Abstract
A high complete remission rate is currently achieved in patients with acute myeloid leukemia (AML). However, many patients eventually relapse due to the persistence of low numbers of residual leukemic cells that are undetectable by conventional cytomorphologic criteria (minimal residual disease [MRD]). Using immunophenotypic multiparametric flow cytometry, we have investigated in sequential studies (diagnosis and follow-up) the impact of MRD detection on the outcome of 53 AML patients that had achieved morphologic remission with standard AML protocols and displayed at diagnosis an aberrant phenotype. Patients were studied at diagnosis with a panel of 35 monoclonal antibodies in triple staining combinations for detection of aberrant or uncommon phenotypic features. According to these features, a patient's probe was custom-built at diagnosis for the identification of possible residual leukemic cells during follow-up. The level of MRD at the end of induction and intensification therapy correlated with the number of relapses and relapse-free survival (RFS). Thus, patients with more than 5 × 10−3 residual cells (5 residual cells among 1,000 normal bone marrow [BM] cells) identified as leukemic by immunophenotyping in the first remission BM showed a significant higher rate of relapse (67% v 20% for patients with less than 5 × 10−3 residual cells; P = .002) and a lower median RFS (17 months v not reached; P = .01). At the end of intensification, with a cut-off value of 2 × 10−3 leukemic cells, AML patients also separated into two distinct groups with relapse rates of 69% versus 32% (P = .02), respectively, and median RFS of 16 months versus not reached (P = .04). In addition, overall survival was also significantly related to the level of residual cells in the marrow obtained at the end of induction and particularly after intensification therapy (P = .008). Furthermore, we have explored whether residual disease was related with the functional expression of multidrug resistance (MDR-1) at diagnosis as assessed by the rhodamine-123 assay. Patients with ≥5 × 10−3 residual leukemic cells at the end of induction therapy had a significantly higher rhodamine-123 efflux (mean, 56% ± 24%) than those with less than 5 × 10−3 residual cells (mean, 32% ± 31%; P = .04). Finally, multivariate analysis showed that the number of residual cells at the end of induction or intensification therapy was the most important prognostic factor for prediction of RFS. Overall, our results show that immunophenotypical investigation of MRD strongly predicts outcome in patients with AML and that the number of residual leukemic cells correlates with multidrug resistance.
A HIGH COMPLETE remission rate is currently achieved in patients with acute myeloid leukemia (AML). However, many patients will eventually relapse due to the persistence of low numbers of residual leukemic cells, which are undetectable using conventional cytomorphologic criteria. This condition is known as minimal residual disease (MRD). Among the techniques suitable for MRD detection, immunophenotyping and polymerase chain reaction (PCR) analysis of leukemia-specific sequences are the most commonly used. Information concerning the value of MRD investigations is significantly lower in AML than it is in acute lymphoblastic leukemia (ALL).1-7 Moreover, most available information on MRD in AML is based on molecular detection of chromosomal translocations such as t(15; 17) or t(8; 21).8,9 Regarding immunophenotypic studies, although there are no leukemia-specific antigens that can be used as target markers for MRD detection, our group,10 as well as several others,2,11-13 have shown that leukemic cells frequently display aberrant or uncommon phenotypic features that allow their distinction from normal cells. For AML, the most relevant type of aberrancies include (1) asynchronous antigen expression, (2) cross-lineage antigen expression, and (3) antigen overexpression.10 14
Initially, studies in immunophenotypical detection of MRD have been based on double antigen stainings analyzed by fluorescence microscopy.15,16 However, more recent data show that multiparametric flow cytometry allows a more precise detection of phenotypic aberrancies.2,11 Moreover, previous MRD studies have been based on the phenotypic characteristics of the predominant blast cell population,11,15,17 ignoring the existence of two or more phenotypically different blast cell subsets at diagnosis in a high proportion of AML cases and not taking into account that relapse could be due to a minor blast cell subset that in turn could be highly resistant to chemotherapy.14 18 Indeed, persistence of residual leukemic cells may be related to multidrug resistance (MDR-1). However, to the best of our knowledge, both parameters (MDR-1 and MRD) have not been previously analyzed simultaneously.
In the present study using immunophenotypic multiparametric flow cytometry, we have investigated the impact on the outcome in AML patients of monitoring MRD in the first remission bone marrow (BM) obtained at the end of induction therapy as well as at the end of intensification treatment. In addition, MRD has been correlated with functional evaluation of multidrug resistance.
MATERIALS AND METHODS
Patients: selection criteria and treatment.A total of 89 patients with unequivocal AML based on morphologic and cytochemical criteria were immunophenotypically studied at diagnosis to identify the presence of aberrant phenotypic features appropriate for MRD investigations providing that the patients were expected to achieve a morphologic complete remission (CR). Accordingly, criteria for inclusion in the MRD study were (1) patients with aberrant or infrequent phenotypes, (2) morphologic CR achieved after induction treatment, and (3) at least two follow-up samples available. Thirty-six patients did not fulfill these prerequisites and, finally, 53 patients were selected for the MRD follow-up investigations.
Patients were uniformly treated according to the protocols AML 87 and 91 of the Spanish Pethema Cooperative Group. The remission-induction therapy included one or two 3/7 courses of anthracycline (60 mg/m2 daunorubicin or 12 mg/m2 idarubicin) and cytosine arabinoside (200 mg/m2 for patients younger than 60 years of age and 100 mg/m2 for the 20 patients older than 60 years of age). Patients who achieved morphologic CR received an identical consolidation course. Finally, either one or two high-dose intensification courses consisting of cytosine arabinoside (3 g/m2/12 hours for a total of 8 doses; 1 g/m2/12 hours for patients older than 60 years of age) and either daunorubicin (45 mg/m2) or idarubicin (12 mg/m2) for 3 days were administered. Overall, a total of 371 MRD immunophenotypic studies were performed (mean, 7 ± 4.3 per patient). Within the 53 patients included, BM samples in morphologic CR were available in 42 cases at the end of induction treatment and in 38 cases after intensification treatment. Morphologic CR was defined by the criteria proposed by Cheson et al19: (1) a peripheral blood (PB) neutrophil count greater than 1.5 × 109/L and a platelet count greater than 100 × 109/L; (2) the absence of leukemic blasts in PB; (3) less than 5% blast cells without detectable Auer rods in a BM sample displaying more than 20% cellularity with maturation of all cell lines in the BM aspirate; and (4) the absence of extramedullary leukemia.
Patients' follow-up ranged between 6 and 70 months (median, 23 months). Overall survival (OS) of the 53 patients included in the MRD study was 74% at 2 years, with a median relapse-free survival (RFS) of 26 months.
Immunophenotypic studies at diagnosis.Immunophenotypic studies at diagnosis were performed on erythrocyte lysed whole BM samples upon staining with directly conjugated monoclonal antibodies (MoAbs). Antigenic expression was systematically analyzed by flow cytometry (FACScan; Becton Dickinson, San Jose, CA) using double and triple stainings with the following fluorochrome-conjugated (fluorescein isothiocyanate [FITC], phycoerythrin [PE], peridin chlorophyll [PerCP], or phycoerythrin-cyanine 5 [PE-Cy5]) combinations of MoAbs: CD15/CD117/CD34, CD15/CD33/CD34, CD15/CD34/HLA-DR, CD34/CD38/CD19, CD34/CD56/CD33, HLA-DR/CD33/CD13, CD7/CD13/CD19, CD65/CD11b/CD4, CD2/CD14/CD13, CD61/glycophorin A/CD45, CD10/CD5/CD20, and CD71/CD11b. Briefly, between 1 and 2 × 106 leukocytes were placed in a final volume of 200 μL of phosphate-buffered saline (PBS)-diluted BM sample and incubated with the appropriate combination of MoAbs for 10 minutes in the dark (room temperature). Afterwards, 2 mL of fluorescence-activated cell sorting (FACS)-lysing solution (Becton Dickinson) was added and another 10 minutes of incubation was performed in the dark (room temperature). The FACS lysing solution, in addition to lysing erythrocytes, fixes the leucocytes. Cells were then centrifuged and washed once in 2 mL of PBS. Finally, they were resuspended in 0.5 mL of PBS. In a second step, once the immunophenotype of the leukemic cells for the above-mentioned panel was established, the need for performing further MoAb combinations that could help to define precisely the aberrant phenotypes detected was evaluated and, if appropriate, performed to define a custom-built phenotypic pattern for follow-up studies. The source and specificity of each MoAb used in the present study were as follows: CD34 (HPCA-2-PE and HPCA-1-FITC), CD33 (leuM9-PE), HLA-DR (anti-HLA-DR-PerCP), CD15 (leuM1-FITC), CD14 (leu3a-PE), CD10 (anti-cALLA-FITC), CD7 (leu9-FITC), CD13 (LEUM7-PE), CD2(leu5b-PE), CD16 (leu11c-PE), CD71 (antitransferrin receptor-FITC), glycophorin A (D2-10-PE), CD5 (leu1-PE), CD56 (leu19-PE), and CD11b (leu15-PE) were purchased from Becton Dickinson; CD117 (95C3-PE) and glycophorin A (D2-10-PE) were from Immunotech (Marseille, France); CD61 (GpIIIa-FITC) was obtained from DAKO (Glostrup, Denmark); and CD45 (GAP8.3-PE/Cy5) and CD19 (SJ25-C1-PE/Cy5) were purchased from Caltag Laboratories (San Francisco, CA). PE/Cy5 staining was used to assess cell viability, because the leukocyte fixation used in our procedure prevents the use of propidium iodide as a cell viability fluorochrome.20
For data acquisition, the LYSIS II software program (Becton Dickinson) was used. The PAINT-A-GATE PRO software program (Becton Dickinson) with the polynomial SSC transformation capability was used for further data analysis. Analysis was performed on gated blast cells according to previously defined methods.21-23 Briefly, leukemic cells were specifically identified on the basis of their scatter (FSC/SSC) and antigenic properties and clearly separated from normal cells, which were excluded from the analysis. In a second step, immunophenotypic characterization of leukemic cells was performed. The aim was to define within leukemic cells those phenotypes that are absent or extremely infrequent in normal BM samples by using a five-dimensional space formed by the two scatter parameters and the three fluorescence-associated characteristics. From the phenotypical point of view, the existence of two or more blast cell populations was established on the basis of a clearly differentiated antigen expression.17 Calibration of the instrument was performed before data acquisition using previously well-established protocols and CD4/CD3/CD8 positive controls as well as bead standards. To exclude the lymphoid origin of the leukemic cells, cytoplasmic CD3 and CD22 were assessed on cells that were fixed and permeabilized before the staining (Fix & Perm; Caltag). In all cases, an isotype-matched negative control with no BM reactivity and the positive control of CD4-FITC/CD3-PE/CD8-PE-Cy5 stained PB lymphocytes were used. This tube was also used as a control for the light-scatter parameters, taking the lymphocytes as a standard.
Aberrant phenotypes and MRD studies.Based on our previous experience10,14,21,22 and that of Terstappen et al,18 four main types of aberrant phenotypes were considered: (1) cross-lineage antigen expression (coexpression of CD2, CD3, CD5, CD7, and CD19 lymphoid-associated markers on myeloid blast cells); (2) asynchronous antigen expression (expression of a combination of myeloid-associated antigens which is not found in the normal myeloid differentiation such as the coexpression of the CD34 and CD56 antigens); (3) antigen overexpression (abnormally high expression of a certain antigen on blast cells); and (4) myeloid cells displaying aberrant light-scatter properties. In addition, phenotypic patterns that are found in normal BM at frequencies lower than less than 1 × 10−3 (1 cell among 1,000) were also analyzed as infrequent phenotypes.10,21 22 To assess the sensitivity of this approach, we performed serial dilutional experiments of leukemic cells with normal BM cells. The detection limit ranged from 1 leukemic cell among 10,000 normal BM cells (10−4) to 1 neoplastic cell among 100,000 normal cells (10−5).
As mentioned above, the custom-built MoAb combinations that precisely defined the singular characteristics of the leukemic clones at diagnosis were used as patient's probes for the identification of possible residual leukemic cells during follow-up. Evaluation of cells displaying leukemia-associated phenotypes was performed using a two-step acquisition procedure as previously described.21 Briefly, in the first step, acquisition of all cells present in the sample was performed and at this point at least 15,000 events/tube were measured. Afterwards, in a second step, a multiparametric live-gate was used to acquire more data on gated cells that were present in low numbers within the original BM sample. Data analysis was based on the identification of cells with identical aberrant phenotypic features to those of leukemic cells at diagnosis. For that purpose, acquisition through an SSC/antigen live gate was performed and information was collected for at least 106 BM nucleated cells.
Functional analysis of multidrug resistance (MDR-1).Functional evaluation of multidrug resistance (MDR-1) was performed at diagnosis in leukemic samples using the rhodamine-123 assay,24 and staining with PE-conjugated and PE-Cy5–conjugated MoAbs specific for the identification of blast cells. Rhodamine-123 fluorescence was measured in a FACScan flow cytometer immediately after staining with appropriate combinations of MoAbs for the identification of leukemic cells. Thorough calibration and compensation was established before the acquisition using erythrocyte lysed whole PB from healthy controls stained for rhodamine-123 and both CD3-PE–conjugated and CD4-PE-Cy5–conjugated MoAb. In all cases, rhodamine-123/CD3/CD4–stained lymphocytes from normal PB samples were measured in parallel. For data analysis the PAINT-A-GATE PRO software was used, and the mean rhodamine fluorescence intensity (MFI) of both gated blast cells from the leukemic samples and CD4+ T cells from normal PB samples was recorded. Elimination of rhodamine-123 was calculated for both the blasts present in leukemia samples and CD4+ T cells from normal PB and expressed as the mean percentage of decrease in rhodamine-123 fluorescence per cell, according to the following formula in which MFI is the mean fluorescence intensity obtained: % of Rhodamine-123 Elimination = 100 − (100 × MFI at 60 minutes/MFI at 15 minutes).
To avoid day to day variability, the percentage of rhodamine 123 elimination by the blast cells was corrected according to the proportion of its elimination obtained for the normal PB CD4+ T cells (mean value ± standard deviation [SD] of 64% ± 18%) measured the same day, using the following formula: % of Corrected Elimination = (% Blast Cells Elimination × Mean % of Normal PB CD4+ T Cells Elimination)/% Normal PB CD4+ T Cells Elimination Obtained the Same Day.
Statistical methods.The χ2 and the Mann-Whitney U test were used to estimate the statistic significance of differences between groups. Survival curves were plotted according to the method of Kaplan and Meier and comparison between the curves was performed using the Breslow and Mantel-Cox tests.25 Subsequently, a multivariate analysis (stepwise regression) was performed to examine the independent effect of MRD levels at the end of induction and intensification therapy with respect to other well-defined prognostic factors (white blood cell [WBC] count, platelets, hemoglobin, levels of blast cells assessed by morphology, MDR-1, and age, all of them obtained at diagnosis) on disease-free survival. Cytogenetics were only available in 60% of the patients and for this reason were not included in the model, except for t(15; 17) that had been assessed by fluorescence in situ hybridization or PCR technique (PML/RAR) in all cases.
RESULTS
Aberrant phenotypes and blast cell subpopulations.From the 53 patients included in the MRD study, 46 cases (87%) displayed an aberrant phenotype at diagnosis, whereas the remaining 7 patients had infrequent phenotypes (<1 × 10−3 cells in normal BM have such characteristics). The distribution of the different leukemic-associated phenotypes (Table 1) was as follows: asynchronous antigen expression was observed in 44 cases (83%), cross-lineage antigen expression in 16 patients (30%), an antigen overexpression in 5 (9%), and an aberrant light scatter pattern was found in 18 cases (34%). The most common asynchronisms were CD117+/CD33+/HLA-DR− (34%) and CD34+CD117+/CD33+/HLA-DR− (21%). Regarding cross-lineage antigen expression, myeloid blast cells coexpressed CD2 in 17% of the cases, CD7 in 9%, CD19 in 4%, and CD22 in 2%. Overexpression of HLA-DR and CD34 was found in 2 cases each (4%). In 20 cases, two or more types of aberrant criteria were present.
AML: Distribution of Aberrant Phenotypes at Diagnosis
| Aberrant Phenotypes (n = 53) . | No. of Cases (%) . | Total % . |
|---|---|---|
| Cross-lineage antigen expression (n = 16) | ||
| CD2 | 9 (17%) | |
| CD5 | 1 (2%) | |
| CD7 | 5 (9%) | 30% |
| CD19 | 2 (4%) | |
| CD22 | 1 (2%) | |
| Antigen overexpression (n = 5) | ||
| CD33 | 1 (2%) | |
| CD34 | 2 (4%) | 9% |
| CD117 | 1 (2%) | |
| HLADR | 2 (4%) | |
| Aberrant light-scatter patterns (n = 18) | ||
| CD2/high FSC/SSC | 8 (15%) | |
| CD7/high FSC/SSC | 5 (9%) | |
| CD13/low FSC/SSC | 5 (9%) | |
| CD14/low FSC/SSC | 1 (2%) | |
| CD15/low FSC/SSC | 2 (4%) | 34% |
| CD19/high FSC/SSC | 1 (2%) | |
| CD33/low FSC/SSC | 3 (6%) | |
| CD34/high FSC/SSC | 2 (4%) | |
| CD117/high FSC/SSC | 3 (6%) | |
| Asynchronous antigen expression (n = 44) | ||
| CD34+CD117+CD11b+ | 4 (8%) | |
| CD34+CD117+CD56+ | 2 (4%) | |
| CD34+CD33+HLADR− | 5 (9%) | |
| CD34+CD33−CD13+ | 10 (19%) | |
| CD34+CD11b+ | 4 (8%) | |
| CD34+CD56+ | 3 (6%) | |
| CD34+CD14+ | 2 (4%) | |
| CD34+CD33+CD13− | 1 (2%) | |
| CD34+CD15+CD117− | 4 (8%) | |
| CD34−CD15+CD117+ | 3 (6%) | 83% |
| CD34+CD117+CD13+HLADR− | 1 (2%) | |
| CD34+CD117+CD33+HLADR− | 11 (21%) | |
| CD117+CD33+HLADR− | 18 (34%) | |
| CD117+CD33+CD13− | 1 (2%) | |
| CD117+CD11b+ | 7 (13%) | |
| CD117+CD56+ | 3 (6%) | |
| CD34−CD14−CD15−CD33+ | 10 (19%) | |
| CD15+CD13−HLADR+ | 2 (4%) | |
| CD14+CD13−HLADR+ | 1 (2%) |
| Aberrant Phenotypes (n = 53) . | No. of Cases (%) . | Total % . |
|---|---|---|
| Cross-lineage antigen expression (n = 16) | ||
| CD2 | 9 (17%) | |
| CD5 | 1 (2%) | |
| CD7 | 5 (9%) | 30% |
| CD19 | 2 (4%) | |
| CD22 | 1 (2%) | |
| Antigen overexpression (n = 5) | ||
| CD33 | 1 (2%) | |
| CD34 | 2 (4%) | 9% |
| CD117 | 1 (2%) | |
| HLADR | 2 (4%) | |
| Aberrant light-scatter patterns (n = 18) | ||
| CD2/high FSC/SSC | 8 (15%) | |
| CD7/high FSC/SSC | 5 (9%) | |
| CD13/low FSC/SSC | 5 (9%) | |
| CD14/low FSC/SSC | 1 (2%) | |
| CD15/low FSC/SSC | 2 (4%) | 34% |
| CD19/high FSC/SSC | 1 (2%) | |
| CD33/low FSC/SSC | 3 (6%) | |
| CD34/high FSC/SSC | 2 (4%) | |
| CD117/high FSC/SSC | 3 (6%) | |
| Asynchronous antigen expression (n = 44) | ||
| CD34+CD117+CD11b+ | 4 (8%) | |
| CD34+CD117+CD56+ | 2 (4%) | |
| CD34+CD33+HLADR− | 5 (9%) | |
| CD34+CD33−CD13+ | 10 (19%) | |
| CD34+CD11b+ | 4 (8%) | |
| CD34+CD56+ | 3 (6%) | |
| CD34+CD14+ | 2 (4%) | |
| CD34+CD33+CD13− | 1 (2%) | |
| CD34+CD15+CD117− | 4 (8%) | |
| CD34−CD15+CD117+ | 3 (6%) | 83% |
| CD34+CD117+CD13+HLADR− | 1 (2%) | |
| CD34+CD117+CD33+HLADR− | 11 (21%) | |
| CD117+CD33+HLADR− | 18 (34%) | |
| CD117+CD33+CD13− | 1 (2%) | |
| CD117+CD11b+ | 7 (13%) | |
| CD117+CD56+ | 3 (6%) | |
| CD34−CD14−CD15−CD33+ | 10 (19%) | |
| CD15+CD13−HLADR+ | 2 (4%) | |
| CD14+CD13−HLADR+ | 1 (2%) |
The total number of aberrant cases is 46. The number of cases with 1 aberrancy is 26 cases, with 2 aberrancies is 5 cases, with 3 aberrancies is 13 cases, and with 4 aberrancies is 2 cases.
At diagnosis, more than one blast cell subpopulation was immunophenotypically identified in 39 cases (74%; 2 in 25 cases [47%], 3 in 11 cases [21%], and 4 in 3 cases [6%]). In 12 of these cases, all blast cell subsets shared a common phenotypic aberrancy that allowed their simultaneous identification for MRD studies. In the remaining 27 cases, each cell subpopulation was followed according to its specific leukemic-associated phenotypic pattern.
Immunophenotypical detection of MRD and patients outcome.A total of 371 BM samples from 53 patients in morphologic CR were analyzed for the assessment of MRD. In this report, analysis has been restricted to BM samples obtained at the end of induction and intensification therapy.
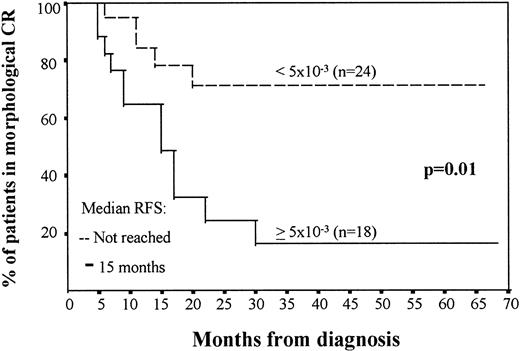
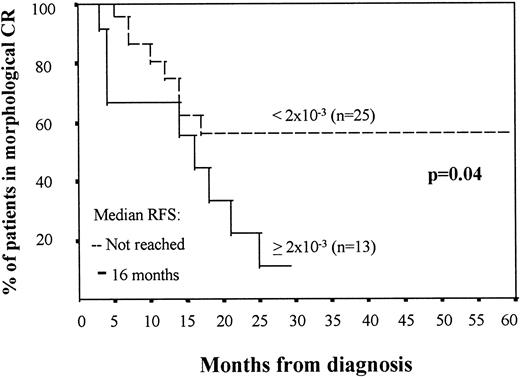
The median number of residual leukemic cells in the BM obtained at the end of induction therapy was 3 × 10−3 (ranging between 4 × 10−2 and 1 × 10−4), whereas at the end of intensification the median number was 1 × 10−3 (ranging between 2.3 × 10−2 and 2 × 10−5 cells). In both cases, the number of residual cells correlated with the incidence of relapses and RFS. It should be emphasized that both after induction and intensification therapy, several cut-off values (1 × 10−3, 2 × 10−3, 3 × 10−3, 4 × 10−3, 5 × 10−3, and 6 × 10−3, all around the median level of residual cells found in CR BM samples in this series) had a significant influence on disease outcome; for this study, we have selected the threshold displaying the highest statistical significance. In the first remission BM sample, using a threshold of 5 × 10−3 residual cells phenotypically identified as leukemic, AML patients were divided into two groups with a different rate of relapse (67% of the patients with ≥5 × 10−3 residual pathologic cells relapsed, whereas only 20% of the cases with <5 × 10−3 leukemic cells relapsed; P = .002) and different RFS (median of 17 months v not reached; P = .01; Fig 1). At the end of intensification therapy in patients in morphologic CR, a cutoff value of 2 × 10−3 residual leukemic cells per total BM nucleated cells also identified two distinct groups with relapse rates of 69% versus 32% (P = .02) and a median RFS of 16 months versus not reached (P = .04; Fig 2). OS was also significantly associated with the level of residual leukemic cells in BM samples obtained at the end of induction and particularly intensification therapy. Patients with high levels of MRD (≥2 × 10−3) at the end of intensification therapy had a median OS of 25 months versus not reached for those with lower levels (<2 × 10−3; P = .008). Multivariate analysis confirmed that the number of residual leukemic cells is independent of other well-defined prognostic factors obtained at diagnosis, such as WBC counts and age for prediction of RFS (P = .007 and P = .04 for the MRD level at the end of induction and intensification therapy, respectively). Multidrug resistance as measured by the rhodamine-123 efflux assay and t(15; 17) did not show a significant influence on the Cox regression model.
RFS in AML patients in morphologic CR depending on the number of leukemic cells in BM aspirate, assessed by immunophenotype, after induction treatment. The two groups of patients are as follows: (A) ≥5 × 10−3 leukemic cells (n = 18), with a median survival of 17 months; and (B) less than 5 × 10−3 leukemic cells (n = 24), in which the median survival has not been reached.
RFS in AML patients in morphologic CR depending on the number of leukemic cells in BM aspirate, assessed by immunophenotype, after induction treatment. The two groups of patients are as follows: (A) ≥5 × 10−3 leukemic cells (n = 18), with a median survival of 17 months; and (B) less than 5 × 10−3 leukemic cells (n = 24), in which the median survival has not been reached.
RFS in AML patients in morphologic CR depending on the number of leukemic cells in BM aspirate, assessed by immunophenotype, after intensification treatment. The two groups of patients are as follows: (A) ≥2 × 10−3 leukemic cells (n = 13), with a median survival of 16 months; and (B) less than 2 × 10−3 leukemic cells (n = 25), in which the median survival has not been reached. The date of BM aspirate after intensification treatment was considered as day 0 for RFS analysis.
RFS in AML patients in morphologic CR depending on the number of leukemic cells in BM aspirate, assessed by immunophenotype, after intensification treatment. The two groups of patients are as follows: (A) ≥2 × 10−3 leukemic cells (n = 13), with a median survival of 16 months; and (B) less than 2 × 10−3 leukemic cells (n = 25), in which the median survival has not been reached. The date of BM aspirate after intensification treatment was considered as day 0 for RFS analysis.
Influence of multidrug resistance at diagnosis on the level of residual disease.We have explored whether residual disease was related with the functional expression of multidrug resistance (MDR-1) at diagnosis as assessed by the rhodamine-123 assay. In 26 patients, information about this parameter and the number of residual blast cells at the end of induction therapy was simultaneously available. Patients with ≥5 × 10−3 residual leukemic cells had a significantly higher rhodamine-123 efflux (mean ± SD, 56% ± 24%) than those with less than 5 × 10−3 residual cells (mean ± SD, 32% ± 31%; P = .04). In addition, in 15 patients, the relationship between MDR-1 and residual disease after intensification therapy could be explored. Again, patients with a high number of residual leukemic cells (≥2 × 10−3) displayed at diagnosis a higher (P = .02) multidrug resistance (mean ± SD, 57% ± 21%) as compared with those with less than 2 × 10−3 residual cells (mean ± SD, 34% ± 27%).
DISCUSSION
Morphologic analysis of BM is very useful for defining a subgroup of patients that do not achieve CR and display a very poor prognosis. However, among those that achieve CR, a high incidence of relapses persists and these cannot be predicted using conventional morphology. Therefore, it is necessary to search for alternative approaches that would allow the detection of lower levels of residual leukemic cells, with a potentially higher value for predicting relapse. In the present study it is shown that the immunophenotypic investigation of MRD is useful to predict outcome in AML patients in morphologic CR and perhaps the term immunologic remission could replace that of morphologic CR or at least be complementary to it.
Few studies have been performed on the clinical value of immunophenotypical investigation of MRD in AML and they are usually based on small series of patients. Both Adriaasen et al16 and Campana et al15 have sequentially studied 15 and 10 AML cases, respectively, that displayed cross-lineage antigen expression (TdT/CD13; TdT/CD33). In most cases, relapse could be predicted based on this phenotypical aberrancy. More recently, Campana and Pui2 have reported on 13 children in CR after BM transplantation showing the value of multiparametric flow cytometry analysis for predicting relapse. Reading et al11 have studied the marrow at first remission in 16 AML patients using three-color antigen stainings. Six cases had greater than 2 × 10−3 phenotypically aberrant cells and all relapsed, whereas only 1 of the remaining 10 patients (<2 × 10−3 aberrant cells) relapsed. Similar results were obtained by Sievers et al26 in pediatric AML patients.
In the present study, including a larger series of patients, it was observed that the number of residual cells displaying leukemic-associated phenotypes correlated with the probability of relapse, remission duration, and the OS. This was found both for the level of residual leukemic cells detected at the end of induction treatment and after intensification therapy. A threshold of MRD of 5 × 10−3 and 2 × 10−3 leukemic cells per total BM nucleated cells after induction and intensification treatments, respectively, allowed the identification of two groups of patients with a significantly different prognosis, which may be particularly important in taking decisions such as BM transplantation for patients at high risk of relapse. In addition, our study showed that this is an independent adverse prognostic factor for RFS. However, larger series of patients, including information on cytogenetic and molecular abnormalities, are needed to define the final value of this new parameter. A major concern of immunophenotypical investigations of MDR is the effort and time required to perform this type of analysis. In our experience, at diagnosis the time taken is doubled because a second step has to be performed to select the best combinations of MoAbs and fluorochromes to establish the phenotypic probes to be used during follow-up. By contrast, analysis of follow-up samples does not imply an increase in the effort required, except that a minimum of 106 cells should be measured (this acquisition takes approximately 10 minutes for the 2 or 3 MoAb combinations usually needed).
To the best of our knowledge, the possible influence of multidrug resistance on the level of residual leukemic cells has not been previously explored. The number of residual leukemic cells would reflect the in vivo resistance of the overall leukemic population to the chemotherapeutic agents used during induction treatment. One of the best characterized mechanisms of chemotherapeutic resistance in AML is that mediated by the multidrug resistance 1 protein (MDR-1).24 27-30 This can be assessed by detection of mRNA transcripts or MDR-1 protein or by measuring the efflux of either drugs or fluorescent dyes, such as rhodamine 123, that are pumped outside the cell through this protein. Most of the previously reported studies in which rhodamine-123 functional assays were used for the assessment of MDR-1 activity did not use simultaneous stainings for the specific identification of leukemic cells; thus, the reported results may not reflect the specific efflux of drugs from the tumor cells. In the present study, rhodamine-123 efflux was specifically assessed for the leukemic cells as identified through double antigenic stainings. Interestingly, according to our results, the level of multidrug resistance measured by the rhodamine-123 assay at diagnosis in patients that were considered to be in morphologic CR correlates with the number of residual cells displaying leukemic associated phenotypes both at the end of induction and intensification therapy.
Although further series including larger numbers of patients are needed to confirm our results, the present study shows that immunologic evaluation of residual disease contributes to the prediction of outcome in AML patients in morphologic CR and that the immunophenotypical assessment of remission is more accurate than the morphologic examination.
Supported in part by national grants from Spain (FISS 95/1640; CITYT SAF 94-308 and AECC-95).
Presented in part at the 1996 ASH meeting (December 6-10, 1996, Orlando, FL).
This study is integrated in the European BIOMED 1 Concerted Action (BMH-CMT 94-1675).
Address reprint requests to J.F. San Miguel, MD, PhD, Servicio de Hematologı́a, Hospital Universitario, Paseo de San Vicente 58-182, 37007 Salamanca, Spain.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal