Abstract
Bone marrow (BM) hypoplasia is a major cause of death in paroxysmal nocturnal hemoglobinuria (PNH). However, little is known about the molecular events leading to the hypoplasia. Considering the close pathologic association between PNH and aplastic anemia (AA), it is suggested that a similar mechanism operates in the development of their BM failure. Recent reports have indicated apoptosis-mediated BM suppression in AA. It is thus conceivable that apoptosis also operates to cause BM hypoplasia in PNH. If this is the case, PNH clones need to survive apoptosis and show considerable expansion leading to clinical manifestations. We report here that granulocytes obtained from 11 patients with PNH were apparently less susceptible than those from 20 healthy individuals to both spontaneous apoptosis without any ligands and that induced by anti-FAS (CD95) antibody in vitro. The patients' BM CD34+ cells were also resistant to apoptosis induced by treatment with tumor necrosis factor-α, interferon-γ, and subsequently with anti-FAS antibody. In lymphocytes, the pathologic resistance was not discriminated from inherent resistance to apoptosis. Granulocytes from 13 patients with AA and 12 patients with myelodysplastic syndrome (MDS) exhibited similar resistance to apoptosis. CD34+ cells from MDS-BM also showed similar tendency. Thus, the comparative resistance to apoptosis supports the pathogenic implication of apoptosis in marrow injury of PNH and related stem cell disorders.
PAROXYSMAL NOCTURNAL hemoglobinuria (PNH) is an acquired stem cell disorder of clonal nature characterized by intravascular hemolysis, bone marrow (BM) hypoplasia, venous thrombosis, frequent episodes of infection, and rare leukemic conversion.1,2 The molecular events leading to hemolysis have recently been clarified3,4 in terms of the mechanisms of both the membrane defects, which cause increased susceptibility to complement and the intravascular complement activation that leads to serious precipitation of hemolysis.5 Intractable BM hypoplasia is the next concern because it is a major cause of death in patients with PNH. Regarding the hypoplasia, a close association has been reaffirmed between PNH and aplastic anemia (AA) based on pathophysiologic analyses by flow cytometry capable of directly identifying PNH cells6-8 and by mutation analysis of the PIG-A gene responsible for the most characteristic membrane defect of PNH cells.3,4,9 For example, severe AA often gives rise to PNH as a late clonal complication, especially after immunosuppresive therapy.10,11 In fact, PNH clone is frequently detected in the BM and peripheral blood of AA patients who do not show hemolysis.6-8 Marrow hypoplasia may be favorable for the emergence of PNH clone.2,8,12,13 Conversely, PNH patients with normocellular marrow often show a substantial loss of hematopoietic progenitor cells and eventually develop obvious marrow hypoplasia.14,15 It is difficult to differentiate between AA and seriously progressed marrow hypoplasia in PNH.16 Such close linkage between PNH and AA indicates not only that BM failure is primary to both diseases, but also that a common mechanism operates in the pathogenesis of their marrow hypoplasia.2 In support of this idea, immunosuppressive therapy was reported to satisfactorily improve BM hypoplasia of patients with AA, PNH, and AA-PNH syndrome.16-18 Moreover, a PNH patient with cyclosporin A (CyA)-responsive BM hypoplasia exhibited a HLA class II haplotype identical to that of AA patients who show a preferable response to CyA.19 Immune-mediated BM suppression thus appears to be a common event in the pathogenesis of the hypoplasia in at least some patients with PNH and AA. Apoptosis-associated suppression of hematopoiesis has been proposed to be responsible for the hypoplasia in AA.10,20,21 Considering the close association between PNH and AA, it is conceivable that apoptosis is also involved in the pathogenesis of marrow hypoplasia in PNH. Apoptosis, programmed cell death, is a mechanism for physiologic exclusion of unnecessary cells in such events as embryogenesis and hematopoiesis.22,23 Therefore, dysregulation of apoptosis could be pathogenic.23,24 For example, persistent interruption of apoptosis leads to the development of lymphoma.24 Conversely, marked enhancement of apoptosis in BM may injure hematopoietic cells, resulting in marrow hypoplasia.24 Accordingly, we considered that PNH clone needs to survive apoptosis in the pathogenesis of BM hypoplasia and then to show considerable expansion leading to clinical manifestations. To assess the pathologic involvement of apoptosis in PNH-BM hypoplasia, we examined the susceptibility to in vitro apoptosis of blood cells from patients with PNH and PNH-related stem cell disorders with BM impairment such as AA and myelodysplastic syndrome (MDS).
MATERIALS AND METHODS
Preparation of blood cells.Peripheral blood granulocytes and lymphocytes were obtained with informed consent from 20 healthy volunteers, 11 patients with PNH, 13 patients with AA, and 12 patients with MDS, using dextran sulfate and Ficoll-Hypaque by the methods described elsewhere.25-27 The purities of granulocyte and lymphocyte fractions exceeded 96% and 85%, respectively, as determined by May-Giemsa staining. On the other hand, BM mononuclear cells were isolated by Ficoll-Hypaque centrifugation and then incubated with magnetic polystyrene beads coated with monoclonal antibodies (MoAb) against CD2 and CD19 (Dynal, Oslo, Norway) to remove lymphocytes and nonspecific adherent cells.6 CD34+ cells were isolated from the lymphocyte-depleted immature cell fraction by incubation with beads coated with MoAb to CD34, as described previously.28 Flow cytometric analysis showed that CD34+ cells accounted for more than 90% and from 60% to 83% of the BM cells from healthy controls and patients with PNH, respectively. CD34+ cells accounted from 40% to 65% in the patients with MDS. Numbers of CD34+ cells isolated from the patients with AA were insufficient for apoptosis assay. Viability of the peripheral leukocytes and BM cells exceeded 98% as determined by Trypan blue dye exclusion. Clinical profiles and laboratory data of the patients with PNH are shown in Table 1. Of the 12 patients with MDS, 4 were diagnosed as having refractory anemia (RA), 3 as RA with an excess of blasts (RAEB), 4 as RAEB in transformation (RAEB in T), and 1 as chronic myelomonocytic leukemia (CMMoL).
Clinical Profiles and Laboratory Data of PNH Patients
| Age/Sex . | Granulocytes . | Erythrocytes . | Platelets . | CD34+ Cells . | ||
|---|---|---|---|---|---|---|
| . | (109/L) . | CD59− (%) . | (1012/L) . | CD59− (%) . | (109/L) . | CD59− (%) . |
| 35/F | 0.8 | 7 | 3.2 | 3 | 165 | ND |
| 60/M | 0.7 | 9 | 2.9 | 4 | 18 | 81 |
| 72/M | 0.6 | 9 | 3.9 | 6 | 161 | 84 |
| 28/M | 0.2 | 50 | 2.4 | 8 | 68 | 93 |
| 83/M | 1.5 | 57 | 2.5 | 20 | 211 | ND |
| 49/F | 2.0 | 91 | 2.9 | 55 | 174 | 46 |
| 21/M | 2.1 | 92 | 2.6 | 50 | 222 | 92 |
| 56/M | 2.8 | 92 | 2.9 | 36 | 346 | 91 |
| 63/F* | 1.0 | 98 | 2.6 | 89 | 59 | ND |
| 74/F† | 7.3 | 98 | 1.8 | 26 | 134 | 93 |
| 60/M | 3.7 | 99 | 1.5 | 34 | 31 | 96 |
| Age/Sex . | Granulocytes . | Erythrocytes . | Platelets . | CD34+ Cells . | ||
|---|---|---|---|---|---|---|
| . | (109/L) . | CD59− (%) . | (1012/L) . | CD59− (%) . | (109/L) . | CD59− (%) . |
| 35/F | 0.8 | 7 | 3.2 | 3 | 165 | ND |
| 60/M | 0.7 | 9 | 2.9 | 4 | 18 | 81 |
| 72/M | 0.6 | 9 | 3.9 | 6 | 161 | 84 |
| 28/M | 0.2 | 50 | 2.4 | 8 | 68 | 93 |
| 83/M | 1.5 | 57 | 2.5 | 20 | 211 | ND |
| 49/F | 2.0 | 91 | 2.9 | 55 | 174 | 46 |
| 21/M | 2.1 | 92 | 2.6 | 50 | 222 | 92 |
| 56/M | 2.8 | 92 | 2.9 | 36 | 346 | 91 |
| 63/F* | 1.0 | 98 | 2.6 | 89 | 59 | ND |
| 74/F† | 7.3 | 98 | 1.8 | 26 | 134 | 93 |
| 60/M | 3.7 | 99 | 1.5 | 34 | 31 | 96 |
Abbreviation: ND, not determined.
The patient also had an inherited C9 deficiency.
The patient underwent heart valve replacement therapy.
Induction of apoptosis.Granulocytes and lymphocytes (1 × 106/mL) were treated with or without several doses of anti-FAS (CD95) IgM MoAb (Medical & Biological Laboratories Co, Nagoya, Japan) up to 100 ng/mL in serum-free RPMI 1640 medium (GIBCO, Grand Island, NY) at 37°C for the indicated periods. In the serum-free RPMI medium, BM CD34+ cells were pretreated for up to 4 days with both 1,000 U/mL interferon-γ (IFN-γ) and 10 ng/mL tumor necrosis factor-α (TNF-α) (both from Boehringer-Mannheim Biochemica, Mannheim, Germany),20 29 and then treated with 100 ng/mL anti-FAS MoAb for an additional 24 hours.
Morphological assessment of apoptosis.Cells (2 × 104) were spun down onto microslide glasses as described28 and then stained with May-Giemsa reagent.29 Apoptotic cells in cytospin preparations were morpholocially identified by cell and nuclear shrinkage, cytoplasmic blebbing, nuclear and cytoplasmic fragmentation, and formation of apoptotic bodies. Apoptotic cells among at least 200 cells were counted by light microscopy and expressed as apoptotic index (percent). This assay was used to identify apoptotic granulocytes.
DNA fragmentation assay.Apoptosis was also confirmed by detection of fragmentation of chromosomal DNA as the classic DNA ladder, as described elsewhere.29 30 Briefly, cells (2 × 106) were immersed in cytolysis buffer consisting of 100 μL of 10 mmol/L Tris-HCl (pH 7.4), 10 mmol/L ethylenediaminetetraacetic acid (EDTA), and 0.5% Triton X-100 (Nacalai Tesque, Kyoto, Japan). The mixture was treated with 400 μg/mL RNase A (Boehringer-Mannheim) and then with 400 μg/mL Proteinase K (Boehringer-Mannheim). DNA was then extracted with phenol-chloroform, precipitated with isopropylalcohol, dissolved in 20 μL of 10 mmol/L Tris-HCl (pH 7.4) and 1 mmol/L EDTA, and separated on 2% agarose gels. DNA was visualized by staining with ethidium bromide.
Flow cytometric determination of apoptotic cells.Apoptosis of CD34+ cells was identified and quantified by flow cytometry with both the fluorescent DNA-binding agent 7-amino actinomycin D (7-AAD; Calbiochem-Novabiochem, La Jolla, CA) as described by Philpott et al31 and with fluorescein isothiocyanate (FITC)-conjugated anti-CD34 MoAb (DAKO, Glostrup, Denmark). Briefly, BM cells (106) were stained with 100 μL of 200 μg/mL 7-AAD for 20 minutes at 4°C and then labeled with FITC-conjugated anti-CD34 MoAb. Cells were analyzed with a cell sorter (FACScan; Becton Dickinson, Mountain View, CA). Scattergrams of gated CD34+ cells were generated by combining forward light scattering with 7-AAD fluorescence, and regions were drawn around clear-cut populations with no, dim, and bright fluorescence. Cells within the negative and dim regions were counted as viable and apoptotic cells, respectively.31 The reliability of flow cytometry was confirmed in advance by simultaneous DNA fragmentation assay with cultured cells sensitive to apoptosis (data not shown). FAS expression on CD34+ cells was also analyzed by two-color flow cytometry with FITC-conjugated anti-CD34 MoAb and phycoerythrin (PE)-conjugated anti-FAS MoAb (IgG1 ) (Medical & Biological Laboratories). For lymphocyte analysis, FITC-conjugated MoAb to CD2 and CD19 (Becton Dickinson) were used.
Western blot analysis.The expression of apoptosis-regulatory proteins was analyzed by Western blotting as described previously.32 Briefly, cells (5 × 106/100 μL) were disrupted at 4°C by sonication in a buffer consisting of 10 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, 5 mmol/L EDTA, and 2 mmol/L phenylmethylsulfonyl fluoride (PMSF ). The lysate was centrifuged at 100,000g at 4°C for 30 minutes and the resultant supernatant was used as the cytosolic fraction. The precipitate was homogenized in the same buffer supplemented with 1% Triton X-100 and centrifuged under the above conditions. This supernatant was used as the particulate membrane fraction. Samples were dissolved in a modified Laemmli's sample buffer without 2-mercaptoethanol (3% sodium dodecyl sulfate [SDS], 5% glycerol, 10 mmol/L Tris-HCl, pH 7.8, and 1 mmol/L PMSF ), separated by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and electroblotted onto nitrocellulose sheets. The sheets were blocked with 5% skimmed milk in phosphate-buffered saline (PBS), incubated with antibody to bcl-2, bax, or bcl-x (Santa Cruz Biotechnology Inc, Santa Cruz, CA), or to p53 (Oncogene Science Inc, Uniondale, NY) and subsequently labeled with peroxidase-conjugated second antibody (Amersham Japan, Tokyo, Japan). The blots were visualized with the ECL Western blotting detection system (Amersham Japan).
Statistical analysis.Student's unpaired t-test was used to determine significance levels. P values less than .05 were considered significant.
RESULTS
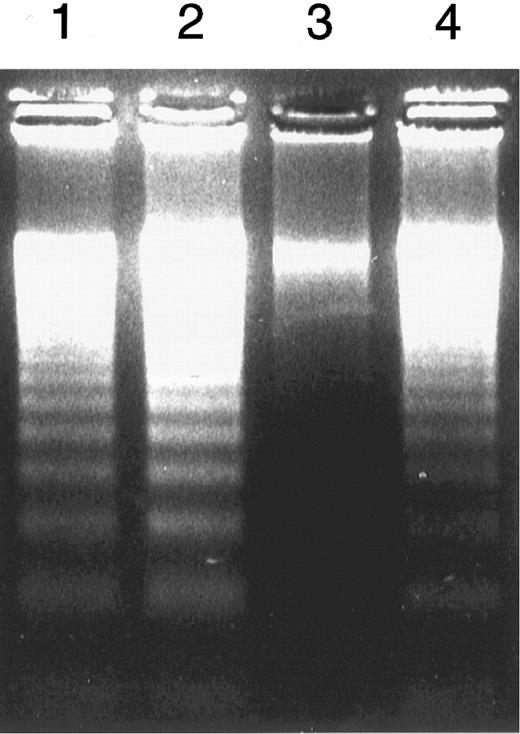
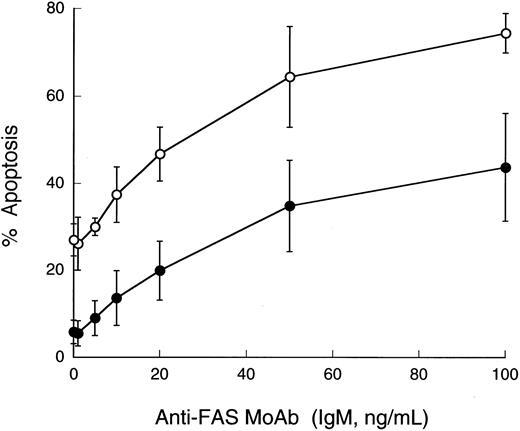
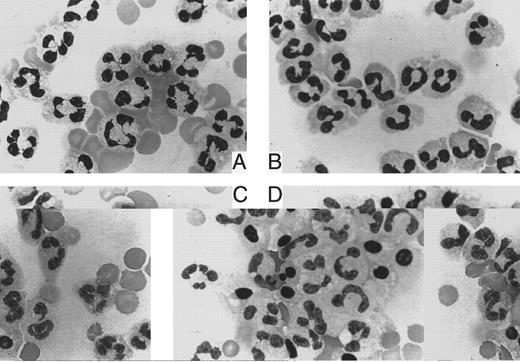
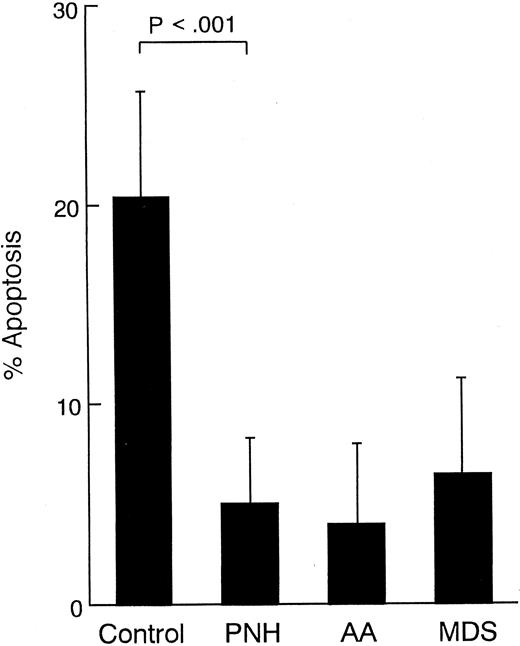
Susceptibility of peripheral blood leukocytes to apoptosis in vitro.Treatment of granulocytes with a low dose of anti-FAS IgM MoAb (50 ng/mL) for 6 hours yielded the classic DNA ladders that are characteristic of apoptosis (Fig 1). The DNA fragmentation was more evident in the control cells than in those from PNH patients. Interestingly, this difference was reproducible despite the absence of the MoAb. Thus, the patients' granulocytes showed reduced apoptosis. The population of apoptotic cells increased dependent on the dose of the antibody in both the control and the patients' granulocytes (Fig 2). At each dose of the antibody, the patients' granulocytes were less susceptible to apoptosis. Under ligand-free conditions, time-dependent apoptosis of the granulocytes was also observed (Fig 3), and the granulocytes from the patients with PNH were comparatively resistant to this spontaneous apoptosis (Fig 3A). This resistance was further confirmed by apoptosis assay in vitro with granulocytes with a high proportion (more than 90%) of cells negative for CD59 (Fig 3B), indicating that PNH cells are resistant to apoptosis. Surprisingly, granulocytes with a low proportion (less than 10%) of cells negative for CD59 also showed similar resistance to apoptosis (Fig 3B). Thus, it appears that there is no close correlation between apoptosis resistance and the population of cells with the PNH phenotype. The observed resistance could not be fully explained only by the CD59-negative cells; ie, the resistance does not seem to be limited to PNH cells in the patients. Microscopic analysis indicated visible differences in granulocyte apoptosis between PNH patients (Fig 4C) and healthy individuals (Fig 4D). As a reference, we examined granulocytes obtained from patients with PNH-related acquired stem cell disorders with marrow dysfunction such as AA and MDS.10 Interestingly, the cells from these patients were also markedly less susceptible to spontaneous apoptosis than controls (Fig 5). On the other hand, in contrast to the granulocytes, no difference in susceptibility to apoptosis was observed in their lymphocytes. That is, both the patients' and control lymphocytes showed considerable resistance to apoptosis with 100 ng/mL or 300 ng/mL of anti-FAS MoAb for 24 hours when analyzed by flow cytometry and for DNA ladder formation (data not shown). This resistance of normal lymphocytes to apoptosis was consistent with previous reports.32
DNA ladder formation. Granulocytes obtained from representative healthy volunteers (lanes 1 and 2) and patients with PNH (lanes 3 and 4) underwent apoptosis in the absence (lanes 1 and 3) and the presence (lanes 2 and 4) of anti-FAS MoAb (IgM, 50 ng/mL) for 6 hours.
DNA ladder formation. Granulocytes obtained from representative healthy volunteers (lanes 1 and 2) and patients with PNH (lanes 3 and 4) underwent apoptosis in the absence (lanes 1 and 3) and the presence (lanes 2 and 4) of anti-FAS MoAb (IgM, 50 ng/mL) for 6 hours.
Apoptosis dependent on the doses of anti-FAS MoAb. Granulocytes were treated for 6 hours with the indicated doses of the antibody. Data are means ± standard deviation (SD) of duplicate samples from 20 healthy volunteers (○) and 11 patients with PNH (•).
Apoptosis dependent on the doses of anti-FAS MoAb. Granulocytes were treated for 6 hours with the indicated doses of the antibody. Data are means ± standard deviation (SD) of duplicate samples from 20 healthy volunteers (○) and 11 patients with PNH (•).
Time-dependent apoptosis. Granulocytes underwent spontaneous apoptosis by incubation for up to 24 hours in the absence of anti-FAS MoAb. (A) Twenty healthy volunteers (○), and 11 patients with PNH (•). (B) Six PNH patients with a high proportion (more than 90%) of CD59− granulocytes (•), and three PNH patients with a low proportion (less than 10%) of such cells (▴). Data are means ± SD of duplicate samples.
Time-dependent apoptosis. Granulocytes underwent spontaneous apoptosis by incubation for up to 24 hours in the absence of anti-FAS MoAb. (A) Twenty healthy volunteers (○), and 11 patients with PNH (•). (B) Six PNH patients with a high proportion (more than 90%) of CD59− granulocytes (•), and three PNH patients with a low proportion (less than 10%) of such cells (▴). Data are means ± SD of duplicate samples.
May-Giemsa staining. Granulocytes from a patient with PNH (A, C) and a healthy donor (B, D) were stained immediately after isolation (A, B) and after a 9-hour incubation in the absence of anti-FAS MoAb (C, D).
May-Giemsa staining. Granulocytes from a patient with PNH (A, C) and a healthy donor (B, D) were stained immediately after isolation (A, B) and after a 9-hour incubation in the absence of anti-FAS MoAb (C, D).
Apoptosis of granulocytes from 20 healthy volunteers (control) and 11 patients with PNH, 13 patients with AA, and 12 patients with MDS. Cells were incubated for 6 hours in the absence of the MoAb to FAS. Data represent means ± SD of duplicate samples.
Apoptosis of granulocytes from 20 healthy volunteers (control) and 11 patients with PNH, 13 patients with AA, and 12 patients with MDS. Cells were incubated for 6 hours in the absence of the MoAb to FAS. Data represent means ± SD of duplicate samples.
Response of BM CD34+ cells to FAS-mediated apoptosis in vitro.To compare the susceptibility to apoptosis between peripheral blood leukocytes and BM cells, we characterized the response to apoptosis in vitro of BM CD34+ cells obtained from patients with PNH by flow cytometry. BM mononuclear cells are, in general, heterogenous in terms of populations of each lineage and at each stage of cell maturation. Moreover, the degree of marrow hypoplasia often varies among patients with PNH. Further, the assay with leukocytes showed a lineage-dependent differential sensitivity to apoptosis as described above and as reported by other investigators.32 Accordingly, we analyzed CD34+ cells rather than whole hematopoietic BM progenitor cells. CD34+ cells from both healthy individuals and PNH patients neither expressed FAS at levels detectable by flow cytometry (Fig 6A and B) nor showed apparent apoptosis in the presence of anti-FAS MoAb (100 ng/mL) for 48 hours. Therefore, we pretreated the cells for 4 days with both IFN-γ and TNF-α, which are known to induce FAS expression.20,21 29 Indeed, FAS was detectable on the CD34+ cells from both patients and control subjects (Fig 6C and D). Cells were then incubated with an antibody to FAS, and viable cells virtually disappeared and the population of apoptotic cells increased in the control CD34+ cells (Fig 7D). In contrast, viable cells were still abundant and the proportion of apoptotic cells was comparatively low in those from PNH marrow (Fig 7C). These findings indicated reduced susceptibility to ligand-induced apoptosis of CD34+ cells from the patients with PNH. Thus, the comparative resistance to apoptosis was a persistent characteristic of peripheral blood granulocytes and BM CD34+ cells from the patients. CD34+ cells from the patients with MDS showed similar results, but to quite a lesser extent (Fig 8). The amount of the CD34+ cells obtained from the patients with AA was too small to use for the apoptosis assay.
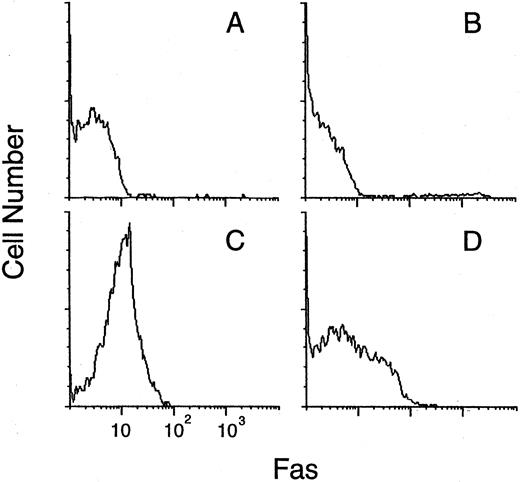
FAS expression by CD34+ cells. BM cells isolated from a patient with PNH (A, C) and a healthy donor (B, D) were analyzed by two-color flow cytometry with FITC-labeled anti-CD34 and PE-labeled anti-FAS MoAbs before (A, B) and after (C, D) treatment with TNF-α and IFN-γ. FAS expression on the gated CD34+ cells is shown in the histograms. A second experiment with cells from four other patients with PNH and four healthy donors gave similar results.
FAS expression by CD34+ cells. BM cells isolated from a patient with PNH (A, C) and a healthy donor (B, D) were analyzed by two-color flow cytometry with FITC-labeled anti-CD34 and PE-labeled anti-FAS MoAbs before (A, B) and after (C, D) treatment with TNF-α and IFN-γ. FAS expression on the gated CD34+ cells is shown in the histograms. A second experiment with cells from four other patients with PNH and four healthy donors gave similar results.
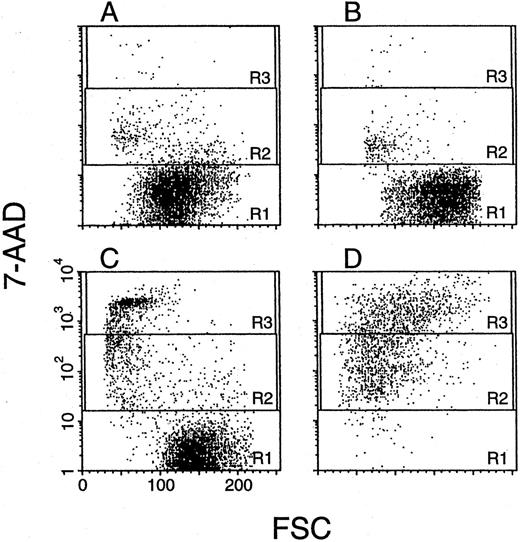
Apoptosis of CD34+ cells from PNH-BM. BM hematopoietic progenitors enriched with CD34+ cells obtained from a PNH patient (A, C) and a healthy volunteer (B, D) were analyzed by two-color flow cytometry with both 7-AAD and FITC-conjugated anti-CD34 MoAb before (A, B) and after (C, D) treatment with TNF-α, IFN-γ, and subsequent anti-FAS MoAb. Scattergrams of gated CD34+ cells were generated by combining forward light scatter (FSC) with 7-AAD fluorescence and show three regions drawn around clear-cut populations having negative (R1, viable cells), dim (R2, apoptotic cells), and bright fluorescence (R3, late-apoptotic or dead cells), as reported originally by Philpott et al.31 A second experiment with cells from four other patients with PNH and four healthy donors gave similar results.
Apoptosis of CD34+ cells from PNH-BM. BM hematopoietic progenitors enriched with CD34+ cells obtained from a PNH patient (A, C) and a healthy volunteer (B, D) were analyzed by two-color flow cytometry with both 7-AAD and FITC-conjugated anti-CD34 MoAb before (A, B) and after (C, D) treatment with TNF-α, IFN-γ, and subsequent anti-FAS MoAb. Scattergrams of gated CD34+ cells were generated by combining forward light scatter (FSC) with 7-AAD fluorescence and show three regions drawn around clear-cut populations having negative (R1, viable cells), dim (R2, apoptotic cells), and bright fluorescence (R3, late-apoptotic or dead cells), as reported originally by Philpott et al.31 A second experiment with cells from four other patients with PNH and four healthy donors gave similar results.
Apoptosis of CD34+ cells from MDS-BM. CD34+ cells were isolated from the BM of patients with MDS (CMMoL, A and D; RA, B and E) and a healthy donor (C, F ). Cells were then treated and analyzed as described in the legend to Fig 7. The population of viable cells (R1) after treatment was 39%, 51%, and 8% in the patients (D, E) and the healthy control (F ), respectively. (A, B, and C) Cells before treatment. A second experiment with cells from three other patients with MDS and three healthy donors gave similar results.
Apoptosis of CD34+ cells from MDS-BM. CD34+ cells were isolated from the BM of patients with MDS (CMMoL, A and D; RA, B and E) and a healthy donor (C, F ). Cells were then treated and analyzed as described in the legend to Fig 7. The population of viable cells (R1) after treatment was 39%, 51%, and 8% in the patients (D, E) and the healthy control (F ), respectively. (A, B, and C) Cells before treatment. A second experiment with cells from three other patients with MDS and three healthy donors gave similar results.
Expression of apoptosis-associated molecules.To determine the reason for observed comparative resistance to apoptosis, we analyzed the expression of p53, bax, bcl-x, and bcl- 2, which are functionally associated with the regulation of apoptosis.24,33 34 However, there were no detectable differences in the levels of expression of these molecules between the PNH and healthy individuals (data not shown).
DISCUSSION
To gain insight into the complex pathophysiology of PNH, it is helpful to elucidate the nature of the affected clones. In this study, we detected reduced susceptibility of blood cells obtained from patients with PNH to both spontaneous and ligand-induced apoptosis in vitro relative to those from healthy volunteers. Among the patients' blood cells, the comparative resistance to apoptosis was evident in the granulocytes, but unclear in the lymphocytes, whereas resistance of their BM CD34+ cells was also revealed by treatment with IFN-γ, TNF-α, and anti-FAS MoAb. The differential resistance among the blood cells may reflect both their inherent sensitivity to apoptosis20,21,29,32 and its disease-associated acquired modulation. The persistence of the resistance between the BM CD34+ cells and the circulating granulocytes suggests that the apoptosis-resistant phenotype is stable at least during the differentiation from CD34+ cells to granulocytes. That is, the resistance appears heritable, although the alternative possibility that the resistant phenotype could merely represent perturbation of any of several pathways of modulation of apoptosis sensitivity could not be excluded. It is of interest whether the resistance to apoptosis of PNH patients' blood cells can be explained by PIG-A gene, which is responsible for the membrane defect of PNH cells.3,4,35,36 PNH granulocytes were obviously resistant to apoptosis. Nevertheless, there were no close correlations in our limited numbers of patients with PNH between resistance to apoptosis and the proportion of cells with the PNH phenotype, ie, negative for glycosylphosphatidylinositol-anchored membrane proteins such as CD59 and decay-accelerating factor.3,4 Thus, the comparative resistance to apoptosis does not seem to be limited to PNH clone, indicating that the resistant phenotype cannot simply be ascribed to PIG-A alone. Of note, the apoptosis-resistant phenotype was also observed in the blood cells from patients with AA and MDS who did not carry PNH cells. That is, the resistant phenotype is shared by PNH and PNH-related stem cell disorders. In the related disorders, a pathogenic involvement of apoptosis in BM impairment has been proposed based on a high incidence of apoptotic cells in BM37,38 and increased expression of FAS on BM cells.20,21 Thus, we concluded that resistance to apoptosis suggests a pathogenic involvement of apoptosis in marrow hypoplasia of PNH, as well as related disorders. Indeed, immunosuppressive therapy often improves BM hypoplasia in patients with PNH and related disorders.16-19 Identification of the reason for the resistance to apoptosis at the molecular level and determination of the events that initiate apoptosis will also shed some light on the complex pathophysiology of PNH.
In the development of PNH, it is conceivable that acquisition of apoptosis resistance confers a survival advantage on PNH clone over other intact clones in the early stage of the apoptosis-mediated BM injury process.10,39 To manifest symptoms characteristic of PNH, however, the survival of PNH clone in the injured marrow is essential, but not sufficient, judging from the presence of clinically silent PNH clones in the marrow of patients with AA, which predisposes to PNH.6,7 It is thus likely that PNH clone needs to show considerable expansion for clinical manifestation.2,10,39 For clonal proliferation, growth advantage of PNH clone has been suggested in the injured marrow.2,10,13,28,39 The growth properties of affected clone appear to be independent of PIG-A. For examle, such proliferation was not confirmed in the BM of chimeric mice generated with Pig-a (the murine homologue of PIG-A )-disrupted embryonic stem (ES) cells.40 Moreover, dominance of a single clone often observed in PNH patients with coexisting multiple PNH clones2,10 41 may indicate that the dominance is independent of PIG-A. To understand molecular events leading to clinical manifestations, growth properties of PNH clone thus remain to be clarified at the molecular level.
ACKNOWLEDGMENT
We thank Fumio Kawano of National Kumamoto Hospital, Makoto Kawakita, and Fumi Inukai in our laboratory for advice and help.
Supported by a grant from Tokyo Biochemical Research Foundation and grants from the Ministry of Education, Science, Sports and Culture of Japan (Tokyo).
Address reprint requests to Hideki Nakakuma, MD, Second Department of Internal Medicine, Kumamoto University School of Medicine, Honjo 1-1-1, Kumamoto 860, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal