Abstract
Primary polycythemias are caused by an acquired or inborn mutation affecting hematopoietic/erythroid progenitors that results in an abnormal response to hematopoietic cytokines. Primary familial and congenital polycythemia (PFCP; also known as familial erythrocytosis) is characterized by elevated red blood cell mass, low serum erythropoietin (EPO) level, normal oxygen affinity of hemoglobin, and typically autosomal dominant inheritance. In this study we screened for mutations in the cytoplasmic domain of the EPO receptor (EPOR; exons 7 and 8 of the EPOR gene) in 27 unrelated subjects with primary or unidentified polycythemia. Two new EPOR mutations were found, which lead to truncation of the EPOR similarly to previously described mutations in PFCP subjects. The first is a 7-bp deletion (del59855991) found in a Caucasian family from Ohio. The second mutation (5967insT) was found in a Caucasian family from the Czech Republic. In both cases the EPO dose responses of the erythroid progenitors of the affected subjects were examined to confirm the diagnosis of PFCP. In one of these families, the in vitro behavior of erythroid progenitors in serum-containing cultures without the addition of EPO mimicked the behavior of polycythemia vera progenitors; however, we show that antibodies against either EPO or the EPOR distinguish the in vitro growth abnormality of polycythemia vera erythroid progenitors from that seen in this particular PFCP family. We conclude that PFCP is a disorder that appears to be associated in some families with EPOR mutations. So far, most of the described EPOR mutations (6 out of 8) associated with PFCP result in an absence of the C-terminal negative regulatory domain of the receptor.
THE TERM polycythemia (or erythrocytosis) refers to an increased number of circulating red blood cells, while primary polycythemias are conditions in which acquired or inherited mutations expressed in erythroid progenitors result in upregulation of erythroid cell numbers with normal or decreased levels of circulating cytokines. Primary familial and congenital polycythemia (PFCP; also known as familial erythrocytosis) is characterized by elevated red blood cell mass, low serum erythropoietin (EPO) level, normal oxygen affinity of hemoglobin, and typically autosomal dominant inheritance.1-4 In searching for the molecular lesion resulting in the PFCP phenotype, abnormalities of EPO or its receptor (EPOR) were considered to be likely candidates.1 Cloning of the murine EPOR cDNA5 and the human EPOR cDNA and gene6,7 enabled analysis of the receptor coding region for mutations in subjects with PFCP. Six mutations of the EPOR have been reported so far in association with primary polycythemia.4,8-12 All of the described mutations were found in exon 8 of the EPOR gene, which codes for a large portion of the intracellular domain of the native EPOR. Four out of the six described mutations (G6002A, 5974insG, C5964G, C5986T) resulted in truncation of the EPOR. These truncated EPORs lack the C-terminal negative regulatory domain of the receptor. This domain plays an important role in down regulation of the signal after ligand-induced signaling through the EPOR.13 D'Andrea et al13 prepared a series of mutant murine receptors to explore functionally important intracellular domains of the receptor. Mutant receptors lacking C-terminal 40 and 91 amino acids exhibited increased mitogenic activity when examined in a myeloid cell line (Ba/F3) for response to EPO. The same increased mitogenic effect was observed when mutant EPORs isolated from PFCP patients (G6002A, 5974insG, C5964G) were examined in the same cell line system.9,10 These studies led to the conclusion that the increased responsiveness of erythroid progenitors of PFCP subjects is caused by the absence of a negative regulatory mechanism in signal transduction through the truncated EPORs. The lack of down regulation of the EPOR after ligand binding results in increased mitogenesis (but not prevention of apoptosis) of cells expressing these abnormal receptors.14 In this study we examined the association of the disease phenotype with EPOR mutations in a number of PFCP subjects.
MATERIALS AND METHODS
Case Reports
Subject 1.The propositus from Ohio family was diagnosed at the age of 8 years after presenting with headaches and fatigue. Her father and three other paternal relatives were also affected; three of them had coronary disease. The ethnic background of the paternal (affected) side of this family was largely Bohemian (Czech) with a minor Irish admixture. Their routine hematological studies, which included normal p50 and decreased erythropoietin, were all consistent with PFCP.
Subject 2.The family was from the Czech Republic. The affected propositus was a 41-year-old former marathon runner. He was diagnosed at age 14 with erythrocytosis, mild splenomegaly, mild hypertension, and normal serum EPO, which were consistent with PFCP. No evidence of a hemoglobin mutation was found either in the propositus or in his father. However, his father, who also had erythrocytosis, died at age 40 of a myocardial infarction.
DNA, Oligonucleotides, Polymerase Chain Reaction
Genomic DNA was extracted from peripheral blood lymphocytes according to standard procedures.15 Template for single-strand conformational polymorphism (SSCP) analysis was prepared by polymerase chain reaction (PCR) using primers described elsewhere8 that were complementary to the EPOR gene in exon 8. Two pairs of primers were used for the 5′-portion of exon 8 EPOR2-FOR 5′-GAGGACCCACCTGCTTCC-3′ (position 5659-5676 bp) and EPOR2-REV 5′-CAAAGCTGGCAGCAGAGG-3′ (position 5942-5959 bp); the 3′-portion of exon 8 was amplified using primers EPOR3-FOR 5′-TCCTGCTCATCTGCTTTGG-3′ (position 5899-5917 bp) and EPOR3-REV 5′-CATCTGCAGCCTGGTGTCC-3′ (position 6213-6231 bp). PCR was performed in 50 μL reactions containing 20 mmol/L Tris-HCl pH 8.4, 50 mmol/L KCl, 1.5 mmol/L MgCl2 , 100 μmol/L dNTP, 300 nmol/L primers, and 2.5 U/reaction Taq DNA polymerase (Life Technologies, Grand Island, NY) overlaid with a drop of mineral oil (Sigma, St Louis, MO). Thirty cycles were performed in a Perkin-Elmer Model 480 thermocycler (Perkin-Elmer, Norwalk, CT) with the following parameters: 1 minute at 94°C initial denaturation, and cycling at 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 30 seconds.
SSCP Screening
SSCP screening for mutations in exon 8 was accomplished after restriction enzyme digestion of PCR products to produce 150 to 200-bp fragments because this size range was reported to provide the highest detection power of the SSCP analysis.16 The 303-bp PCR product derived from the 5′-portion of exon 8 was cleaved with Ava II, Ban II and Sph I. The full-length PCR product and the restriction fragments were analyzed on SSCP gels. The 333-bp PCR product of the 3′-portion of exon 8 was digested with Ava II, Hae III, HinfI, Rsa I, and BstNI and then loaded on a 6%, 8%, or 12% native polyacrylamide gel (depending on the analyzed fragment size) containing 5% (vol/vol) glycerol in 0.5 × Tris/borate/EDTA (TBE) buffer. The gels were run at 22 to 25 V/cm at 15°C using 15 × 15-cm gels in a SE600 electrophoresis apparatus (Hoeffer Scientific Instruments, San Francisco, CA). The DNA was visualized by silver staining.15
Ribonuclease Detection of Mutations
Ribonuclease (RNase) detection of mutations was performed using the Mismatch Detect Non-Isotopic RNase Cleavage Assay Kit (Ambion Inc, Austin, TX) according to manufacturer's instructions. Briefly, the procedure includes PCR amplification of the target DNA sequence using a nested set of primers in which T7 and SP6 promoter sequences are added to the 5′ and 3′ ends of the resulting PCR product. In vitro transcription is then performed using the PCR product as a template for synthesizing sense and antisense RNA from a normal control subject and subjects with PFCP. Normal sense and patients' antisense RNA (or, alternatively, normal antisense and patients' sense RNA) are hybridized and cleaved with RNase A. Samples with a mutation are cleaved by RNase A, whereas samples without sequence alterations remain intact. The cleavage is detected on a standard agarose gel. The following pairs of primers were used to prepare the PCR product for in vitro transcription, which included exons 7 and 8 encoding the cytoplasmic portion of the EPOR: 5′-GCCTCTATGACTGGGAGTGG-3′ and 5′-TTGGATCCCTGATCATCTGC-3′ (position 5336-5355 and 6225-6244 of the EPOR gene); and 5′-ATTTAGGTGACACTATAGGGGAATTGGTGAGTATTCAAT-3′ and 5′-TAATACGACTCACTATATCATATTGGATCCCTGATCATC-3′ (positions 5358-5379 and 6228-6249 of the EPOR gene; SP6 and T7 consensus promoter sequences are underlined). PCR was performed in two rounds using primer pair 1 for the first round and primer pair 2 for the second round. For the first-round PCR, 0.5 μg of genomic DNA was used, and 1 μL of 10 times diluted first round PCR product was used as template for the second round. Reaction conditions and cycling parameters were described above.
Cloning and Sequence Analysis
Samples exhibiting gel migration anomaly (detected by SSCP) and RNase cleavage (using RNase detection) were cloned using TA Cloning Kit (Invitrogen Corp, San Diego, CA) and sequenced with Sequenase 2.0 (USB, Cleveland, OH) according to manufacturer's recommendations.
EPO Dose Response of Erythroid Progenitors
Peripheral blood mononuclear cells from affected family members and a normal control were isolated on Histopaque (Sigma) density gradient (1.077 g/mL). Erythropoiesis was examined by in vitro cultures as described elsewhere1,2 with the modifications that mononuclear cells were cultured at a final concentration of 2 × 105 cells/mL in MethoCult 4531 medium (StemCell Technologies Inc, Vancouver, Canada) in 35-mm Petri dishes in the presence of EPO as follows: 0, 30, 60, 125, 250, 500, 1,000, and 3,000 mU/mL. Cultures were maintained in a humidified atmosphere of 5% carbon dioxide in air at 37°C. Erythroid colonies were scored after 14 days by standard criteria.17 18
Cultures of Erythroid Progenitors in the Presence of Anti-EPO and Anti-EPOR Antibodies
Peripheral blood mononuclear cells were isolated and plated as described above using 0 and 3 U/mL EPO concentrations. Before plating, the cells were preincubated for 1 hour at room temperature with an anti-EPOR monoclonal antibody (50 ng/mL), recognizing the ligand binding domain of the receptor (kind gift of S. Jones, Genetics Institute, Boston, MA). Additionally, the MethoCult 4531 medium was preincubated (50 ng/mL) with an anti-EPO polyclonal antibody (kind gift of E Goldwasser, University of Chicago, Chicago, IL) for 1 hour at room temperature. The experimental details were identical to that previously published.19 After the preincubations the antibody treated or untreated cells were plated in medium with or without anti-EPO antibody.
RESULTS
Screening of the EPOR Intracellular Domain for Mutations
Exons 7 and 8, which code for the intracellular portion of the EPOR, were screened in 27 patients with the phenotype of primary or otherwise undefined polycythemia using SSCP analysis and RNase A detection. Abnormal migration of DNA fragments on SSCP gels was observed in two PFCP subjects (subjects 1 and 2). RNase cleavage of exons 7 and 8 was also detected in both these PFCP subjects, indicating a possible mutation in the EPOR coding region.
Sequence Analysis of the EPOR Mutations
PCR products of subjects 1 and 2 were subcloned and sequenced. A 7-bp deletion (del5985-5991) was found in subject 1 (Fig 1). This deletion produced a frameshift, which changed the amino acid sequence and introduced a premature stop codon that resulted in truncation of the EPOR in its negative regulatory domain (Fig 2). An insertion of T (5967insT) was found in subject 2 (Fig 1) that also caused a frameshift, changed amino acid sequence, premature termination, and truncation of the EPOR (Fig 2). To exclude artifacts caused by the high frequency of spontaneous mutations introduced by Taq DNA polymerase, the veracity of these mutations was confirmed by repeat PCR amplification of different genomic DNA aliquots followed by subcloning and sequence analysis.
C-terminal amino acid sequences of the EPOR of subject 1, subject 2, and other published EPOR mutations associated with PFCP (black box termination codon; shaded letters, homology to the wild type EPOR; unshaded letters, nonhomologous amino acids).
C-terminal amino acid sequences of the EPOR of subject 1, subject 2, and other published EPOR mutations associated with PFCP (black box termination codon; shaded letters, homology to the wild type EPOR; unshaded letters, nonhomologous amino acids).
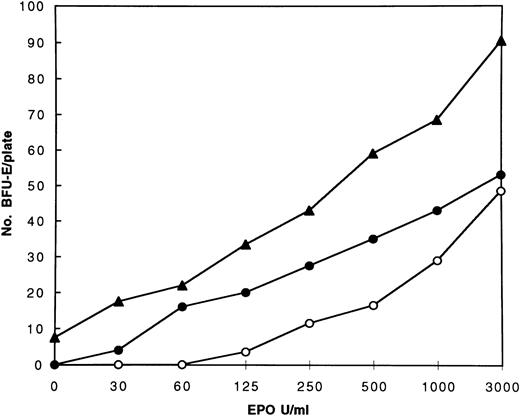
EPO Dose Responses of Erythroid Progenitors
The increased responsiveness of erythroid progenitors to EPO stimulation, examined in vitro in the presence of serum, has been a hallmark of PFCP in all cases studied so far in our laboratory. To confirm the diagnosis of PFCP in the subjects with the del5985-5991 and 5967insT mutations, the EPO dose responses of erythroid progenitors were examined. The erythroid progenitors of both subjects showed increased responsiveness to EPO when compared with a normal control (Fig 3). The hypersensitive response of erythroid progenitors of subject 1 (del5985-5991) was typical for PFCP with no erythroid colonies formed in the absence of EPO. The erythroid progenitors of subject 2 (5967insT) also exhibited a hypersensitive response to EPO and formed low numbers of BFU-E colonies even without the addition of EPO to the medium. This behavior mimicked the responses of polycythemia vera erythroid progenitors, which are characterized by EPO-independent erythroid colony formation in serum containing clonogenic cultures.20 21
EPO dose response curves of the erythroid progenitors of the PFCP subjects 1 (•) and 2 (▴) and a normal control (○).
EPO dose response curves of the erythroid progenitors of the PFCP subjects 1 (•) and 2 (▴) and a normal control (○).
To differentiate the growth abnormalities of erythroid progenitors of subject 2 from those associated with polycythemia vera, antibodies to either EPO or EPOR were used to inactivate traces of EPO present in the serum or to block the EPOR, respectively. The results of the BFU-E responses in these antibody containing cultures are summarized in Table 1. Both anti-EPO and anti-EPOR completely blocked EPO independent colony formation in cultures established from the subject 2 (5967insT EPOR mutation), but no effect of either antibody was seen on EPO independent BFU-E colonies of a subject with polycythemia vera similar to what was reported using the same antibodies by Fisher et al.19
Number of BFU-E Colonies in Methylcellulose Cultures of Subject 2 (5967insT EPOR mutation), a Polycythemia Vera Subject, and a Normal Control Subject in the Absence or Presence of Anti-EPO or Anti-EPOR Antibodies
| EPO . | Subject 2 . | Polycythemia Vera . | Normal Control . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| U/mL . | No Ab . | Anti-EPO . | Anti-EPOR . | No Ab . | Anti-EPO . | Anti-EPOR . | No Ab . | Anti-EPO . | Anti-EPOR . |
| 0 | 3/7 | 0/0 | 0/0 | 42/35 | 30/41 | 38/36 | 0/0 | 0/0 | 0/0 |
| 3 | 38/46 | 22/17 | 15/19 | 105/92 | 88/72 | 95/82 | 40/31 | 22/25 | 21/16 |
| EPO . | Subject 2 . | Polycythemia Vera . | Normal Control . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| U/mL . | No Ab . | Anti-EPO . | Anti-EPOR . | No Ab . | Anti-EPO . | Anti-EPOR . | No Ab . | Anti-EPO . | Anti-EPOR . |
| 0 | 3/7 | 0/0 | 0/0 | 42/35 | 30/41 | 38/36 | 0/0 | 0/0 | 0/0 |
| 3 | 38/46 | 22/17 | 15/19 | 105/92 | 88/72 | 95/82 | 40/31 | 22/25 | 21/16 |
Abbreviation: Ab, antibody.
In the remaining 25 patients with the PFCP phenotype, several patients exhibited hypersensitivity of erythroid progenitors to EPO (data not shown). In these patients, however, we were unable to detect EPOR mutations, and in some we were even able to rule out linkage of the PFCP phenotype with the EPOR gene.22 23
DISCUSSION
We report two new EPOR mutations in two families with autosomal dominant primary polycythemia. The first is a 7-bp deletion (del5985-5991) found in a white family from Ohio. The second mutation, a T insertion (5967insT), was found in a Czech white family. Both mutations cause a frameshift, resulting in a changed amino acid sequence and a premature termination codon leading to truncation of the EPOR by 59 amino acids in the first case (del5985-5991) and by 65 amino acids in the second case (5967insT; Fig 2). Interestingly, four out of the six described mutations so far (or, including this study, six out of eight) caused truncation of the EPOR C-terminal by 59-83 amino acids (Fig 2). All mutations resulting in a truncated EPOR were associated with PFCP, unlike two missense mutations (C6148T, A6146G) where a causative effect of the EPOR mutation on the polycythemia phenotype could not be shown.4,11 Murine EPORs lacking up to 91 C-terminal amino acids induced increased mitogenic responses in a myeloid cell line (Ba/F3) expressing these mutant receptors (relative to wild-type murine EPOR).13 These observations were extended in studies of naturally occurring human EPOR mutations9 10 in which three mutant EPORs associated with PFCP (G6002A, 5974insG, and C5964G) were examined in Ba/F3 cells. All of these truncated human EPORs were shown to induce increased mitogenic responses to EPO stimulation.
Examination of EPO dose responses of the erythroid progenitors (BFU-E) from both subjects with EPOR mutations showed increased sensitivity to EPO stimulation, confirming the diagnosis of PFCP, because hypersensitivity of BFU-Es to EPO is considered to be one of the hallmarks of primary familial and congenital polycythemias. Interestingly, subject 2 (5967insT mutation) also exhibited a low number of EPO-independent erythroid colonies, thus mimicking the behavior of erythroid progenitors in polycythemia vera.20,21 Association of EPO-independent BFU-E colony formation with PFCP has rarely been observed in our laboratory and elsewhere.24 In our subject 1 and in all PFCP subjects previously studied in our laboratory, we have seen either no, or in a rare case only one, isolated EPO-independent BFU-E colony under standard culture conditions. The presence of a significant number of EPO-independent BFU-E colonies in PFCP subject 2 was surprising, because EPO-independent colony formation is a hallmark of polycythemia vera20 21 and does not occur in other polycythemic conditions. This paradox was resolved by using anti-EPO and anti-EPOR antibodies. When either one of these two antibodies was incorporated in the erythroid cultures, the EPO-independent colony formation was abolished in subject 2, whereas it was unaffected in a polycythemia vera subject. These results suggested that in subject 2, the erythropoiesis is primarily regulated by EPO/EPOR stimulation and confirmed that subject 2 complies with the PFCP phenotype. These results further confirm the usefulness of BFU-E clonogenic cultures in diagnosis of PFCP (EPO hypersensitivity of BFU-Es) and polycythemia vera (EPO hypersensitivity with the presence of EPO-independent colonies), providing that in selected situations, such as in our subject 2, the culture studies are extended to include anti-EPO and anti-EPOR antibodies.
After we reported the del5985-5991 mutation,25 the same mutation was independently described in an apparently unrelated family.26 Using the available polymorphic marker in the 5′ region of the EPOR gene,27 a possible founder effect of the del5985-5991 mutation could not be ruled out (data not shown). A more comprehensive EPOR haplotype analysis would be necessary to confirm or exclude a founder effect of this mutation; however, at this time other polymorphic markers closely linked to the EPOR gene are not available.
From this and other previously published studies we conclude that the increased responsiveness of erythroid progenitors of PFCP subjects is caused by the absence of a negative regulatory mechanism in signal transduction through truncated EPORs. The majority (six out of eight) of the known EPOR mutations associated with PFCP result in truncations of the C-terminal of the EPOR. The other two previously described mutations were missense mutations and were not shown to be associated with the PFCP phenotype. Thus, the truncation of the intracellular domain of the EPOR represents the most common lesion in EPOR-gene–linked PFCP cases.
All of the reported mutations associated with PFCP have occurred in exon 8 of the EPOR gene. However, it is likely that mutations in other regions of the EPOR gene or mutations in other genes could generate PFCP phenotype.22,23 Other EPOR mutations may be present in the extracellular ligand binding domain, as suggested in murine EPOR studies.28 The finding of only two cytoplasmic domain mutations in 27 unrelated PFCP subjects analyzed in this study supports this suggestion. The genetic defect responsible for the PFCP phenotype in the other 25 cases remains to be determined.
ACKNOWLEDGMENT
We thank Lubomir Sokol, Michal Mrug, Xylina Gregg, Lina Tze, and Yongli Guan for their technical assistance and advice. We also thank Bernard Forget (Yale University) for providing us with the genomic DNA of an independently characterized PFCP subject with the del5985-5991 mutation.
Supported by a Veterans Administration Hospital Merit Grant, the United States Public Health Service, and National Institute of Health Grant Nos. HL51650 and HL50077.
Presented in a preliminary form at the 37th Annual Meeting of the American Society of Hematology, December 1995, Seattle, WA.
Address reprint requests to Robert Kralovics, University of Alabama, Division of Hematology/Oncology, 1900 University Blvd, THT #513, Birmingham, AL 35294.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal