Abstract
The mouse mutant hemoglobin deficit (gene symbol hbd ) is characterized by a severe microcytic anemia that is inherited in an autosomal-recessive manner. To assess the mutation's effect on hematopoiesis, unfractionated bone marrow (BM) from either a mutant C57BL6/J-hbd/hbd, Gpi1b/Gpi1b (phenotype symbol HBD), or normal C57BL6/J -+hbd/+hbd, Gpi1b/Gpi1b mouse was injected intravenously into irradiated congenic C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a mice. The congenic recipients of mutant or normal marrow obtained complete red blood cell (RBC) and leukocyte reconstitution, with the exception of one recipient of HBD marrow. After 24 weeks posttransplantation, the normal recipients of HBD marrow obtained a microcytic anemia similar to the donor. These results suggest that the HBD phenotype is caused by a BM defect. We observed that the erythroid lineage derived from donor HBD marrow repopulated more slowly than the normal marrow at 4 weeks posttransplantation. To determine if this difference was a result of an erythropoietic defect, competitive repopulation was performed using either mutant or normal marrow competed against normal congenic marrow. For the erythroid lineage, no significant contribution from HBD marrow was observed. To assess if the RBC block was based on a deficiency of myeloid progenitors, both in vitro and in vivo assays were performed: absolute numbers of bone progenitors were increased, suggesting that the defect results in a late block to erythroid differentiation.
THE HEMOGLOBIN-DEFICIT mouse (gene symbol hbd ) originated as a spontaneous mutation in the AY strain and was bred onto the C57BL6/J background.1 2 The defect is inherited in an autosomal-recessive manner. Homozygous animals (phenotype symbol HBD) exhibit a microcytic and hypochromic anemia that persists from birth into adulthood. Reticulocytosis as well as increased red blood cell (RBC) counts are also evident. The HBD anemia is not caused by simple iron deficiency: serum iron levels are above normal and intraperitoneal iron-dextran injections do not cure the phenotype.
To assess a potential defect in iron metabolism, Garrick et al3 assayed for both iron and transferrin acquisition in vitro in normal and HBD reticulocytes. Reticulocyte-rich RBCs were incubated with saturated 125I-labeled diferric mouse transferrin and 59Fe-saturated mouse plasma. Transferrin acquisition for HBD reticulocytes was unaffected compared with controls whereas iron acquisition was significantly reduced. The investigators concluded from these data that a defect in iron acquisition occurs distal to binding of transferrin to the membrane receptor.
A defect in iron acquisition similar to that of the HBD mouse has also been observed in vitro for the microcytic anemia mutant (gene symbol mk, phenotype symbol mk).4 Acquisition of iron into mk/mk reticulocytes was significantly lower than normal controls. Aquisition of iron into normal reticulocytes was not different if incubated in normal or mutant plasma, suggesting that the defect is at the transferrin receptor. Additional analysis in vivo on mk mice showed that iron injections failed to improve the blood values and that the transfer of normal bone marrow (BM) to mutant mice only partially improved the phenotype.5 Blood values near normal were obtained in mk recipients of normal marrow if iron was injected. The data from these studies point to a generalized defect in iron acquisition.6
The mk mouse also has a defect in mucosal iron uptake. The in vitro uptake of iron into the mucosa of everted duodenal sacs is decreased compared with normal controls. This effect is presumably compensated by a net increase in in vitro uptake of iron across the everted sac. In vivo iron retention is decreased, suggesting that this in vitro compensation is not adequate and results in the anemic phenotype.7
The sex-linked anemia mouse appears to have a defect in iron absorption in the gut.8 Iron dextran injections remarkably improve the phenotype, suggesting that the defect is localized to the gut.9 In vitro experiments similar to those used for the mk mouse have shown a defect in transport across the mucosa while uptake into the mucosa appears to be unaffected.10
The biochemical effects of these mutations on iron acquisition have been clearly established. Given these findings, each model represents a unique opportunity to assess the resultant biologic effects of iron deficiency. The purpose of this study is to further define the HBD defect by analyzing, in vivo and in vitro, the effects of the mutation on BM progeny as well as progenitor and stem cells. To resolve this question, BM from HBD mice was transplanted into normal myeloablated recipients. We report here that the recipients of HBD marrow developed a long-term anemic phenotype that was comparable to the phenotype of the HBD donor. We conclude from these data that the HBD mutation results in a BM defect and that this defect may contribute substantially to the anemic phenotype of the HBD mouse.
In addition to phenotype analysis, engraftment of donor marrow was analyzed. Compared with normal controls, the HBD erythroid component was slower to reconstitute at the 4-week time point. Competitive repopulation as well as progenitor colony assays were performed to further assess the possible defect in erythropoiesis.
MATERIALS AND METHODS
Animals.Animals were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained at the National Institutes of Health in a facility that is approved by the American Association for the Accreditation of Laboratory Animal Care. Mice carrying the hbd mutation had been backcrossed at the Jackson Laboratory onto the C57BL6/J background for greater than 16 generations.
Comparison of Blood Parameters for HBD Mice in the NIH Colony and the Original Report
| . | Hct (%) . | MCV (fL) . | Hgb (g/dL) . | MCHC (%) . | Protoporphyrin . |
|---|---|---|---|---|---|
| C57BL6/J-hbd/hbd | 39.6 ± 1.7 | 34.3 ± 1.3 | 10.3 ± 0.7 | 26.0 ± 0.7 | 298.6 ± 25.2 (ZPP) |
| C57BL6/J-+hbd/+hbd | 46.8 ± 1.7 | 48.2 ± 1.3 | 15.4 ± 0.7 | 32.8 ± 0.9 | 43.6 ± 5.2 (ZPP) |
| AB/Jena-Halle-hbd/hbd | 37.0 | 43.3 | 10.7 | 28.9 | 611 (FEP) |
| C57BL6/J-+hbd/+hbd | 45.7 | 51.6 | 14.1 | 30.9 | 50 (FEP) |
| . | Hct (%) . | MCV (fL) . | Hgb (g/dL) . | MCHC (%) . | Protoporphyrin . |
|---|---|---|---|---|---|
| C57BL6/J-hbd/hbd | 39.6 ± 1.7 | 34.3 ± 1.3 | 10.3 ± 0.7 | 26.0 ± 0.7 | 298.6 ± 25.2 (ZPP) |
| C57BL6/J-+hbd/+hbd | 46.8 ± 1.7 | 48.2 ± 1.3 | 15.4 ± 0.7 | 32.8 ± 0.9 | 43.6 ± 5.2 (ZPP) |
| AB/Jena-Halle-hbd/hbd | 37.0 | 43.3 | 10.7 | 28.9 | 611 (FEP) |
| C57BL6/J-+hbd/+hbd | 45.7 | 51.6 | 14.1 | 30.9 | 50 (FEP) |
Data in rows 1 (n = 5) and 2 (n = 5) were derived from our colony at the NIH and represent the mean ± SE. Data from rows 3 (n = 8) and 4 (n = 14) are from the original report.2
Abbreviations: Hct, hematocrit; Hgb, hemoglobin concentration. For the protoporphyrin values, (ZPP) = zinc-protoporphyrin in μmol ZPP/mol heme and (FEP) = free-erythrocyte protoporphyrin.
Blood Parameters for Donors and Their Respective Recipients at Four-Week Intervals Posttransplantation
| . | Donor . | Baselines . | 4 wk . | 8 wk . | 12 wk . | 16 wk . | 20 wk . | 24 wk . |
|---|---|---|---|---|---|---|---|---|
| ZPP | ||||||||
| +/+ | 50 | 27.5 ± 2.3 | 59 ± 7.1 | 39.3 ± 3.3 | 36 ± 2.8 | 34.7 ± 5.7 | 25.4 ± 5.4 | 43.8 ± 5.3 |
| hbd/hbd | 325 | 24.7 ± 2.7 | 181.7 ± 8.7 | 249.3 ± 36.5 | 243.3 ± 27.9 | 272.5 ± 27.9 | 251.3 ± 13.2 | 300.5 ± 34.5 |
| MCV | ||||||||
| +/+ | 47.1 | 50.4 ± 0.4 | 47.3 ± 1.0 | 48.7 ± 1.7 | 50.3 ± 2.5 | 43.4 ± 1.3 | 47.5 ± 2.0 | 48.8 ± 3.0 |
| hbd/hbd | 29.4 | 51.4 ± 1.0 | 39.0 ± 0.6 | 34.7 ± 1.4 | 34.5 ± 0.9 | 33.9 ± 1.5 | 34.5 ± 1.5 | 32.5 ± 1.6 |
| MCHC | ||||||||
| +/+ | 28.2 | 29.4 ± 1.3 | 33.6 ± 0.6 | 32.6 ± 1.6 | 31.0 ± 1.2 | 36.2 ± 1.6 | 31.4 ± 0.8 | 33.4 ± 2.2 |
| hbd/hbd | 27.4 | 31.4 ± 0.7 | 28.5 ± 0.3 | 27.6 ± 0.9 | 28.7 ± 1.6 | 29.2 ± 1.5 | 25.8 ± 1.2 | 28.8 ± 1.4 |
| . | Donor . | Baselines . | 4 wk . | 8 wk . | 12 wk . | 16 wk . | 20 wk . | 24 wk . |
|---|---|---|---|---|---|---|---|---|
| ZPP | ||||||||
| +/+ | 50 | 27.5 ± 2.3 | 59 ± 7.1 | 39.3 ± 3.3 | 36 ± 2.8 | 34.7 ± 5.7 | 25.4 ± 5.4 | 43.8 ± 5.3 |
| hbd/hbd | 325 | 24.7 ± 2.7 | 181.7 ± 8.7 | 249.3 ± 36.5 | 243.3 ± 27.9 | 272.5 ± 27.9 | 251.3 ± 13.2 | 300.5 ± 34.5 |
| MCV | ||||||||
| +/+ | 47.1 | 50.4 ± 0.4 | 47.3 ± 1.0 | 48.7 ± 1.7 | 50.3 ± 2.5 | 43.4 ± 1.3 | 47.5 ± 2.0 | 48.8 ± 3.0 |
| hbd/hbd | 29.4 | 51.4 ± 1.0 | 39.0 ± 0.6 | 34.7 ± 1.4 | 34.5 ± 0.9 | 33.9 ± 1.5 | 34.5 ± 1.5 | 32.5 ± 1.6 |
| MCHC | ||||||||
| +/+ | 28.2 | 29.4 ± 1.3 | 33.6 ± 0.6 | 32.6 ± 1.6 | 31.0 ± 1.2 | 36.2 ± 1.6 | 31.4 ± 0.8 | 33.4 ± 2.2 |
| hbd/hbd | 27.4 | 31.4 ± 0.7 | 28.5 ± 0.3 | 27.6 ± 0.9 | 28.7 ± 1.6 | 29.2 ± 1.5 | 25.8 ± 1.2 | 28.8 ± 1.4 |
Blood parameters for normal and mutant donors are presented in column 1. The congenic recipients of either C57BL6/J-+hbd/+hbd or -hbd/hbd marrow were assayed at 4-week intervals (columns 2-8). Abbreviations and units are the same as in Table 1. For recipients of -hbd/hbd marrow at all time points, n = 6. For recipients of -+hbd/+hbd marrow up to 16 weeks, n = 6. For recipients of +hbd/+hbd marrow at 20 and 24 weeks, n = 5. Values are represented as the mean ± SE. For the ZPP and MCV parameters, P < .01. For MCHC, P ≤ .01 at 4, 16, and 20 weeks, P < .05 at 8 weeks, P = .25 at 12 weeks, and P = .1 at 24 weeks. The P values are based on the t-test for equal variance (two-tailed).
Blood parameters.Blood was collected from the peri-orbital venous sinus with heparinized hematocrits tubes (Oxford Labware, St Louis, MO). RBC counts were performed in azide-free isotonic diluent (Fisher Scientific, Pittsburgh, PA) using a Coulter Counter Model ZM (Hialeah, FL). Hemoglobin concentration was determined using a hemoglobin standard diluted in Drabkin's reagent (Sigma, St Louis, MO). Mean cell volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were calculated based on direct measurements of hematocrit values, RBC counts, and hemoglobin concentrations. Zinc protoporphyrin (ZPP) levels were measured with a Protofluor-Z (Helena Labs, Beaumont, TX). This fluorometer measures the ZPP complex that absorbs at 415 nm and emits an excitation wavelength of 595 nm.
Transplantation.Recipient animals were given 800 R whole-body irradiation using a 137Cs source. Donor marrow from adult mice was flushed from both femurs and tibias into 2.0 mL of phosphate-buffered saline (PBS) (pH 7.4) using a 27-gauge needle. A total of 2.0 × 106 nucleated BM cells was injected into each animal's tail vein. Blood counts and RBC enzymes were analyzed at 4-week intervals. Engraftment was determined indirectly by assaying the percent donor-type glucose phosphate isomerase (GPI1) isotype in the peripheral blood. Donor mice of two genotypes with the same genetic background were used: C57BL6/J-+hbd/+hbd, Gpi1b/Gpi1b and C57BL6/J-hbd/hbd, Gpi1b/Gpi1b. Recipient mice were of the genotype: C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a.
Isotype analysis.Isotype analysis of GPI1 (encoded by genes Gpi1a or Gpi1b ) in RBCs and leukocytes was performed as described.11 Enrichment for RBCs was performed by cutting 8.0 mm of packed RBCs from a spun 60-μL hematocrit tube (Oxford Labware). The packed RBCs were lysed in water before application to cellulose acetate strips. For leukocytes, the buffy coat was removed from a spun hematocrit tube and the cells were suspended in 100 mL of 1× PBS (pH 7.4). Two rounds of RBC lysis were performed with ACK lysing buffer (Biofluids cat. No. 304, Rockville, MD). Enriched leukocytes were lysed by freezing and thawing twice before application onto cellulose acetate membranes (Helena Labs). The stained membranes were scanned with a densitometer GS 300 (Hoefer Scientific Instruments, San Francisco, CA) and integrated using software GS370D version 3.0 (Hoefer Scientific Instruments) for the Macintosh computer (Apple Computer, Inc, Cupertino, CA).
Colony-forming units-spleen (CFU-S) assay.Nucleated BM cells (105) were injected intravenously into recipients that had been exposed to 800 R whole body irradiation. Mice were killed 10 days after injection. The spleens were then removed and placed into Tellyesniczky/Fekete fixative. After fixation, CFU-S were counted under a dissecting microscope.
Colony assays.Nucleated BM cells (4.5 × 104) were plated into three different 33-mm dishes. The methylcellulose media (StemCell Technologies, Vancouver, BC, Canada) contained 0.9% methylcellulose, 1.0% bovine serum albumin, 10−4 mol/L 2-mercaptoethanol, 2.0 mmol/L L-glutamine, 3 U/mL erythropoietin, fetal bovine serum, insulin, recombinant murine interleukin-3 (IL-3), stem cell factor (SCF ), and recombinant human IL-6. Three colony types (colony-forming unit granulocyte/macrophage [CFU-GM], CFU granulocyte/erythroid/monocyte/macrophage [CFU-GEMM], and burst-forming units-erythroid [BFU-E]) were counted after 7 days of incubation at 37°C and 5.0% CO2 . Colonies were scored according to morphology observed using an inverted microscope.
RESULTS
In a previous report, the HBD phenotype was assayed in mice that had been bred onto a mixed genetic background.2 The mutants from our colony had been backcrossed onto the C57BL6/J background for more than 16 generations at the Jackson Laboratory. To determine if the genetic change had affected the phenotype, blood counts of the HBD mice from our colony were measured and compared with those from the original report (Table 1). RBCs from HBD mice in our colony were smaller than those of previously described mutants. Reduction of the MCHC was more pronounced in our mutants compared with that in the previous report. We also assayed ZPP, an indirect measure of the free erythrocyte protoporphyrin (FEP). The ZPP was higher in the mutant animals. Although the overall phenotype was more pronounced on the C57BL6/J background, we concluded from these results that the anemic phenotype has remained acceptably stable.
Reconstitution of mice injected with either mutant or normal marrow. The percentage isotype Gpi1b was determined for normal recipient mice (genotype: C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a ) that were transplanted with either HBD or normal marrow. Identification numbers for individual mice are indicated at the right of each graph. (A) Leukocyte reconstitution for recipients of normal marrow. (B) Leukocyte reconstitution for recipients of mutant marrow. (C) RBC reconstitution for recipients of normal marrow. (D) RBC reconstitution for recipients of mutant marrow.
Reconstitution of mice injected with either mutant or normal marrow. The percentage isotype Gpi1b was determined for normal recipient mice (genotype: C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a ) that were transplanted with either HBD or normal marrow. Identification numbers for individual mice are indicated at the right of each graph. (A) Leukocyte reconstitution for recipients of normal marrow. (B) Leukocyte reconstitution for recipients of mutant marrow. (C) RBC reconstitution for recipients of normal marrow. (D) RBC reconstitution for recipients of mutant marrow.
To determine if the BM from HBD mice was capable of producing an anemia in a normal environment, C57BL6/J-hbd/hbd, Gpi1b/Gpi1b marrow was transplanted into normal congenic recipients of the same genetic background (C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a ). A control transplant of normal C57BL6/J-+hbd/+hbd, Gpi1b/Gpi1b marrow into normal congenic recipients was performed concurrently. By 24 weeks posttransplantation, recipients of HBD marrow had converted to an anemic phenotype (Table 2). These data show that the transplant of BM stem cells from mutant to normal mice was sufficient to produce an anemic phenotype.
In addition to phenotype analysis, engraftment of RBC and leukocyte lineages was followed at 4-week intervals up to 24 weeks posttransplantation (Fig 1). Recipients of either normal C57BL6/J-+hbd/+hbd, Gpi1b/Gpi1b or mutant C57BL6/J-hbd/hbd, Gpi1b/Gpi1b marrow showed complete leukocyte reconstitution by 4 weeks posttransplantation (Fig 1A and B). Of the six recipients of HBD marrow, five showed stable reconstitution of the leukocyte lineage for 24 weeks. Mouse no. 494 failed to show stable engraftment. All recipients of normal marrow obtained long-term stable reconstitution. The major difference between engraftment of the normal and mutant marrow types was in the kinetics of reconstitution of the RBC lineages. At 8 weeks posttransplantation, the recipients of normal marrow had obtained 100% reconstitution (Fig 1C). In contrast, complete RBC reconstitution of the HBD marrow was obtained at 12 weeks (Fig 1D). As with leukocyte reconstitution, recipient no. 494 did not obtain long-term reconstitution of the donor RBC lineage.
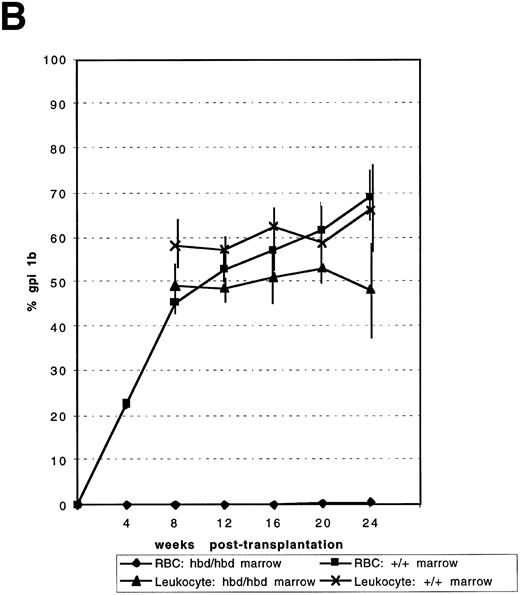
Competitive repopulation of HBD and normal marrow against normal congenic marrow. In two independent experiments (A and B), mixtures of either mutant and congenic or normal and congenic marrow were injected into irradiated C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a recipient mice. The percentage of isotype Gpi1b in the peripheral RBCs and leukocytes indicated the competitive ability of the mutant or normal marrows compared with congenic marrow. Data points indicate the mean for mixtures as indicated under the figure. Error bars represent the SEM. In (A), n = 6 for both the normal:congenic mix and HBD:congenic mixes. The P value for the leukocytes for the HBD:congenic versus the normal:congenic was .0007 at 24 weeks posttransplantation. In (B), n = 6 for normal:congenic and HBD:congenic mixes. At 16 weeks, n = 5 and at 24 weeks n = 4 for normal:congenic mix. The P value for HBD:congenic versus normal:congenic leukocytes was 0.27 at 24 weeks.
Competitive repopulation of HBD and normal marrow against normal congenic marrow. In two independent experiments (A and B), mixtures of either mutant and congenic or normal and congenic marrow were injected into irradiated C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a recipient mice. The percentage of isotype Gpi1b in the peripheral RBCs and leukocytes indicated the competitive ability of the mutant or normal marrows compared with congenic marrow. Data points indicate the mean for mixtures as indicated under the figure. Error bars represent the SEM. In (A), n = 6 for both the normal:congenic mix and HBD:congenic mixes. The P value for the leukocytes for the HBD:congenic versus the normal:congenic was .0007 at 24 weeks posttransplantation. In (B), n = 6 for normal:congenic and HBD:congenic mixes. At 16 weeks, n = 5 and at 24 weeks n = 4 for normal:congenic mix. The P value for HBD:congenic versus normal:congenic leukocytes was 0.27 at 24 weeks.
To assess if the delay in erythroid engraftment was caused by a defect in hematopoietic ontogeny, competitive repopulation was performed by mixing equal numbers of nucleated BM cells from donor C57BL6/J-hbd/hbd, Gpi1b/Gpi1b and competitor C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a mice. In parallel, equal numbers of C57BL6/J-+hbd/+hbd, Gpi1b/Gpi1b donor BM cells were mixed with C57BL6/J-+hbd/+hbd, Gpi1a/Gpi1a, Igha/Igha, Thy1a/Thy1a competitor marrow. These mixtures were injected into myeloablated congenic recipients. The percentages of isozymes Gpi1A and Gpi1B were assayed at 4-week intervals up to 24 weeks posttransplantation. In two experiments, no contribution to the RBC population was observed from the C57BL6/J-hbd/hbd, Gpi1b/Gpi1b component for all time points assayed (Fig 2A and B). In addition, the competitive ability of the leukocyte component from HBD marrow was decreased in both experiments. The normal marrow components contributed greater than the expected 50% in both experiments.
To further assess the erythroid defect, myeloid progenitors were assayed from normal and mutant mice (Table 3). An estimation of the absolute number of these progenitors as well as primitive hematopoietic stem cells (PHSC) was also determined (Table 4). The CFU-S contain a mixed population of hematopoietic cells and are thought to represent an early multipotential progenitor that is distinct and more mature than the pluripotent repopulating stem cells.12,13 CFU-S were generated by injecting normal or HBD marrow into lethally irradiated C57BL6/J-+hbd/+hbd recipients. Compared with normal controls, HBD BM was more concentrated in CFU-S. If corrected for cellularity, we estimate that there are twice the normal number of CFU-S in HBD mice. Titration effects of fractionated marrow on CFU-S formation have previously been observed, suggesting that certain cell types inhibit or enhance colony formation.14 To assess if unfractionated HBD marrow contains an inhibitor or enhancer of colony formation, CFU-S formation was titrated around the point reported in Table 3. The slope was not significantly different from 1.0, suggesting that this effect is not present in unfractionated HBD marrow (data not shown). In vitro colonies derived from BM are thought to represent a precursor that is more mature than the CFU-S progenitor. BM from the HBD mouse is more concentrated in CFU-GM, CFU-GEMM, and BFU-E than is the marrow of normal mice (Table 3). Based on cellularity, the absolute number of these colony-forming progenitors is about three times that of the normal mouse.
Myeloid Progenitor Assays of C57BL6/J-hbd/hbdand C57BL6/J-+hbd/+hbd Marrow
| . | C57BL6/J-+/+ (n = 8) . | C57BL6/J-hbd/hbd (n = 8) . |
|---|---|---|
| CFU-GM | 54.8 ± 2.1 | 100.5 ± 4.0 |
| BFU-E | 4.6 ± 0.84 | 7.5 ± 0.96 |
| CFU-GEMM | 1.13 ± 0.64 | 2.13 ± 0.74 |
| CFU-S | 11.71 ± 0.73 (n = 14) | 15.42 ± 0.76 (n = 14) |
| . | C57BL6/J-+/+ (n = 8) . | C57BL6/J-hbd/hbd (n = 8) . |
|---|---|---|
| CFU-GM | 54.8 ± 2.1 | 100.5 ± 4.0 |
| BFU-E | 4.6 ± 0.84 | 7.5 ± 0.96 |
| CFU-GEMM | 1.13 ± 0.64 | 2.13 ± 0.74 |
| CFU-S | 11.71 ± 0.73 (n = 14) | 15.42 ± 0.76 (n = 14) |
BM cells (4.5 × 104) from either a C57BL6/J-+hbd/+hbd or C57BL6/J-hbd/hbd mouse were grown in methylcellulose media containing recombinant growth factors. Three colony types were scored: CFU-GM, CFU-GEMM, and BFU-E. Each value represents the mean number of colonies per 4.5 × 104 BM cells plated from two combined experiments ± SE. P < .05 for all comparisons except CFU-GEMM where P = .32. For CFU-S, either C57BL6/J-hbd/hbd and C57BL6/J-+hbd/+hbd marrow was injected into irradiated C57BL6/J-+hbd/+hbd mice. Ten days postinjection spleen colonies were counted. The means are based on two independent experiments. For the CFU-S comparison, P = .0016.
Estimation of Absolute CFU-S, CFU-C, and PHSC in Two Femurs and Two Tibias From Either Normal or Mutant Mice
| Marrow Type . | CFU-GM4-150 . | BFU-E . | CFU-GEMM . | CFU-S . | PHSC4-151 . |
|---|---|---|---|---|---|
| HBD | 156,300 | 11,666 | 3,313 | 10,794 | 399 |
| Normal | 53,580 | 4,497 | 1,104 | 5,150 | 440 |
| Marrow Type . | CFU-GM4-150 . | BFU-E . | CFU-GEMM . | CFU-S . | PHSC4-151 . |
|---|---|---|---|---|---|
| HBD | 156,300 | 11,666 | 3,313 | 10,794 | 399 |
| Normal | 53,580 | 4,497 | 1,104 | 5,150 | 440 |
Absolute numbers were calculated by multiplying the CFU concentration (Table 3) by the BM cellularity for two tibias and two femurs. The cellularity was calculated based on the mean of BM isolations for the transplant studies. For hbd/hbd mice (n = 3), marrow cellularity was 7.0 × 107 cells. For +hbd/+hbd mice, marrow cellularity was 4.4 × 107.
For PHSC numbers, the mean engraftment for white blood cells from both competitive repopulation experiments was determined for the HBD and normal marrows. The fraction of HBD to control was multiplied by the estimated frequency of PHSC in the normal mouse.20 21 Both the estimate for normal and HBD mice was multiplied by their respective cellularities.
The absolute number of PHSC was also estimated (Table 4). This determination was based on the previous estimates of PHSC concentration as well as the cellularity of the HBD and the assumption that the concentration of PHSC remains the same. If this assumption is correct, we estimate that there is a 10% decrease in absolute PHSC numbers in the HBD mouse.
DISCUSSION
The results presented here show that HBD BM is capable of producing microcytic RBCs in a normal host environment and that erythropoiesis is defective in this transplant model. Although these results do not discount a micro-environmental defect for the HBD mouse, we show here that the BM effect is substantial and complex.
Long-term persistence of anemia in recipients of HBD marrow indicates that a defect resides in BM-derived cells. Only a short-term anemia would have been expected in the recipient if the BM defect was caused by the microenvironment. Control animals that received normal marrow did not obtain a microcytic phenotype. These results indicate that neither the transplant procedure nor the congenic marker loci on the C57BL6/J background substantially affect the phenotype.
The recipients of mutant marrow present an anemic phenotype as early as 4 weeks posttransplantation. In fact, histogram analysis of RBC populations from recipients indicates a distinct microcytic population at all intermediate time points before complete reconstitution of microcytic RBCs (data not shown). These results indicate that the transfer and expansion of late HBD progenitors is sufficient to produce a defective RBC population in the normal environment. These results suggest that the established defect is not reversible for late HBD progenitors in a normal environment.
Concomitant with our observations that the HBD marrow-derived cells produce a microcytic phenotype in normal recipients was our observation of the delayed erythropoiesis in the transplant model. To further define this potential defect, the competitive repopulation assay was performed which measures the ability of two different donor populations to compete in the host environment.15 We show a striking defect in long-term competitive ability of the HBD erythroid lineage. A similar defect has been observed for the α-thalassemic mice, although for a different reason. The competitive defect is the result of RBC hemolysis, which is not a significant factor in HBD mice.16 No evidence of excess hemolysis in the HBD mouse has been observed through analysis of fecal urobilinogen excretion.2 A severe erythroid defect is also observed in mutants of the W series but is secondary to a PHSC defect.17 However, we doubt that the HBD anemia is caused by stem cell lesion. First, we were unable to repopulate an unconditioned HBD mouse with congenic marrow (data not shown). Second, we show here that the HBD marrow can completely engraft into myeloablated hosts by 12 weeks posttransplantation. Excluding a stem cell defect and hemolysis, we examined the myeloid progenitor population to explain the erythroid defect. A defect in this population was not observed. In fact, we estimate an absolute increase in progenitors that form the erythroid colonies. These results indicate that the block to differentiation is at a late step to differentiation.
One explanation for the erythroid expansion defect is that the HBD hematopoietic cells are unable to compete for a circulating component, resulting in a late block to erythroid expansion. This hypothesis is supported by both our phenotype transfer and competitive repopulation results. For the phenotype transfer, HBD engraftment is delayed but finally completed as the normal recipient marrow is ablated from the radiation exposure. For the competitive repopulation, where the normal and HBD donor marrows are equally represented, the HBD erythroid lineage would not be able to compete with normal marrow. In vitro uptake of radiolabeled iron is lower in HBD reticulocytes compared with normal controls whereas transferrin uptake is unaffected.3 It is possible that iron is the component that is outcompeted by the normal cells.
A defect in erythropoieis has also been observed for the flexed-tail (fl) mutant. This mutant has a hypochromic anemia in the fetus and newborn that is resolved in adults. A delay in production of cells that incorporate iron into heme is observed in spleen colonies derived from transplanted (fl/fl) adult marrow.18,19 Because the anemia is restricted to early development, these results suggest that the defect is only present in an environment of rapid cell proliferation. These CFU-S derived from these adult mice were smaller and deficient in erythrocytes whereas the granulopoiesis was not affected, suggesting a specific defect on the committed erythroid progenitors.18 In contrast, the CFU-S derived from HBD mice are not smaller in size and do not indicate a gross reduction in erythroid colonies (data not shown). The flexed-tail defect may block erythropoiesis at an earlier step than the HBD mutant.
In conclusion, we have shown a defect in the HBD BM component both in phenotype and erythropoiesis. Given the previous results on a defective iron acquisition in the HBD mouse, the HBD mutant offers an opportunity to study this link between iron metabolism and erythropoiesis. The future determination of the HBD gene product as well as its biochemical function will be crucial.
ACKNOWLEDGMENT
We thank Drs Neal Young, Cindy Dunbar, and Daniel Dunn for suggestions and comments.
Address reprint requests to Michael L. Bloom, PhD, National Heart, Lung, and Blood Institute, Hematology Branch, Bldg 10, Room 7C 118, Bethesda, MD 20892.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal