Abstract
Electron microscopic (EM) studies were performed to clarify the interactions of membrane proteins in the red blood cell membrane structure in situ of a homozygous patient with total deficiency of protein 4.1 who carried a point mutation of the downstream translation initiation codon (AUG → AGG) of the protein 4.1 gene [the 4.1 (−) Madrid; Dalla Venezia et al, J Clin Invest 90:1713, 1992]. Immunologically, as expected, protein 4.1 was completely missing in the red blood cell membrane structure in situ. A markedly disrupted skeletal network was observed by EM using the quick-freeze deep-etching method and the surface replica method, although the number of spectrin molecules was only minimally reduced (395 ± 63/μm2; normal, 504 ± 36/μm2). The number of basic units in the skeletal network was strikingly reduced (131 ± 21/μm2; normal, 548 ± 39/μm2), with decreased small-sized units (17 ± 4/μm2; normal, 384 ± 52/μm2) and increased large-sized units (64% ± 14%; normal, 5% ± 1%). Concomitantly, immuno-EM disclosed striking clustering of spectrin molecules with aggregated ankyrin molecules in the red blood cell membrane structure in situ. Although no quantitative abnormalities in the number and size distribution of the intramembrane particles were observed, there was a disappearance of regular distribution, with many clusters of various sizes, probably reflecting the distorted skeletal network. Therefore, protein 4.1 suggests by EM to play a crucial role in maintenance of the normal integrity of the membrane structure in situ not only of the skeletal network but also of the integral proteins.
THE RED BLOOD CELL (RBC) membrane is composed of three major components: integral proteins, skeletal proteins, and anchoring proteins.1 The skeletal network is basically composed of spectrin, protein 4.1, and actin, with other accessory proteins (tropomyosin, tropomodulin, adducin, and band 4.9).1
The skeletal network in the intact RBC membrane in situ has been shown to be a dense, sweater-like meshwork directly laminating the inner leaflet of the lipid bilayer.2-4 When the meshwork is detached from the lipid bilayer and artificially stretched, the extended skeleton has been described primarily as a hexagonal lattice.2,5-7 This lattice is basically composed of long spectrin filaments and junctional complexes, which are located at the center and six corners of the hexagons.2 5-7
The locations of these membrane proteins under this stretched condition have been identified by immunogold labeling.2,8,9 Ankyrin and band 3 were located 80 nm from the distal end of the extended spectrin molecules.2,10-12 These findings are in good agreement with biochemical results.13,14 Protein 4.1 bound to the distal ends of spectrin tetramers.12,15,16 The ternary complex of actin-spectrin-4.1 along with actin-binding proteins, including adducin, is considered the junctional complex.2,8 9
However, contrary to these observations made under artificially extended conditions,2,5-8,17 it has been shown that the basic structure of the RBC membrane in situ does not necessarily show a hexagonal structure.2-4,18-20 Instead, it is now well established that the membrane in normal subjects is composed of numerous basic units resembling cages, with the filaments in a three-dimensional folded configuration.3,4,18-20 When examined by electron microscopy (EM) with the quick-freeze deep-etching (QFDE) method,3,4,18-20 the skeletal network in normal RBCs shows a fairly uniform distribution of filamentous structures and also uniformity of apparent branchpoints of the filamentous elements in an essentially orderly fashion. The immunogold method has recently been used in EM with the surface replica (SR) method, which is equally useful for examining the skeletal units.18 With this technique, it has become easy to identify the exact location of major membrane proteins in a native state in situ.
Protein 4.1, which is one of the key proteins of the skeletal network, has been studied extensively and has been proven to be an intriguing multifunctional structural protein using biochemistry and molecular biology.1,21-33 It is present in approximately 200,000 copies per cell and interacts with the skeletal proteins, spectrin and actin, to form the junctional complex. It also associates with integral proteins, glycophorin C, and band 3.1,21 In vitro proteolysis studies have shown protein 4.1 to be composed of four major domains of 30, 16, 10, and 22/24 kD.31 It participates in interaction with spectrin and actin at its 10-kD domain21,29,32 and is also connected to transmembrane proteins by its 30-kD domain.21,30 33 Therefore, protein 4.1 appears to play an important role in association with skeletal proteins horizontally, but also anchors the skeletal network to transmembrane proteins vertically. Despite such detailed information, which has been obtained mostly by in vitro experiments, the exact molecular arrangement of membrane proteins (especially protein 4.1) and their interactions with the intact membrane structure in situ have not been determined.
Clinically, various abnormalities in protein 4.1 have been reported,21,34-43 including structural mutations of protein 4.142,43 and quantitative abnormalities such as 4.1 (−) hereditary elliptocytosis (HE); ie, a heterozygote with reduced protein 4.121,36,37,40,41 or a homozygote with no protein 4.1.34,35,38,39 Homozygous 4.1 (−) HE is extremely rare: in three children of an Algerian family,34,35 in an American child,38 and in a Spanish man (allele Madrid).39 The allele Madrid was identified as a point mutation of the downstream translation initiation codon (AUG → AGG) that induced the missing protein 4.1 in a homozygous state.39 Just like deletion experiments in nature, the homozygous 4.1 (−) Madrid case may serve as an excellent model for elucidating the crucial roles played by protein 4.1 in association with skeletal proteins in the skeletal network; in interactions with band 3, which still have been controversial; and in its competition with ankyrin.
In this patient, therefore, the skeletal network was examined by EM using the QFDE method and the SR method with the immunogold method. The intramembrane particles (IMPs) were also examined by EM using the freeze fracture (FF ) method.
MATERIALS AND METHODS
Subjects.The propositus [homozygous 4.1 (−) Madrid], who was born in Spain in 1948, suffered from uncompensated hemolytic anemia until 1979, at which time splenectomy yielded a remarkable hematologic improvement, as reported previously.39 Blood smears indicated elliptocytosis with anisopoikilocytosis and fragmented RBCs. Whole blood was preserved in isotonic Alsever's solution immediately after blood was drawn in Madrid, Spain, and was carried carefully at 4°C by hand to Kawasaki Medical School (Kurashiki, Japan). EM studies were immediately initiated within 40 hours after the blood was taken. A normal control specimen, which was drawn at the same time and treated by the same procedures, yielded the same results as fresh normal specimens.
Analysis of RBC membrane proteins.RBC ghosts were prepared, and membrane proteins were analyzed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using the methods of Fairbanks et al44 and Laemmli.45 Western blot analysis of RBC protein 4.1 was performed, as described previously.39 The contents of each membrane protein fraction, especially spectrin, ankyrin, band 3, band 4.1, band 4.2, and actin, were determined on SDS-PAGE gels densitometrically.46 The results were expressed as relative ratios of the contents of the membrane proteins.
QFDE method for skeletal network.RBC ghosts were frozen quickly with liquid nitrogen. Deep etching and replication were performed with Balzers BAF 301 (Balzers, Fürstentum, Liechtenstein), as described previously.19 20 Etching was performed at −100°C at 5 × 10−5 Pa for 5 minutes. Rotary replication was performed with platinum at an angle of 20° (2 nm) and with carbon at an angle of 90° (10 nm). Film thickness was controlled by the frequency shift on a Balzers quartz crystal monitor (QSD 201D). The replica was placed onto copper grids (400 mesh) and subjected to transmission electron microscopy (JEM-2000 EXII; JEOL, Tokyo, Japan) at 200 kV.
Surface replica method for skeletal network and immunoelectron micrography.White ghosts were placed on a cover slip coated with 0.1% poly-L-lysine. After fixation with 2% glutaraldehyde solution and further fixation with 1% osmium tetroxide, the fixed ghosts were subjected to sequential dehydration with alcohol up to critical point drying. Then they were treated with platinum-paladium and coated with carbon. The replica was examined with a transmission electron microscope (JEM-2000 EXII; JEOL) at 120 kV, as described previously.18
For immunogold labeling,18 washed ghosts were placed on a coverslip coated with 0.1% poly-L-lysine and fixed with 2% paraformaldehyde and 0.1% glutaraldehyde. After blocking with 1% bovine serum albumin, antibodies against the membrane proteins were applied to the fixed ghosts. The following antibodies were used for this study: antihuman spectrin rabbit polyclonal IgG antibody18; antihuman protein 4.1 mouse monoclonal IgM antibody, which was provided by Dr W. Nunomura (Tokyo Women's Medical College, Tokyo, Japan); and antihuman ankyrin rabbit polyclonal IgG antibody, which was provided by Prof S. Lux (Children's Hospital, Boston, MA). Gold conjugate antirabbit IgG (10 nm; Biocell, Cardiff, UK) or gold conjugate antimouse IgM (15 nm) was applied. The ghosts were finally fixed with a 2% glutaraldehyde solution and subjected to dehydration, and the replica was prepared, as described previously.18
FF method for IMPs.Intact RBCs were examined for IMPs by fixation in 1.0% glutaraldehyde, followed by impregnation with 10% to 40% glycerol, as described previously.18-20 RBC suspensions were rapidly frozen in liquid nitrogen, and FF replicas were prepared in a Balzers BAF 301 apparatus (Balzers), after which they were examined with an electron microscope (JEM-2000 EXII; JEOL). In this method, IMPs at the inner (so-called “P”) face were examined.
All values are reported as the mean ± SD. n refers to the number of specimens analyzed for a particular quantitation on individual skeletons or IMPs. The actual numbers of measurements or observations are noted and indicated by obs.
RESULTS
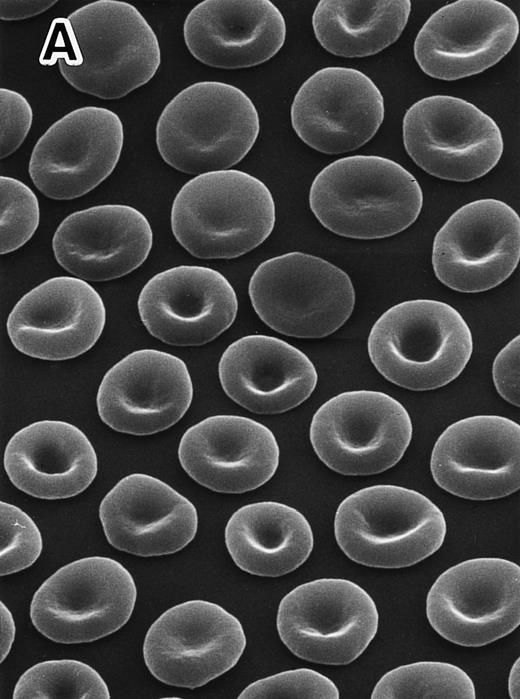
Scanning electron micrographs in peripheral RBCs.Marked anisocytosis, poikilocytosis with elliptocytosis, and fragmented RBCs were evident in the 4.1 (−) Madrid (Fig 1B), compared with discocytes in a normal subject (Fig 1A). Some of these changes appear to be modified by splenectomy, which in this case was performed in 1979 with remarkable hematologic improvement, although the findings of the original RBC morphology before the splenectomy were not available as a reference for this study.
Scanning electron micrographs of peripheral RBCs in a normal subject and the homozygous patient with total protein 4.1 deficiency. The patient was splenectomized in 1979. RBCs were fixed with 1% glutaraldehyde solution immediately after blood was drawn and transferred at 4°C. Striking poikilocytosis and anisocytosis were noted on the background of elliptocytosis in the 4.1 (−) Madrid case (B), compared with normal discocytes in a representative normal subject (A). Original magnification × 1,500.
Scanning electron micrographs of peripheral RBCs in a normal subject and the homozygous patient with total protein 4.1 deficiency. The patient was splenectomized in 1979. RBCs were fixed with 1% glutaraldehyde solution immediately after blood was drawn and transferred at 4°C. Striking poikilocytosis and anisocytosis were noted on the background of elliptocytosis in the 4.1 (−) Madrid case (B), compared with normal discocytes in a representative normal subject (A). Original magnification × 1,500.
Protein analysis.The total absence of protein 4.1 was reconfirmed by SDS-PAGE and Western blotting with antiprotein 4.1 polyclonal antibodies, as reported previously39 (not shown). A 55-kD protein (p55) was missing in the region of band 4.5, and glycophorins C and D were sharply diminished, as described previously39 (not shown).
Although the method of quantitating proteins by densitometry of coomassie blue-stained gels is not an exquisitely sensitive technique, the amount of spectrin (α chain and β chain) was significantly decreased by 21.4% (P < .05) on SDS-PAGE with the Fairbanks gels (Table 1). The amount of actin was also diminished by 18.4% (P < .05) on the Laemmli gels (Table 1). The protein 4.2 content appeared to be lower than that of normal controls as judged by the Laemmli gels, but the difference was not significant. The amount of ankyrin appeared to be normal on the Fairbanks gels under a usual steady-state condition with essentially normal reticulocyte counts (43 × 109/L).
Membrane Protein Profiles in Protein 4.1 (−) Madrid
| Proteins . | Normal Subjects . | Protein 4.1 (−) Madrid . |
|---|---|---|
| . | Mean ± 1 SD . | Average . |
| . | (n = 13) . | (n = 2) . |
| On the Fairbanks gels | ||
| Spectrins (α + β)/band 3 | 85.8 ± 3.5 | 67.4 (−21.4%*) |
| Ankyrin/band 3 | 13.3 ± 1.0 | 14.0 |
| On the Laemmli gels | ||
| Band 4.2/band 3 | 14.4 ± 1.1 | 12.9 |
| Actin/band 3 | 14.7 ± 1.2 | 12.0 (−18.4%*) |
| Proteins . | Normal Subjects . | Protein 4.1 (−) Madrid . |
|---|---|---|
| . | Mean ± 1 SD . | Average . |
| . | (n = 13) . | (n = 2) . |
| On the Fairbanks gels | ||
| Spectrins (α + β)/band 3 | 85.8 ± 3.5 | 67.4 (−21.4%*) |
| Ankyrin/band 3 | 13.3 ± 1.0 | 14.0 |
| On the Laemmli gels | ||
| Band 4.2/band 3 | 14.4 ± 1.1 | 12.9 |
| Actin/band 3 | 14.7 ± 1.2 | 12.0 (−18.4%*) |
SDS-PAGE was performed in the protein 4.1 (−) Madrid case on two independent occasions in triplicate. Results were compared with those in normal subjects (n = 13). Each membrane protein band stained with Coomassie blue was determined densitometrically at 570 nm after SDS-PAGE was performed, as previously described.39 The relative ratios are given as percentages, and mean values ± 1 SD are shown.
Statistically significant reduction (P < .05).
Electron micrographs by the surface replica method with anti-protein 4.1 monoclonal antibody.RBC ghosts were applied to immuno-EM using the surface replica method with anti-protein 4.1 antibody conjugated with immunogold particles. Representative results are shown in Fig 2. The immunogold particles (protein 4.1) were totally missing in the RBCs of protein 4.1 (−) Madrid (Fig 2B), in contrast to the normal subjects (Fig 2A), in whom the immunogold particles with the anti-protein 4.1 antibody were found to be present normally by colocalizing at the skeletal network. In the normal subjects, the number of immunogold particles with anti-protein 4.1 antibody was 186 ± 29/μm2. Therefore, the total deficiency of protein 4.1, which had been suggested by biochemical analyses on SDS-PAGE as reported previously,39 was proven morphologically by the immuno-EM.
Electron micrographs of RBC membrane skeletons by the surface replica method with antihuman protein 4.1 mouse monoclonal IgM antibody. No protein 4.1 was detected in the 4.1 (−) Madrid (B) as compared with normal subjects (A). Original magnification × 100,000. Bars indicate 0.1 μm.
Electron micrographs of RBC membrane skeletons by the surface replica method with antihuman protein 4.1 mouse monoclonal IgM antibody. No protein 4.1 was detected in the 4.1 (−) Madrid (B) as compared with normal subjects (A). Original magnification × 100,000. Bars indicate 0.1 μm.
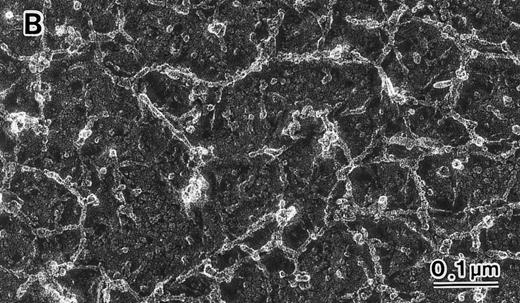
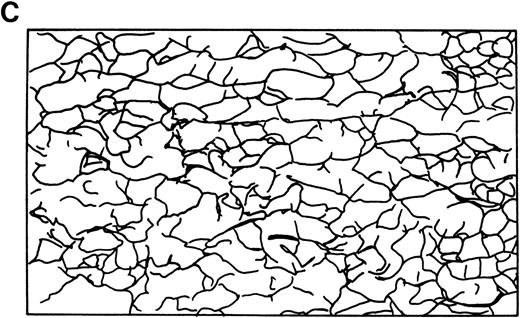
Skeletal networks examined by EM with the QFDE method.RBC membrane ghosts were subjected to EM using the QFDE method, and representative results are shown in Fig 3. In the normal subjects, the filaments (mostly spectrin, as identified by immuno-EM) of the intact skeletal network were present in multistereotactic dimensions rather than in a single plane, as shown in Fig 3A and C. The filaments in the normal subjects were 48 ± 9 nm in length and 7 ± 1 nm in diameter and appeared to be in a folded configuration. The skeletal network in normal RBCs (n = 20, obs 272) showed a fairly uniform distribution of filamentous structures and also uniformity of apparent branchpoints of the filamentous elements in an essentially orderly fashion. The skeletal network in the normal subjects showed numerous basic units, resembling cages, the number of which was 548 ± 39/μm2, as shown in Table 2. These cage-like structures consisted essentially of two major types of units; ie, small (20 to 44 nm) and medium (45 to 68 nm) -sized units as determined by the interdistance (or diameter) of the longer axis of each structure. In the normal subjects, two thirds of these units were of small size (70% ± 10%), and the remaining one third were of medium size (25% ± 6%). There were only a few large-size units (5% ± 1%) in the normal subjects, as shown in Table 2.
Skeletal networks by EM with the QFDE method. Markedly disrupted skeletal networks are shown representatively in the 4.1 (−) Madrid (B) as compared with normal subjects (A). These results are also shown schematically in normal (C) and in the 4.1 (−) Madrid (D). Original magnification × 150,000. Bars indicate 0.1 μm.
Skeletal networks by EM with the QFDE method. Markedly disrupted skeletal networks are shown representatively in the 4.1 (−) Madrid (B) as compared with normal subjects (A). These results are also shown schematically in normal (C) and in the 4.1 (−) Madrid (D). Original magnification × 150,000. Bars indicate 0.1 μm.
Marked Decrease in the Number of Apparent Skeletal Units With Increase in Their Size in the Homozygous Protein 4.1 (−) Madrid
| Apparent Cytoskeletal Units . | Normal . | Protein 4.1 (−) Madrid . |
|---|---|---|
| . | (n = 20) . | (n = 1) . |
| No. (/μm2) | 548 ± 39 | 131 ± 21 |
| Size (%) in diameter of the longer axis | ||
| Small (20-44 nm) | 70 ± 10 | 13 ± 4 |
| Medium (45-68 nm) | 25 ± 6 | 23 ± 6 |
| Large (69-92 nm) | 5 ± 1 | 27 ± 10 |
| Extra-large (93-240 nm) | 0 | 37 ± 15 |
| Apparent Cytoskeletal Units . | Normal . | Protein 4.1 (−) Madrid . |
|---|---|---|
| . | (n = 20) . | (n = 1) . |
| No. (/μm2) | 548 ± 39 | 131 ± 21 |
| Size (%) in diameter of the longer axis | ||
| Small (20-44 nm) | 70 ± 10 | 13 ± 4 |
| Medium (45-68 nm) | 25 ± 6 | 23 ± 6 |
| Large (69-92 nm) | 5 ± 1 | 27 ± 10 |
| Extra-large (93-240 nm) | 0 | 37 ± 15 |
RBC ghost membranes were subjected to EM studies using the QFDE method. The number of apparent skeletal units in the homozygous protein 4.1 (−) Madrid (n = 1, obs 52) as compared with that in the normal subjects (n = 20, obs 272) was counted. The results are shown as the number of apparent skeletal units per square micrometer. The size distribution of these skeletal units was also evaluated, and the results are shown in percentages. Mean values and 1 SD are shown.
In contrast, in total deficiency of protein 4.1, the uniform distribution of filamentous structures was lost, and apparent branchpoints of the filamentous elements were markedly disrupted or distorted, as shown in Fig 3B and D. The abnormality of the skeletal network in band 4.1 deficiency was quantitated by counting the number of apparent skeletal units still left as nearly recognizable and tolerable for these counting procedures. The number of these skeletal units was markedly reduced in the 4.1 (−) Madrid case (131 ± 21/μm2), as shown in Fig 3B.
The relative size distribution of these skeletal units was also quantitated by measuring the interdistance (or diameter) of the longer axis in each unit (Table 2). In the 4.1 (−) Madrid case, the skeletal units of basic small size (20 to 44 nm) were markedly reduced (17 ± 4/μm2) compared with the number in the normal subjects (384 ± 52/μm2). In their place, units of large size (69 to 92 nm) and of extra-large size (93 to 240 nm), which were rarely observed in the normal subjects (5% ± 1%), were markedly increased in the 4.1 (−) Madrid case (64% ± 14%). It should be noted that the structures being called small, large, or extra-large cages may be composed of entirely different components (and/or the same components organized differently in relationship to one another) in the 4.1-deficient sample compared with the normal sample.
Aggregated spectrins in the skeletal network in situ.Intact RBCs of the normal subjects and of the 4.1 (−) Madrid case were examined by EM with the surface replica method. The skeletal networks were further labeled by the immunogold particles conjugated with antispectrin polyclonal antibody. Representative results are shown in the normal subjects (Fig 4A) and in the 4.1 (−) Madrid case (Fig 4B).
Immuno-EM of RBC membrane skeletons using the surface replica method with antihuman spectrin rabbit polyclonal IgG antibody. Aggregated spectrin was noted in the 4.1 (−) Madrid case ([B] n = 1, obs 12) as compared with normal subjects ([A] n = 20, obs 52). Original magnification × 100,000. Bars indicate 0.1 μm.
Immuno-EM of RBC membrane skeletons using the surface replica method with antihuman spectrin rabbit polyclonal IgG antibody. Aggregated spectrin was noted in the 4.1 (−) Madrid case ([B] n = 1, obs 12) as compared with normal subjects ([A] n = 20, obs 52). Original magnification × 100,000. Bars indicate 0.1 μm.
The filaments of the skeletal meshwork in situ were identified as spectrin by immunogold labeling. The immunogold particles (spectrin) in the normal subjects (n = 20, obs 52) were almost evenly distributed to represent a normal orderly arranged skeletal network (Fig 4A), as observed by EM with the QFDE method (Fig 3A). The number of immunogold particles (spectrins) was 504 ± 36/μm2 in the normal subjects. In contrast, the immunogold particles for spectrin labeling in the 4.1 (−) Madrid case (n = 1, obs 12) were definitely distributed in uneven fashion, mostly by forming large clusters, or by lining up close together in necklace fashion (Fig 4B). It was evident that many areas of the membrane plane remained open and free of any immunogold particles, indicating that the skeletal meshwork was completely disrupted or distorted in the absence of protein 4.1. The findings were nearly identical to those by EM using the QFDE method. The number of immunogold particles was slightly diminished to 395 ± 63/μm2 (−21.6% of the mean value of normal controls) in this patient, which was compatible with the biochemical results (Table 1).
Increased clustering of ankyrin in the skeletal network in situ.Immuno-EM with antiankyrin antibody was also applied to normal cells and the 4.1 (−) Madrid RBCs. Representative results in the normal subjects (Fig 5A) and the 4.1 (−) Madrid case (Fig 5B) are shown.
Immuno-EM of RBC membrane skeletons using the surface replica method with antihuman ankyrin rabbit polyclonal IgG antibody. Marked aggregation of ankyrin was observed in the 4.1 (−) Madrid case ([B] n = 1, obs 10) as compared with normal subjects ([A] n = 20, obs 50). Original magnification × 100,000. Bars indicate 0.1 μm.
Immuno-EM of RBC membrane skeletons using the surface replica method with antihuman ankyrin rabbit polyclonal IgG antibody. Marked aggregation of ankyrin was observed in the 4.1 (−) Madrid case ([B] n = 1, obs 10) as compared with normal subjects ([A] n = 20, obs 50). Original magnification × 100,000. Bars indicate 0.1 μm.
Ankyrin was almost evenly distributed in the normal subjects (Fig 5A). The number of immunogold particles (ankyrin) was 150 ± 34/μm2 in normal subjects (n = 20, obs 50). In contrast, large open areas without immunogold particles (ankyrin) were widely present in the 4.1 (−) Madrid case (Fig 5B; n = 1, obs 10). Although the number of immunogold particles of ankyrin appeared to be slightly diminished to 123 ± 21/μm2 (−19.8% of the normal controls), the most striking feature was the rather clustered distribution of immunogold particles, which were still attached to the basic units of the skeletal meshwork. There was a minor discrepancy in the ankyrin quantitation by SDS-PAGE (same as normal control) and by the immunogold method [slightly less in the 4.1 (−) Madrid]. It might be due to the experimental condition, in which the ankyrin labeling in situ was abnormal in the 4.1 (−) Madrid probably by the limitation of the epitopes for ankyrin molecules.
IMPs examined by EM with the FF method.Intact RBCs were subjected to EM using the FF method, and representative results are shown in Fig 6. In the normal subjects (n = 20; Fig 6A), the number of IMPs at the inner (so-called “P”) face was 5,390 ± 420/μm2 (obs 121), most (71% ± 8%) of which were basically small (4 to 8 nm) in size (Table 3). In the RBCs of the protein 4.1 (−) Madrid case, the number of IMPs present was normal (5,275 ± 329/μm2, n = 1, obs 28), as shown in Fig 6B. The size distribution of IMPs appeared to be unaffected; ie, 68% ± 9% of small size (4 to 8 nm in diameter), 29% ± 5% of medium size (9 to 20 nm), and 3% ± 2% of large size (>21 nm), as shown in Table 3. Therefore, no quantitative IMP abnormalities were observed in this patient. However, there was a striking disappearance of the regular distribution of IMPs of small size that was a major component of IMPs. In the patient (Fig 6B), IMPs were distributed like clustered icebergs with irregularly and widely open channels of water between them. In the normal subjects (Fig 6A), on the other hand, IMPs were more regularly distributed. In this patient, the IMPs appeared to form clusters of various sizes that were composed by 3 to 10 IMPs (Fig 6B).
Intramembrane particles at the inner (so-called “P”) face by EM with the FF method. No quantitative abnormalities were detected in the 4.1 (−) Madrid case (B), as compared with normal subjects (A). However, regular distribution was lost with many clusters of various sizes in the 4.1 (−) Madrid case (B). Many irregularly and widely open membrane areas with a much smaller number of IMPs were also observed (B). Original magnification × 150,000. Bars indicate 0.1 μm.
Intramembrane particles at the inner (so-called “P”) face by EM with the FF method. No quantitative abnormalities were detected in the 4.1 (−) Madrid case (B), as compared with normal subjects (A). However, regular distribution was lost with many clusters of various sizes in the 4.1 (−) Madrid case (B). Many irregularly and widely open membrane areas with a much smaller number of IMPs were also observed (B). Original magnification × 150,000. Bars indicate 0.1 μm.
Number and Size of IMPs in the Homozygous Protein 4.1 (−) Madrid
| IMPs . | Normal . | Protein 4.1 (−) Madrid . |
|---|---|---|
| . | (n = 20) . | (n = 1) . |
| No. (/μm2) | 5,390 ± 420 | 5,275 ± 329 |
| Size (%) | ||
| Small (4-8 nm) | 71 ± 8 | 68 ± 9 |
| Medium (9-20 nm) | 27 ± 3 | 29 ± 5 |
| Large (>21 nm) | 2 ± 1 | 3 ± 2 |
| IMPs . | Normal . | Protein 4.1 (−) Madrid . |
|---|---|---|
| . | (n = 20) . | (n = 1) . |
| No. (/μm2) | 5,390 ± 420 | 5,275 ± 329 |
| Size (%) | ||
| Small (4-8 nm) | 71 ± 8 | 68 ± 9 |
| Medium (9-20 nm) | 27 ± 3 | 29 ± 5 |
| Large (>21 nm) | 2 ± 1 | 3 ± 2 |
Intact RBCs were subjected to EM studies using the FF method. The number of IMPs at the inner (so-called “P”) face in the homozygous protein 4.1 (−) Madrid (n = 1, obs 28) as compared with that in the normal subjects (n = 20, obs 121) was counted. The results are shown as the number of IMPs per square micrometer. The size of IMPs was also evaluated, and the results are shown in percentages. Mean values and 1 SD are shown.
The uneven distribution of IMPs at the inner (so-called “P”) face in the patient was proven by counting the numbers of IMPs in surface areas of a specific size by EM using the freeze fracture method, as shown in Table 4. In normal subjects, approximately 80% of each membrane face area contained 6 to 11 IMPs per 33 nm2. Only 2.4% of the membrane areas contained 0 to 3 IMPs per 33 nm2. In contrast, in the 4.1 (−) Madrid RBCs, nearly 40% of the membrane face areas contained 0 to 5 IMPs, although the total numbers of IMPs were nearly identical at a larger scale (ie, per square micrometer), as shown in Table 3.
Uneven Distribution of IMPs in the Total Protein 4.1 Deficiency
| No. of IMPs . | Frequency (%) . | |
|---|---|---|
| (per 33 nm2 ) . | Normal . | Protein 4.1 (−) Madrid . |
| . | (n = 5) . | (n = 1) . |
| 0-3 | 2.4 ± 1.0 | 11.7 ± 2.54-150 |
| 4-5 | 18.1 ± 3.0 | 27.0 ± 3.24-150 |
| 6-11 | 79.5 ± 9.8 | 61.3 ± 7.64-150 |
| No. of IMPs . | Frequency (%) . | |
|---|---|---|
| (per 33 nm2 ) . | Normal . | Protein 4.1 (−) Madrid . |
| . | (n = 5) . | (n = 1) . |
| 0-3 | 2.4 ± 1.0 | 11.7 ± 2.54-150 |
| 4-5 | 18.1 ± 3.0 | 27.0 ± 3.24-150 |
| 6-11 | 79.5 ± 9.8 | 61.3 ± 7.64-150 |
After EM using the FF method was performed, the number of IMPs (per each 33 nm2 ) at the inner (so-called “P”) face was counted to determine the distribution of IMPs on the membrane faces in 10 microphotographs from each normal subject (n = 5, obs 45,000) and the 4.1 (−) Madrid case (n = 1, obs 9,000). The relative frequency in each category was expressed in percentages.
Statistical significance in P < .05.
DISCUSSION
Protein 4.1 is known as one of the most important proteins because of its interaction with spectrin, actin, and integral proteins in the lipid bilayer.1,21 It has been shown that protein 4.1 binds tightly to β-spectrin very near the actin binding site,47,48 probably within the N-terminal domain,32 and also that its 10-kD domain strengthens the spectrin-actin binding.29,49 Using EM, it has been shown that protein 4.1 and actin bind at the end of the β-spectrin molecule.47 The ternary complex appears to be regulated by the extent of phosphorylation by protein kinase A,50,51 by tyrosine kinase,52 and by Ca2+ and calmodulin.53-55 Protein 4.1 also appears to have binding sites for band 3, glycophorin A (GPA), and glycophorin C (GPC), probably at its 30-kD domain.56-58 Protein 4.1-deficient RBCs have also been reported to be deficient in GPC and p55, but not in GPA or band 3.38,39,59,60 Protein 4.1 also interacts with myosin at its 10-kD domain.61
However, most of the findings described above have been obtained by in vitro experiments. Therefore, it should be clarified whether these interactions of protein 4.1 with other membrane proteins actually occur in the membrane structure in situ. If so, the primary deficiency of protein 4.1 should yield a tremendous disruption of the skeletal network, resulting in severe hemolysis.
There have been several reports of homozygous patients with hereditary elliptocytosis, in which no protein 4.1 was detected.34,35,38,39,41 Clinically, these homozygous patients with total deficiency of protein 4.1 demonstrate a severe, transfusion-dependent hemolytic anemia with markedly fragmented elliptocytes.34 39 In these patients, the exact states of the skeletal network in situ have never been shown in detail, especially by EM, although the biochemical and genetic results from in vitro experiments have strongly suggested marked impairment of the skeletal network.
The present Spanish patient (designated as member II.9 of family SA) had been proven to be a homozygote of complete protein 4.1 deficiency due to a point mutation at the downstream translation initiation codon (AUG → AGG) of the protein 4.1 gene, leading to a total lack of RBC protein 4.1, as reported previously.39
Therefore, we studied him by EM using the surface replica method, which was combined with the immunogold method using the anti-protein 4.1 antibody, because this procedure using antispectrin antibody had been successfully applied by us to clarify the impaired skeletal network in β-spectrin Le Puy.18 In this homozygous 4.1 (−) patient, immuno-EM proved that protein 4.1 was totally missing (Fig 2B), as expected from the biochemical and genetic data.39
The skeletal network was initially visualized by EM with the negative staining method, especially in in vitro experiments.5,6,62 The method, which was combined with the immuno-EM, yielded excellent results to demonstrate the binding characteristics of several membrane proteins in vitro. However, the negative staining method was not free from artificial procedures, by which the RBC membrane ghosts were treated with chemical reagents to make the membrane skeleton spread by losing the in situ native structure of the membrane. Therefore, the replica method with the QFDE method for EM is believed to provide the best resolution for visualization of the in situ condition of the skeletal network. In this study, the intact structure of the skeletal network was clearly shown in normal RBC membranes (Fig 3A). However, in the 4.1 (−) Madrid case, the skeletal meshwork was totally disrupted or distorted, as shown by disappearance of normal intact basic units, elongation of fibrous filaments, and disconnection of each basic unit of the skeletal network (Fig 3B), although the amount of spectrin was only minimally decreased biochemically (Table 1). Schematic diagrams of the skeletal network in normal and in the 4.1 (−) Madrid are also shown in Fig 7. In addition, no functional abnormalities of spectrin, such as the dimer-dimer association, were detected, as reported previously.39 Therefore, the marked abnormalities of the skeletal network seem to be derived from the total protein 4.1 deficiency due to the primary genetic lesion, because other proteins, especially anchoring proteins such as ankyrin and band 4.2, appeared to be nearly normally maintained (Table 1).
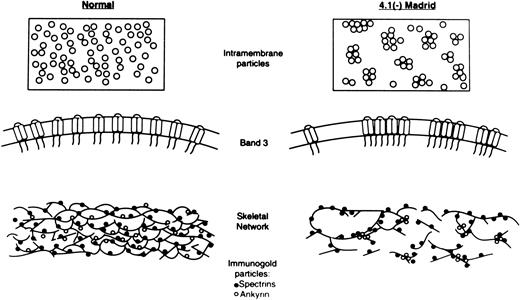
Schematic diagrams of states of IMPs and the skeletal network in RBCs of normal and the 4.1 (−) Madrid case. The upper and the middle portions indicate the sizes and distribution patterns of IMPs in normal (left) and the 4.1 (−) Madrid case (right). The clustering of the normal-sized IMPs is evident in the 4.1 (−) Madrid case. The bottom portions represent the states of the skeletal network of normal (left) and the 4.1 (−) Madrid case (right). (•) Antispectrin antibody-conjugated immunogold particles; (○) antiankyrin antibody-conjugated immunogold particles. The skeletal network in the 4.1 (−) Madrid case is markedly disrupted, which is proven by the abnormal distribution of the immunogold particles for spectrin. Ankyrin tends to cluster more than spectrin in the 4.1 (−) Madrid case.
Schematic diagrams of states of IMPs and the skeletal network in RBCs of normal and the 4.1 (−) Madrid case. The upper and the middle portions indicate the sizes and distribution patterns of IMPs in normal (left) and the 4.1 (−) Madrid case (right). The clustering of the normal-sized IMPs is evident in the 4.1 (−) Madrid case. The bottom portions represent the states of the skeletal network of normal (left) and the 4.1 (−) Madrid case (right). (•) Antispectrin antibody-conjugated immunogold particles; (○) antiankyrin antibody-conjugated immunogold particles. The skeletal network in the 4.1 (−) Madrid case is markedly disrupted, which is proven by the abnormal distribution of the immunogold particles for spectrin. Ankyrin tends to cluster more than spectrin in the 4.1 (−) Madrid case.
The marked abnormalities in the 4.1 (−) Madrid case as detected by EM with the QFDE method (Fig 3B) were confirmed by immuno-EM with the surface replica method using antispectrin antibody (Fig 4B). The filaments, which were identified as spectrin by immuno-EM, were distributed unevenly, forming their large clusters or abnormally lining up close together (Fig 7). However, the number of immunogold particles (spectrins) was only slightly diminished (−21.6%) in the 4.1 (−) Madrid case, as compared with the normal subjects. These results clearly imply that protein 4.1 does actually play a critical role in maintaining the normal integrity of the skeletal network even in situ, as suggested by previous results obtained from in vitro experiments.
It has been proposed that the skeletal network, which is composed mostly of spectrin, is linked to the integral proteins, such as band 3, glycophorins, and others, via interaction with anchoring proteins. A critical role of protein 4.2 in connecting the skeletal network with the integral proteins (especially band 3) has recently been suggested from EM studies of RBCs with a total deficiency of protein 4.2.19 Possible interaction of protein 4.2 with spectrin has been reported directly by a binding assay of protein 4.2 to spectrin.63 In this 4.1 (−) Madrid case, the amount of protein 4.2 present was nearly normal and not deficient (Table 1).
Ankyrin has been reported to be one of the major anchoring proteins connecting the skeletal network (especially spectrin) to the integral proteins (especially band 3). Therefore, it would be interesting to know whether the skeletal network could be normally supported by the presence of ankyrin even in the total absence of protein 4.1. The amount of ankyrin present in this splenectomized patient with a normal reticulocyte count was nearly normal, but the disruption of the skeletal network was extremely marked, indicating that the critical role played by protein 4.1 in construction of the skeletal network cannot be taken over by ankyrin. In addition, under a condition of the total absence of protein 4.1, ankyrin tended to cluster, as shown by the immuno-EM with antiankyrin antibody (Fig 5B). The extent of the clustering of ankyrin appeared to be more marked than that of spectrin. This may imply that, in addition to the primary abnormalities of the skeletal network itself, the distribution of ankyrin was more directly affected in the absence of protein 4.1 (Fig 7).
Finally, the interaction of the skeletal network and the integral proteins (especially band 3) under the total deficiency of protein 4.1 must be discussed. It has also been speculated that protein 4.1 may play a biologic role in connecting the skeletal network to the integral proteins (especially band 3) in the lipid bilayer, although there is much controversy regarding this possibility. Surprisingly, the EM with the FF method (Fig 6B) showed no quantitative abnormalities in IMPs in the 4.1 (−) Madrid case (Table 3), unlike the markedly decreased p55 and GPC, although protein 4.1 has been reported to have its binding to band 3.33 The number of IMPs in this patient was normal, corresponding to the biochemical results, which showed nearly normal band 3 content on SDS-PAGE. The sizing of the IMPs was also identical to that of normal subjects. The sizing of the IMPs is considered to be one of the typical indices for determining the extent of oligomerization of band 3. Therefore, it appears evident that protein 4.1 has no substantial effect on the oligomerization of band 3 (Fig 7), although this interpretation may be reserved by the fact that the number of copies of band 3 (approximately 1,000,000/RBC) is too much greater than that of protein 4.1 (approximately 200,000/RBC) to appreciate significant differences in the oligomeric state of band 3.
However, the most striking feature of the abnormalities in the IMPs was their uneven distribution (Fig 6B and Table 4). It has been reported that band 3 consists of a mobile fraction (one third) and an immobile fraction (two thirds), which is fixed to the skeletal network mostly by ankyrin.63-66 Therefore, a condition involving the marked disruption of the skeletal network with clustering of spectrin and ankyrin should easily affect the state of the distribution of band 3, resulting in an abnormal distribution pattern of IMPs. When examined using a smaller scale (33 nm2 ), some membrane areas should contain the clustered IMPs, which should be composed mostly of the immobile band 3 attached to the distorted skeletal network and/or of the mobile band 3 trapped in collapsed compartments of skeletal proteins. However, other areas should contain a much smaller number of IMPs (Fig 6B and Table 4). The abnormal distribution pattern of the IMPs in the 4.1 (−) Madrid case, therefore, appears to mostly reflect the markedly impaired skeletal disruption (Fig 7).
Another consideration is a possible competitive interaction between ankyrin and protein 4.1. Ankyrin has its binding to band 3 near the N-terminus and also at a putative central hinge.33 Protein 4.1 also has its binding to band 3 predominantly near the N-terminus and also near the junction of the cytoplasmic domain and the membrane domain of band 3.33 Therefore, protein 4.1 may be competitive with ankyrin with regard to its binding to band 3. In the absence of protein 4.1, ankyrin (∼100,000 copies/RBC) may have more opportunities to bind to band 3 molecules, unless it otherwise has to share its binding to band 3 with protein 4.1. The increased binding of ankyrin to band 3 in the absence of protein 4.1 may enhance the clustering of band 3, which is connected to or is trapped in the collapsed compartments of skeletal proteins.
Other integral proteins, such as p55 and GPC, which were also diminished secondary to the absence of protein 4.1, might be involved in the abnormalities of the IMPs to some extent, despite the fact that we have no direct evidence.
In summary, marked disruption of the skeletal network in situ was precisely shown in the protein 4.1 (−) Madrid case by EM using the QFDE method and the surface replica method combined with the immunogold method using antibodies against spectrin, protein 4.1, and ankyrin. The abnormal distribution of IMPs (especially band 3) was probably mostly a reflection of the impaired skeletal network, as shown in Fig 7 schematically.
Therefore, protein 4.1 appears to be crucial in maintaining the normal integrity of the membrane structure, both of the skeletal network and of the integral proteins.
ACKNOWLEDGMENT
The authors thank Prof S. Lux for his kind gift of antihuman ankyrin rabbit polyclonal IgG antibody, Dr W. Nunomura for antihuman protein 4.1 mouse monoclonal IgM antibody, K. Uehira and S. Eda for their excellent technical assistance, and Profs W. Doerfler and S. Eber for their useful discussion.
Supported in part by Grants-in-Aid for Scientific Research (No. 07457236, 07670180, 09470235, and 09670164) and for the International Scientific Research Program: Joint Research (No. 06044212, 08044328, and 09044346) from the Ministry of Education, Science, Sports and Culture of the Japanese Government; by a research grant for Idiopathic Disorders of Hematopoietic Organs from the Japanese Ministry of Health and Welfare; by a research grant from the Uehara Memorial Foundation; by research grants from Kawasaki Medical School (No. 7-104 and 8-107); and by the Japanese-German Cooperative Science Promotion Program from the Japan Society for the Promotion of Science.
Address reprint requests to Yoshihito Yawata, MD, PhD, Division of Hematology, Department of Medicine, Kawasaki Medical School, 577 Matsushima, Kurashiki City, 701-01, Japan.






![Fig. 4. Immuno-EM of RBC membrane skeletons using the surface replica method with antihuman spectrin rabbit polyclonal IgG antibody. Aggregated spectrin was noted in the 4.1 (−) Madrid case ([B] n = 1, obs 12) as compared with normal subjects ([A] n = 20, obs 52). Original magnification × 100,000. Bars indicate 0.1 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2471/3/m_bl_0017f4a.jpeg?Expires=1765885466&Signature=YavMjds93Lrlyn6KUwKp7SQrvAKUMXNUNWsK7QBRkML5XqntxjPM5Vxza6yFfLVVHFFGOslb5Xgb3dx595KAmC6folgxEszputfAvpvFF6u9~kimj~YXYGd6mn9wdTl5ExUgcXpbAZx734smMPaRkLq1g9-iO3WPXqyWMwNh0E~5Po7QrzQ73e1WRTA7GCZm~swGE2O9TgRUzt6R2YNAJpDpLBzOTuziNlG97fwMtwBWmuE2BqJIx0Yfn8H3FJiREPObIxff3uVJHPSc5uhS7IsKBLVl5pKzjdQPqIrdTNYtIwl2ds4oIAQEiC3V9hfW5Rsf~tdkgdiHJZa96B~6bA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Immuno-EM of RBC membrane skeletons using the surface replica method with antihuman spectrin rabbit polyclonal IgG antibody. Aggregated spectrin was noted in the 4.1 (−) Madrid case ([B] n = 1, obs 12) as compared with normal subjects ([A] n = 20, obs 52). Original magnification × 100,000. Bars indicate 0.1 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2471/3/m_bl_0017f4b.jpeg?Expires=1765885466&Signature=a61Uvm8FAeXGe8lYreQr4hrSwepYD50~iPgewf~kvyAJKNYJMgFjdJcZE60TQbpx9Dy4O8~nkRaLsRGuQOVXIb-HpgIYo-oC1RwTEUUwxNZ4HUJAyVf2i9P7TzrdMjfNAYm3XT4~4ZugDZ9w3fmtLhbY6kSIloAcXYW~hojRMeaeqnh697nE~xWqWvkiiY4zP6Lvnp49pds9XfKGJJZNV99zlTpa4k65n0Pas3xNG9~5CbcyOxFV1-ZHm2aBlhYbvVgTA3pNzc1sevngI9kQfQ~zqXcS0P8BxHL-nwCouM6X-oeSAtNw-y4TfZObxr0btkEcSlYqTwOdmAU0loZr8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Immuno-EM of RBC membrane skeletons using the surface replica method with antihuman ankyrin rabbit polyclonal IgG antibody. Marked aggregation of ankyrin was observed in the 4.1 (−) Madrid case ([B] n = 1, obs 10) as compared with normal subjects ([A] n = 20, obs 50). Original magnification × 100,000. Bars indicate 0.1 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2471/3/m_bl_0017f5a.jpeg?Expires=1765885466&Signature=Ey2hPIRkk68CQqLYubkDWAtHQ329FESHBdxyGHeDWfIng1-uVgQ1X~H0~o6zsXW1uoP1fIbpq1GCO~HrTQ8Ui~pvH5zce17j1cojt0-wIljB0HD3OAsOjGNVLBH1JFZGZAEz8-TlY2Q9s~WsLiFobZ~fCLxmMUNfsWIy7vCOPIyDhBzquaRPrHtBcRpWBN0tOzuhPTQJvDs5oHZ8AZhHFJC5QvMpwoZMeQ-NvL-i0V7W2XLSEsnZPi06ghD-3tfTMxcbOw8loPkchuOehnias4I55PxNKPESl5WZLLIeqoGfIEG9epfxhwysGBjZTRAU9cCQcCf4EHpD8stVuKIiWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Immuno-EM of RBC membrane skeletons using the surface replica method with antihuman ankyrin rabbit polyclonal IgG antibody. Marked aggregation of ankyrin was observed in the 4.1 (−) Madrid case ([B] n = 1, obs 10) as compared with normal subjects ([A] n = 20, obs 50). Original magnification × 100,000. Bars indicate 0.1 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2471/3/m_bl_0017f5b.jpeg?Expires=1765885466&Signature=xGZQ5tHKLhrdYPCYHGc~N7oa7albkjefTXVyBlQ5XlKYKeyFFggBHxnyLtM6VfLmPoFSeRCGsY4B9btEkLWa2V3RvpfQAtpIDQ6fypRih~Ex4W4pyZvBhk5jzCXtwj6M5vz0~dk6k1re9iL0Pg1gOGrv~lxTSe9X87ESmtFCtl8GT78W-zadfevcstnJMcj6v4VMbsGLCKUKwzckLrP6ZQ1nKNQFP~TWVI83uabDaeaP7S1KTwi8d4y63YeafEpPSi32NQYmT0oZzeJfeBapLqigQ-dthGiHl5dpyMdd14Y3yFA-jGpjly5~XLLpjt0Af0c~3H5Yvk0kJeFRnip1yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal