Abstract
Virally infected cells degrade intracellular viral proteins proteolytically and present the resulting peptides in association with major histocompatibility complex (MHC) class I molecules to CD8+ cytotoxic T lymphocytes (CTLs). These cells are normally prone to CTL-mediated elimination. However, several viruses have evolved strategies to avoid detection by the immune system that interfere with the pathway of antigen presentation. Epstein-Barr virus (EBV) expresses a predominantly late protein, the BCRF1 gene product vIL-10, that is similar in sequence to the human interleukin-10 (hIL-10). We show here that vIL-10 affects the expression of one of the two transporter proteins (TAPs) associated with antigen presentation. Similarly, hIL-10 showed the same activity. Expression of the LMP2 and TAP1 genes but not expression of TAP2 or LMP7 is efficiently downregulated, indicating a specific IL-10 effect on the two divergently transcribed TAP1 and LMP2 genes. Downregulation of TAP1 by IL-10 hampers the transport of peptide antigens into the endoplasmatic reticulum, as shown in the TAP-specific peptide transporter assay, their loading onto empty MHC I molecules, and the subsequent translocation to the cell surface. As a consequence, IL-10 causes a general reduction of surface MHC I molecules on B lymphocytes that might also affect the recognition of EBV-infected cells by cytotoxic T cells.
EPSTEIN-BARR VIRUS (EBV) is a human tumor virus that establishes a life-long latent infection and affects the control of proliferation of infected cells. In particular, EBV induces and maintains proliferation of infected human B lymphocytes in vivo and in vitro. In cell culture, this process is termed immortalization, but early events during infection and immortalization of primary human B lymphocytes are far from understood.1 We applied the differential display technique2 to identify cellular genes that are regulated early in response to EBV infection. Primary B lymphocytes from tonsils, adenoids, and peripheral blood were incubated with supernatants of the EBV-positive marmoset cell line B95.8.3 These supernatants contain infectious EBV released by B95.8 cells that support the lytic cycle of the virus efficiently.
Differentially expressed RNAs present only in infected or noninfected cells were cloned and sequenced. One of the differentially expressed RNAs was identified as a partial cDNA encoding a subunit of the transporter associated with antigen presentation (TAP). The TAP proteins belong to the ATP-dependent family of transporters and are known to play a central role in the transport of peptides derived from proteolytic degradation in the proteasome.4-7 These peptides are translocated by the TAP heterodimer from the cytosol across the membrane into the lumen of the endoplasmatic reticulum (ER), where they are loaded onto empty class I major histocompatibility complex (MHC) molecules in conjunction with β2-microglobulin.8,9 Subsequently, this complex is transported to the cell surface and presented to class I-restricted CD8+ cytotoxic T lymphocytes (CTLs).10,11 As a consequence, lack of antigen transporters is known to result in the retention of MHC class I molecules in the ER and presentation of empty MHC molecules on the cell surface with a dramatically reduced half-life.12,13 We show here that EBV produces interleukin-10 (IL-10) to downregulate TAP, which in turn reduces the cell surface density of class I MHC molecules and affects cellular antigen presentation. Several mechanisms of immune escape have been described for different viruses.14-20 The use of a cytokine as a mechanism of virus-mediated immunosuppression provides a novel molecular basis for the activities of IL-10 as well as for the advantages for EBV in having adopted the homologue of the cellular IL-10 gene.
MATERIALS AND METHODS
Cell preparation and virus infection.Primary human B lymphocytes were isolated from routine tonsillectomies and adenectomies by generating single-cell suspensions and T-cell depletion by rosetting with whole sheep blood. The preparations were analyzed by fluorescence-activated cell sorting (FACS) and were found to be greater than 95% positive for the pan-B marker CD19 (DAKO, Hamburg, Germany). A total of 1 to 5 × 108 cells were incubated with 200 mL of cell-free B95.8 supernatant for 48 hours at 37°C. Control cells were incubated with cell culture medium only. Cell-free B95.8 supernatant was generated by pelleting densely grown B95.8 cells at 350g and passing the supernatant through a 0.45-μm filter (Nalgene, Bruxelles, Belgium). Virus-free supernatant (VFS) was obtained by ultracentrifugation of cell-free B95.8 supernatant for 4 hours at 30,000g. After ultracentrifugation, viral DNA was not detectable by polymerase chain reaction (PCR) with primer pair combinations specific for B95.8 DNA (data not shown). Supernatant from DG75 cells was generated exactly as described for B95.8 cell-free supernatant. All supernatants were supplemented with 10% fetal calf serum.
Concentration and synthesis of IL-10.Concentrations of IL-10 in supernatants from B95.8, DG75, and Cos7 cells (after transfection with expression plasmids for vIL-10 and hIL-10) were determined and calculated with sandwich enzyme-linked immunosorbent assays (ELISAs) exactly as recommended by the manufacturer (Pharmingen, San Diego, CA). Recombinant IL-10s were diluted in culture medium to concentrations as they are present in B95.8 supernatant. Mock-treated control cells were incubated with RPMI 1640 medium with 10% fetal calf serum supplemented with supernatant from untransfected Cos7 cells. Recombinant vIL-10 and hIL-10 were produced by transient transfection of Cos7 cells with expression plasmids obtained from Dr Kevin Moore (DNAX, Palo Alto, CA) or were purchased from Biomol (Heidelberg, Germany) and used according to the manufacturer's instructions.
RNA preparations and Northern blotting.Total cellular RNA was prepared as described.21 For Northern blots, 10-μg RNA samples were used for electrophoresis through 1% agarose formaldehyde gels that were transferred to Hybond N+ membranes (Amersham Buchler, Braunschweig, Germany). Molecularly cloned cDNAs for TAP1 and TAP2 were labeled using a random priming kit (Boehringer Mannheim, Mannheim, Germany). Hybridizations were performed as described.21
Reverse transcription-PCR (RT-PCR).Total RNA from primary B lymphocytes was isolated after 48 hours of incubation with native B95.8 supernatant or VFS from the same cells. The RNA preparations were treated with RNase-free DNase (Boehringer Mannheim) for 30 minutes at 37°C. After inactivation of the enzyme, 1 μg RNA was reverse transcribed (+RT; Superscript Plus; GIBCO BRL, Gaithersburg, MD) with an oligo-dT primer for 60 minutes at 37°C. Reverse transcription was omitted for control purposes where indicated (−RT). PCR with one tenth of the volume (2 μL) was performed in a buffer containing 1.5 mmol/L MgCL2 , 100 pmol of each primer, 0.2 mmol/L final concentration of each dNTP, and 0.5 μL Goldstar Taq-polymerase (Eurogentec, Seraing, Belgium) in a final volume of 50 μL in a Perkin Elmer thermal cycler (Perkin Elmer, Norwalk, CT). PCR primers were 5′-TGGAGCGAAGGTTAGTGGTCAC-3′ and 5′-ATGGTCTTTGGCTTCAGGGTCC-3′ for BCRF1. Amplified bands were analyzed by electrophoresis through a 1.5% agarose gel and ethidium bromide staining.
Western blot analysis.Cell extracts were separated on 10% polyacrylamide-sodium dodecyl sulfate gels, transferred to Hybond C (Amersham), and probed with a TAP1-specific murine monoclonal antibody. Detection was preformed using an ECL system (Amersham). Protein concentrations were determined using an BCA Protein Assay kit (Pierce, Rockford, IL).
FACS analysis.Peripheral blood mononuclear cells were isolated from heparinized blood by Ficoll density centrifugation. Before FACS analysis, the cell sample was incubated for 2 hours in a cell culture flask to remove the monocyte/macrophage fraction by adhesion. CD3+ cells were depleted with a CD3-specific monoclonal antibody (Dianova, Hamburg, Germany) coupled to Dynabeads (Dynal, Hamburg, Germany). After purification, the content of B cells was greater than 95% as determined with a CD19-specific antibody. After incubation with different supernatants for 48 hours, the cells were washed in cold phosphate-buffered saline (PBS) and incubated with the anti-MHC class I antibody w6/32 (ATCC, Rockville, MD) directed against nonpolymorphic determinants of MHC class I molecules or a negative control antibody, diluted in PBS/10% FCS for 1 hour on ice. After a single washing, the cells were incubated with a secondary antimouse IgG-fluorescein isothiocyanate–conjugated antibody (DAKO, Hamburg, Germany) for 45 minutes. Cells were analyzed after a final washing. Flow cytometry was performed using a FACS flow cytometer and the CellQuest analysis program (Becton Dickinson, Heidelberg, Germany).
Peptide iodination and translocation.The peptide RRYQNSTEL was synthesised on a multiple peptide synthesizer (MultiSynTech, Bochum, Germany) using conventional Fmoc-chemistry. The purity was determined by high-performance liquid chromatography to be greater than 95%. Twelve micrograms of peptide was radiolabeled by chloramine T-catalyzed iodination22 with 3 × 107 Bq Na 125I to a specific activity of 3 × 1014 Bq/mol. Free iodine was removed by gel filtration through a Sephadex G-10 column (Pharmacia, Uppsala, Sweden).
The peptide translocation assay was essentially performed as described.8 23 Briefly, for each sample, 1 × 107 cells were harvested by centrifugation, washed once, and resuspended in 1 mL cold RPMI. An aqueous solution of Streptolysin O (Murex, Dartford, UK) was preactivated for 10 minutes at 37°C with 4 mmol/L dithiothreitol (DTT) and added at a final concentration of 1 U/mL. After incubation on ice for 10 minutes, cells were washed twice with cold RPMI, resuspended in 650 μL warm translocation buffer (78 mmol/L KCl, 4 mmol/L MgCl2 , 4 mmol/L CaCl2 , 5 mmol/L EGTA, 1 mmol/L DTT, 4 mg/mL bovine serum albumin, 50 mmol/L HEPES/KOH, pH 7.0) and permeabilized for 5 minutes at 37°C. Permeabilization was 75% to 85% as determined by trypan blue exclusion. To 150-μL aliquots, 2 U apyrase (Sigma, Deisenhofen, Germany) or 5 mmol/L ATP and radiolabeled peptide was added to a final concentration of 300 nmol/L. After incubation for 2.5 minutes at 37°C, 800 μL ice-cold translocation buffer was added to stop the reaction. After centrifugation, cell pellets were lysed in 1 mL lysis buffer (1% NP-40, 150 mmol/L NaCl, 5 mmol/L MgCl2 , 50 mmol/L Tris-Cl, pH 7.0) and the amount of glycosylated peptide was quantified by γ-counting after binding to Concanavalin A-Sepharose (ConA; Pharmacia) and elution with 200 mmol/L α-methylmannoside.
RESULTS
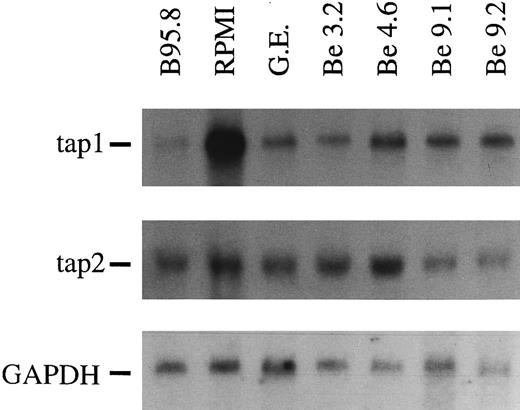
A soluble factor present in B95.8 supernatant downregulates the expression of TAP1.In our attempts to identify genes whose expressions are affected by EBV shortly after infection, we used the differential display technique.2 Among several differently expressed RNAs, we identified a partial cDNA of one of the antigen transporters (TAP). To confirm this initial finding, Northern blots with total cellular RNAs were performed. As shown in Fig 1, we observed a dramatic decrease in TAP1 expression within 48 hours of EBV infection relative to its expression in noninfected primary human B cells (Fig 1, lanes 1 and 2). Reduction of TAP1 specific mRNA levels to 1/10 to 1/20 as compared with untreated B cells was apparent in B-cell preparations from more than 25 different donors. Reduction was complete as early as 20 hours after infection when MHC class I levels were still high (data not shown) and was maintained in EBV-immortalized B-cell lines at a slightly increased level (Fig 1 and below). Reduction of mRNA expression was specific for TAP1 and could not be observed for the TAP2 gene (Fig 1). Similarily, mRNA steady-state levels of MHC class I genes were only marginally affected (not shown). The magnitude of downregulation of TAP1 mRNA was unexpected and could indicate that the majority of primary B cells were infected with EBV. Alternatively, the lower steady-state levels of TAP1 mRNA could be caused by a soluble factor released either from the infected B lymphocytes and/or from the EBV-infected B95.8 cell line.
In Northern blots, TAP1 mRNA levels in primary tonsillar B lymphocytes are reduced after 48 hours of incubation with B95.8 supernatant (lane 1, labeled B95.8) in comparison with the same B-cell preparation incubated with cell culture medium (lane 2, labeled RPMI) only. Immortalized B-cell lines established by EBV infection in vitro also show a reduced TAP1 expression, although the reduction was not as severe as in primary B lymphocytes incubated with B95.8 supernatant (lanes 3 through 7, labeled G.E., Be 3.2, Be 4.6, Be 9.1, and Be 9.2, respectively). The steady state of TAP2 mRNA is unaltered or only slightly affected in the same RNA preparations. GAPDH-specific hybridization of the Northern blot indicated that the amount of mRNA loaded is about the same in all lanes.
In Northern blots, TAP1 mRNA levels in primary tonsillar B lymphocytes are reduced after 48 hours of incubation with B95.8 supernatant (lane 1, labeled B95.8) in comparison with the same B-cell preparation incubated with cell culture medium (lane 2, labeled RPMI) only. Immortalized B-cell lines established by EBV infection in vitro also show a reduced TAP1 expression, although the reduction was not as severe as in primary B lymphocytes incubated with B95.8 supernatant (lanes 3 through 7, labeled G.E., Be 3.2, Be 4.6, Be 9.1, and Be 9.2, respectively). The steady state of TAP2 mRNA is unaltered or only slightly affected in the same RNA preparations. GAPDH-specific hybridization of the Northern blot indicated that the amount of mRNA loaded is about the same in all lanes.
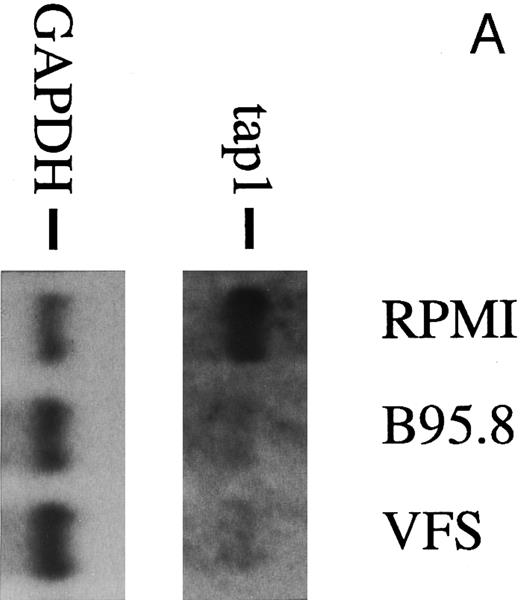
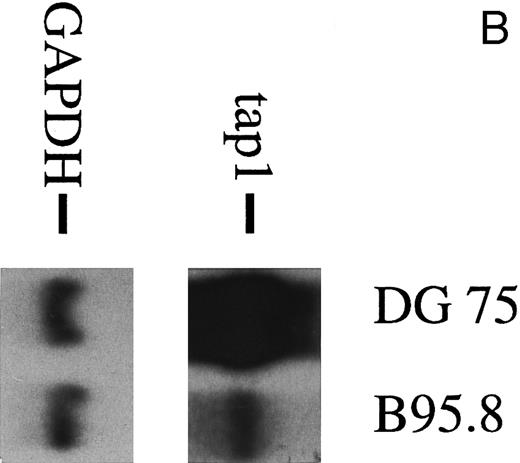
To analyze the nature of the effect more closely, human primary B lymphocytes were incubated with untreated B95.8 supernatant or B95.8 supernatant after the removal of virus particles by ultracentrifugation. After incubation with VFS or untreated B95.8 supernatant, expression of the TAP1 gene was equally diminished (Fig 2A). No such effect was seen when cells were incubated with supernatants from the EBV-negative Burkitt's lymphoma (BL) cell line DG7524 (Fig 2B). We concluded that only a factor produced by the B95.8 cell line could be responsible for the observed reduction of TAP1 expression and that this factor is soluble. A candidate for such a factor is the viral homologue of the cellular human IL-10 (hIL-10). This gene product is encoded by the EBV open reading frame BCRF1, exhibits a high sequence similarity to hIL-10, and has similar activities.25 26 Thus, vIL-10 has often been considered as a possible agent for EBV to evade the host's immune system, but its potential mechanism has remained obscure.
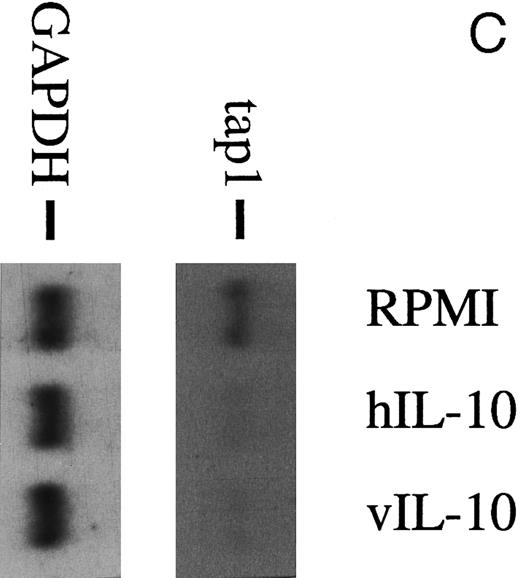
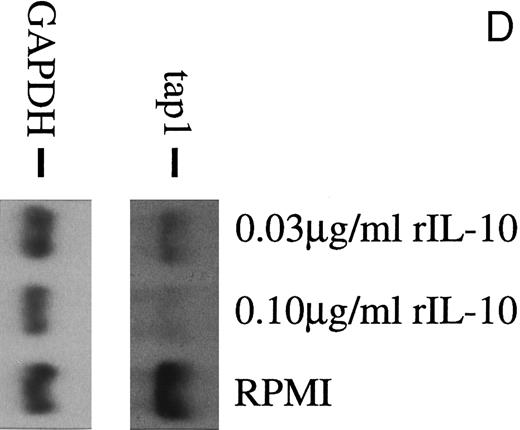
(A) TAP1 mRNA levels in primary B cells from two different donors are strikingly reduced after incubation with either B95.8 supernatant (labeled B95.8) or VFS (labeled VFS) in comparison with cells incubated with culture medium only (labeled RPMI). (B) The TAP1 mRNA level is not reduced with cell culture supernatant derived from the EBV-negative, IL-10–negative cell line DG7524 in comparison with reduced levels detected after treatment with B95.8 supernatant. (C) Cell culture medium (RPMI with 10% fetal calf serum) with appropriate dilutions of recombinant vIL-10 and hIL-10 from transiently transfected Cos7 cells causes the same reduction of TAP1 steady-state mRNA levels in primary B lymphocytes as does treatment with B95.8 shown in (A). B lymphocytes from two different donors were analyzed in parallel. (D) TAP1 mRNA steady-state level is reduced in tonsillar B cells after incubation with commerially available recombinant human IL-10 in a dose-dependent manner.
(A) TAP1 mRNA levels in primary B cells from two different donors are strikingly reduced after incubation with either B95.8 supernatant (labeled B95.8) or VFS (labeled VFS) in comparison with cells incubated with culture medium only (labeled RPMI). (B) The TAP1 mRNA level is not reduced with cell culture supernatant derived from the EBV-negative, IL-10–negative cell line DG7524 in comparison with reduced levels detected after treatment with B95.8 supernatant. (C) Cell culture medium (RPMI with 10% fetal calf serum) with appropriate dilutions of recombinant vIL-10 and hIL-10 from transiently transfected Cos7 cells causes the same reduction of TAP1 steady-state mRNA levels in primary B lymphocytes as does treatment with B95.8 shown in (A). B lymphocytes from two different donors were analyzed in parallel. (D) TAP1 mRNA steady-state level is reduced in tonsillar B cells after incubation with commerially available recombinant human IL-10 in a dose-dependent manner.
Recombinant vIL-10 and hIL-10 both reduce the expression of TAP1.Recombinant hIL-10 and vIL-10 were produced in Cos7 cells transiently transfected with expression plasmids for vIL-10 or hIL-10. The release of hIL-10 and vIL-10 into the supernatant was confirmed using a sandwich ELISA with monoclonal antibodies specifically directed against hIL-10 or vIL-10 as described in the Materials and Methods. Freshly isolated B lymphocytes were incubated in cell culture medium containing recombinant hIL-10 or vIL-10 at concentrations as in B95.8 supernatants (approximately 100 to 200 pg/mL), which corresponds to IL-10 serum levels during acute EBV infections.27 After 48 hours, the steady-state level of TAP1 mRNA in Northern blot hybridizations was clearly reduced with both vIL-10 and hIL-10 (Fig 2C), similar to the levels seen after incubation with B95.8 supernatant, VFS (Fig 2A), and highly purified, recombinant hIL-10 (Fig 2D). Protein levels of TAP1 were less dramatically reduced to about one third of that observed in untreated cells after treatment for 48 hours, indicating that the half life of the TAP1 gene product is much longer than its mRNA half life (Fig 3). These experiments indicate that downregulation of TAP1 is most probably caused by vIL-10 that is present in B95.8 cell culture supernatants.
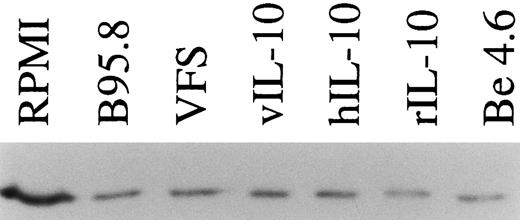
TAP1 protein levels in primary tonsillar B lymphocytes are reduced to about one third after incubation with IL-10 or IL-10 containing supernatants (supernatant from B95.8 cells, VFS, supernatant-derived Cos7 cells transiently transfected with expression plasmids of hIL-10 and vIL-10, or 0.03 μg/mL purified rIL-10) in comparison with cell culture medium (RPMI). After 48 hours of incubation, TAP1 protein is reduced but still detectable, presumably due to its extended protein half life. An in vitro-generated LCL (Be 4.6, lane 7) produces significantly lower levels of TAP1 as compared with primary B cells. Equal amounts of total cellular protein were loaded in each lane. TAP1 protein was visualized after Western blotting with a monoclonal antibody directed against TAP1.
TAP1 protein levels in primary tonsillar B lymphocytes are reduced to about one third after incubation with IL-10 or IL-10 containing supernatants (supernatant from B95.8 cells, VFS, supernatant-derived Cos7 cells transiently transfected with expression plasmids of hIL-10 and vIL-10, or 0.03 μg/mL purified rIL-10) in comparison with cell culture medium (RPMI). After 48 hours of incubation, TAP1 protein is reduced but still detectable, presumably due to its extended protein half life. An in vitro-generated LCL (Be 4.6, lane 7) produces significantly lower levels of TAP1 as compared with primary B cells. Equal amounts of total cellular protein were loaded in each lane. TAP1 protein was visualized after Western blotting with a monoclonal antibody directed against TAP1.
BCRF1 is expressed early during infection.vIL-10, the gene product of the BCRF1 EBV gene, is thought to be expressed during the lytic cycle of EBV,28 but has also been found to be expressed in primary B cells during early stages of infection.29 We used an RT-PCR approach to confirm the expression of BCRF1 early after infection of primary B cells. After only 2 days, the expression of BCRF1 was clearly detectable in cells infected with supernatant from B95.8 cells but not with VFS (Fig 4). BCRF1 is not detectably expressed in established B cells immortalized by EBV in vitro or in EBV-positive Burkitt's lymphoma cell lines (BL); instead, high amounts of cellular IL-10 are often produced.30 It thus appears that BCRF1 is expressed very early during B-cell infection but is later functionally replaced by cellular IL-10. As a consequence, TAP1 mRNA expression and protein levels are markedly reduced in in vitro established B-cell lines immortalized by EBV compared with primary human B lymphocytes (Fig 1, lanes 3 through 7, and Fig 3).
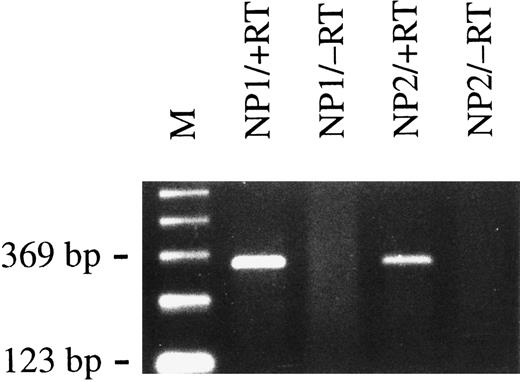
Primary B lymphocytes from adenoids express BCRF1 early after infection with B95.8 virus as determined by RT-PCR with BCRF1-specific primers (+RT). Total cellular RNA preparations from nasal polyps of two different donors (NP1 and NP2) were used. No PCR amplification product could be detected when reverse transcriptase was omitted, as shown in lanes labeled −RT, as a negative control. M indicates the 123-bp ladder as a molecular weight marker.
Primary B lymphocytes from adenoids express BCRF1 early after infection with B95.8 virus as determined by RT-PCR with BCRF1-specific primers (+RT). Total cellular RNA preparations from nasal polyps of two different donors (NP1 and NP2) were used. No PCR amplification product could be detected when reverse transcriptase was omitted, as shown in lanes labeled −RT, as a negative control. M indicates the 123-bp ladder as a molecular weight marker.
IL-10 downregulates LMP2 but not LMP7, MHC class I, or MHC class II molecules.Intuitively, it seemed unlikely that IL-10 would specifically target only the TAP1 gene, because IL-10 has been reported to downregulate CD1, a class I-like but TAP-independent molecule, cytokines such as IL-1α, IL-1β, IL-6, tumor necrosis factor α, and MHC class II molecules on monocytes.31-34 Therefore, we analyzed the status of the LMP2 gene (Fig 5) that shares a bidirectional promoter element with TAP1,35 the mRNA levels of LMP7, and MHC class I. Steady-state mRNA levels of LMP2 and TAP1 were found to be downregulated by vIL-10 and hIL-10 containing supernatants to almost the same extent (Fig 5). In contrast, B cells incubated with B95.8 supernatant or recombinant IL-10 cytokine still expressed 80% to 90% of the levels of class I heavy chain and LMP7 mRNAs in comparison with controls (data not shown). We also monitored the level of MHC class II molecules in B-cell preparations by FACS analysis and did not find a reduction of MHC class II at the cell surface (data not shown), which is in agreement with a previous report.34
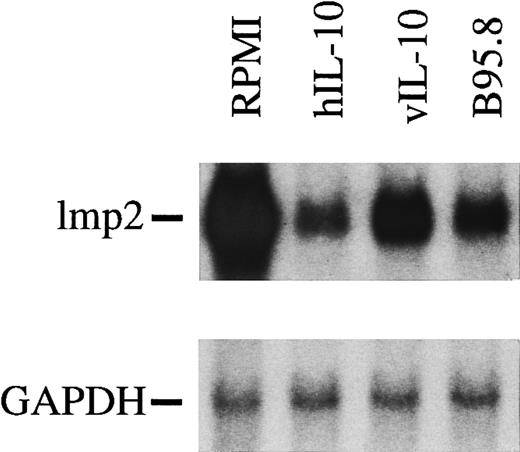
mRNA levels of lmp2 in B cells are significantly reduced after incubation with viral IL-10 (vIL-10), cellular IL-10 (hIL-10), or B95.8 supernatants containing IL-10 (B95.8) in comparison to cells that had been incubated with RPMI only. hIL-10 seems to have the most dramatic effect on the reduction of lmp2 expression.
mRNA levels of lmp2 in B cells are significantly reduced after incubation with viral IL-10 (vIL-10), cellular IL-10 (hIL-10), or B95.8 supernatants containing IL-10 (B95.8) in comparison to cells that had been incubated with RPMI only. hIL-10 seems to have the most dramatic effect on the reduction of lmp2 expression.
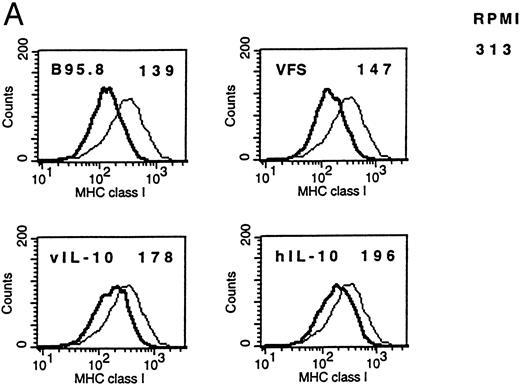
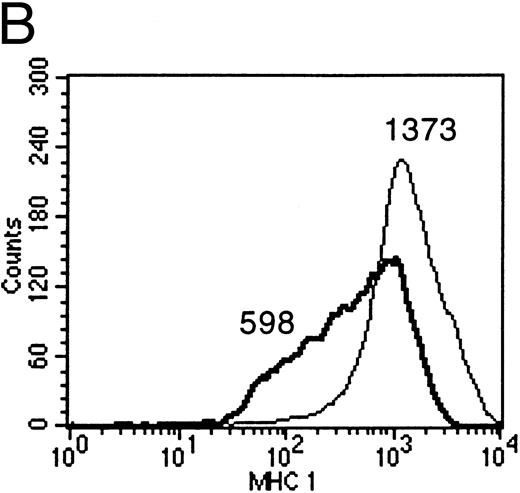
IL-10 downregulates membrane-associated MHC molecules on primary B lymphocytes.Empty class I MHC molecules are retained in the ER and only the ternary complex of MHC heavy chain, β2-microglobulin, and bound peptide is stably presented at the cell surface. Cells deficient for TAP function are not capable of presenting antigens efficiently for this reason.10 Therefore, IL-10–mediated reduction of TAP1 protein was expected to cause a measurable reduction of membrane-associated class I MHC molecules only if reduced TAP1 protein levels bear biologic significance. FACS analysis with antibodies directed against nonpolymorphic domains of MHC class I molecules confirmed the reduction of surface class I MHCs after incubation of B cells with supernatants containing recombinant vIL-10, hIL-10, B95.8, or VFS (Fig 6A). The same effect was observed when purified recombinant human IL-10 was used (Fig 6B).
(A) Flow cytometric analysis shows the downregulation of surface MHC class I molecules on peripheral blood B cells after incubation for 2 days with B95.8 supernatant, VFS, or hIL-10 (200 pg/mL) or vIL-10 (200 pg/mL) derived from transiently transfected Cos7 cells. Results are given as mean fluorescence intensity values. The mean value of a reference antibody was less than 5 as a negative control (not shown). The mean fluorescence value of the control cells incubated with cell culture medium was 313 (RPMI 313). (Thin line) MHC class I expression on control cells incubated with cell culture medium, containing 1% of supernatant from untransfected Cos7 cells. (Thick line) MHC class I expression on the same cells treated with either B95.8, VFS, vIL-10, or hIL-10, which showed mean fluorescence intensity values of 139, 147, 178, and 196, respectively. (B) The same effect was observed with cells incubated in cell culture medium containing purified recombinant human IL-10 (0.03 μg/mL rIL-10) in comparison with cell culture medium only. Mean fluorescence values were 1,373 and 598, respectively.
(A) Flow cytometric analysis shows the downregulation of surface MHC class I molecules on peripheral blood B cells after incubation for 2 days with B95.8 supernatant, VFS, or hIL-10 (200 pg/mL) or vIL-10 (200 pg/mL) derived from transiently transfected Cos7 cells. Results are given as mean fluorescence intensity values. The mean value of a reference antibody was less than 5 as a negative control (not shown). The mean fluorescence value of the control cells incubated with cell culture medium was 313 (RPMI 313). (Thin line) MHC class I expression on control cells incubated with cell culture medium, containing 1% of supernatant from untransfected Cos7 cells. (Thick line) MHC class I expression on the same cells treated with either B95.8, VFS, vIL-10, or hIL-10, which showed mean fluorescence intensity values of 139, 147, 178, and 196, respectively. (B) The same effect was observed with cells incubated in cell culture medium containing purified recombinant human IL-10 (0.03 μg/mL rIL-10) in comparison with cell culture medium only. Mean fluorescence values were 1,373 and 598, respectively.
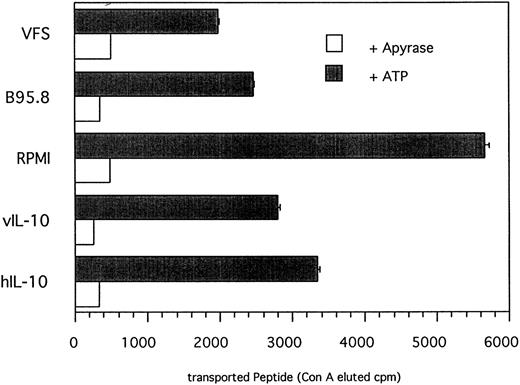
IL-10 reduces the amount of peptide translocated into the ER.Peptides that are presented in association with MHC class I molecules are generated by proteolytic degradation in the cytoplasm by proteasomes. Subsequently, antigenic peptides are translocated into the ER by TAP heterodimers acting as peptide transporters and loaded onto empty MHC I molecules. The addition of N-linked glycan onto peptides containing an N-linked glycosylation consensus site (NST; single letter code) also takes place in the ER.36 TAP activity can be determined in a peptide translocation assay13 that should allow us to discriminate between direct IL-10 effects on functional TAP levels and indirect effects on the expression of LMP2 involved in peptide processing. To measure TAP activity directly, cells pretreated either with recombinant IL-10, EBV supernatant, VFS, or RPMI only were permeabilized with Streptolysin O and the 125I-labeled peptide RRYQNSTEL was added. Glycosylated peptides were then trapped with immobilized, matrix-coupled Concanavalin A and the amount of translocated peptide, which is a direct measure of TAP activity, was determined by γ-counting of lectin-eluted peptide. As shown in Fig 7, incubation of primary B lymphocytes with recombinant IL-10, B95.8 supernatant, or VFS resulted in an approximately 50% reduction of translocated peptide in comparison to cells incubated with RPMI only. Because peptide translocation was strictly ATP-dependent, the amount of translocated peptide can be directly attributed to TAP activity. The magnitude of reduced TAP activity is in agreement with the results of the 51Cr-release assay indicating that IL-10–induced downregulation of TAP1 is capable of affecting antigen peptide transport in a functionally relevant manner.
TAP-dependent peptide translocation is significantly reduced in primary B lymphocytes incubated with B95.8 supernatant, VFS, or recombinant hIL-10 and vIL-10 in comparison with control cells incubated with cell culture medium RPMI containing supernatant from untransfected Cos7 cells. Primary B lymphocytes were incubated with the different supernatants as indicated for 2 days and permeabilyzed with Streptolysin O, and the 125I-labeled peptide RRYQNSTEL was added. Peptides that are translocated into the endoplasmatic reticulum become N-glycosylated at the position NST. Glycosylated peptides were retained on Concanavalin A-coupled sepharose matrix and eluted with α-methyl mannoside, and the eluted radioactivity was determined in a γ-counter. Results are given as ConA-eluted cpm. The addition of apyrase shows that the translocation of peptide is strictly ATP-dependent. The representative results of one of two experiments are shown.
TAP-dependent peptide translocation is significantly reduced in primary B lymphocytes incubated with B95.8 supernatant, VFS, or recombinant hIL-10 and vIL-10 in comparison with control cells incubated with cell culture medium RPMI containing supernatant from untransfected Cos7 cells. Primary B lymphocytes were incubated with the different supernatants as indicated for 2 days and permeabilyzed with Streptolysin O, and the 125I-labeled peptide RRYQNSTEL was added. Peptides that are translocated into the endoplasmatic reticulum become N-glycosylated at the position NST. Glycosylated peptides were retained on Concanavalin A-coupled sepharose matrix and eluted with α-methyl mannoside, and the eluted radioactivity was determined in a γ-counter. Results are given as ConA-eluted cpm. The addition of apyrase shows that the translocation of peptide is strictly ATP-dependent. The representative results of one of two experiments are shown.
DISCUSSION
BCRF1, the viral homologue of the human IL-10 gene, is expressed late during the lytic phase of EBV's life cycle before the release of virus particles and initially in EBV-infected B lymphocytes. EBV-immortalized B-cell lines that emerge from infection of primary B cells consistently produce high levels of hIL-10 and therefore do not downregulate TAP1 when IL-10 is added exogenously. IL-10 has been shown to have various immunosuppressive effects.31,37-40 IL-10 has also been found to interfere with the MHC class I antigen presenting pathway, but the mechanism of this observation remained unclear.41 We now provide an explanation in that hIL-10 as well as vIL-10 are equally capable of downregulating TAP1 expression, thereby interfering with the loading of MHC class I molecules, resulting in empty and unstable MHC class I/β2-microglobulin complexes.10 However, the reduction in TAP activity in the peptide translocation is only twofold to threefold within a period of 48 hours compared with a far greater decrease in TAP1 mRNA levels, which most likely reflects the stability of the TAP1 protein. Downregulation of TAP1 and reduction of MHC class I surface levels by IL-10 could also be shown in another experimental setting using the EBV-negative Burkitt's lymphoma cell line BL4142 cells, which express very little TAP1 and do not produce IL-10.30 A 51Cr-release assay with BL41 as target cells and CTLs from an allogeneic donor showed a protective effect of IL-10 against target cell lysis that is known to depend on the amout of surface MHC class I molecules (data not shown). This finding also indicates that IL-10 can modulate TAP1 expression even on the background of very different initial TAP1 levels.
Immortalized EBV-infected B-lymphocyte cell lines express hIL-10 in vitro (data not shown) but nevertheless show elevated levels of TAP1 mRNA compared with primary B lymphocytes treated with IL-10 (Fig 1). These cell lines also express the viral LMP1 gene product that is suspected to activate the TAP1 promoter through the NFκ-B/Rel pathway.35,43 Thus, the effect of vIL-10 on TAP1 expression is most likely reversed by LMP1 and, as a consequence, in vitro established immortalized human B lymphocytes are functional as antigen-presenting cells in CTL-mediated immune assays and do not respond to exogenously added IL-10.31 In contrast, EBV-infected human B lymphocytes do not express LMP1 in vivo but other immunogenic viral proteins namely LMP2A and EBNA1.44 IL-10 could therefore affect crucial TAP1 levels in vivo, suggesting that it might contribute to the persistence of EBV-infected cells in the human host. Because an MHC class I-restricted cytotoxic T-cell response is dependent on proper antigen presentation, the downregulation of TAP1 may also contribute to EBV-associated lymphomas that have been shown to express high levels of IL-10 in immunosuppressed patients.45
The viral routes of escape from CTL recognition already identified use different mechanisms than this used by EBV.46 Our data indicate that IL-10 is capable of modulating the expression of TAP1 levels in different B cells, but clearly IL-10 is not capable of abrogating TAP1 expression totally. The differences observed in MHC class I expression, sensitivity to cytotoxicity (data not shown), and peptide transport nevertheless indicate that IL-10 can efficiently modulate TAP1 functions in a biologically significant manner. This novel finding also shows a previously unknown mechanism for IL-10–induced immunosuppression that either affects transcriptional activation of the TAP1 gene or the turnover rate of its transcript. It is likely but remains to be shown that IL-10 abrogates MHC class I peptide antigen presentation by the same mechanism not only in B lymphocytes but also in other antigen-presenting cells.
ACKNOWLEDGMENT
The authors thank Dr K. Moore for expression plasmids for hIL-10 and vIL-10 and Dr J. Trowsdale for providing TAP1 and TAP2 cDNAs. We are grateful to Dr B. Sugden, Dr H. Lindhofer, and Dr H.-J. Delecluse for critically reading the manuscript.
Supported by Grant No. CA70723 from the National Institutes of Health, by Grant No. 10-1016 from Deutsche Krebshilfe, by Grant No. Ha 1354/3-1 from the Deutsche Forschungsgemeinschaft, and by institutional grants.
Address reprint requests to Reinhard Zeidler, PhD, GSF-National Research Center for Environment and Health, Marchioninistrasse 25, D-81377 München, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal