance data in its next treatment protocol.
Concerning the validation of the MTT assay, it is important that we also found a highly significant correlation between the antileukemic activity of prednisolone in vitro and the clinical response to a systemic monotherapy with that drug.29
We conclude that the MTT assay provides drug-resistance profiles which accurately predict the clinical outcome after chemotherapy in childhood ALL. The nonlaborious and objective MTT assay may be a valuable tool for risk-group stratification. The MTT assay results are available within 1 week from obtaining the leukemic cells, which allows an early change of therapy. The presented data may also stimulate studies regarding other applications of cellular drug-resistance assays, such as rational improvements of current regimens,8-11 38 selection of patients for phase II trials or adjuvant chemotherapy, and assay-selected chemotherapy.
ACKNOWLEDGMENT
The authors thank F.R. Rosendaal (Departments of Clinical Epidemiology and Hematology, University Hospital Leiden, Leiden, The Netherlands), for statistical assistance. The Dutch Childhood Leukemia Study Group (DCLSG) provided the patient samples. Board members of the DCLSG are H. Van Den Berg, M.V.A. Bruin, J.P.M. Bökkerink, P.J. Van Dijken, K. Hählen, W.A. Kamps, F.A.E. Nabben, A. Postma, J.A. Rammeloo, I.M. Risseeuw-Appel, A.Y.N. Schouten-Van Meeteren, G.A.M. De Vaan, E. Th. Van't Veer-Korthof, A.J.P. Veerman, M. Van Weel-Sipman, and R.S. Weening. Computer equipment was provided by Olivetti Nederland BV (Amstelveen, The Netherlands).
Address reprint requests to G.J.L. Kaspers, MD, PhD, Department of Pediatrics, Free University Hospital, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Supported by the Dutch Cancer Society (IKA 89-06) and by the project VONK (VU Onderzoek Naar Kinderkanker).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal