Abstract
Dendritic cells (DC) are important initiators of specific primary immune responses because they are the only APC that can efficiently activate naive Th cells. DC have the capacity to produce interleukin-12 (IL-12), a cytokine that plays a pivotal role in the development of Th1-mediated cellular immune responses. The present study focuses on the conditions under which human DC produce bioactive IL-12 p70 and, consequently, direct the development of naive T helper (Th) cells toward the Th1 phenotype. Bacteria or bacterial compounds such as Staphylococcus aureus Cowan strain I (SAC) or lipopolysaccharide (LPS) induced substantial IL-12 levels in DC, which could be further upregulated by interferon-γ (IFN-γ), whereas induction of IL-12 production via CD40 ligation required IFN-γ as an obligatory, complementary signal. Also, activated naive Th cells were poor inducers of IL-12 production, unless exogenous IFN-γ was present, whereas activated memory Th cells were effective inducers of IL-12 production and did not require exogenous IFN-γ. Next, the cytokine profiles of matured Th cells that were primed by DC under different conditions were examined. DC promoted the development of naive Th cells into memory Th0 cells that produced both the type 1 cytokine IFN-γ and the type 2 cytokine IL-4. In contrast, after activation with SAC, DC efficiently directed the development of Th1 cells through the release of IL-12. An APC-independent Th cell maturation model, using either recombinant IL-12 or supernatants of SAC-activated DC and neutralizing anti-IL-12 antibodies, confirmed that DC-derived IL-12 was the major Th1 skewing factor. Together, these data indicate that the contact between DC and naive Th cells during the initiation of specific immune responses does not result in the efficient induction of IL-12 production and that, consequently, exogenous IL-12–inducing factors are required to promote primary Th1-mediated cellular immune responses.
DENDRITIC CELLS (DC) are highly specialized antigen-presenting cells (APC) of the immune system.1 DC pick up and process antigen (Ag) with high efficiency in peripheral tissues and continuously migrate to lymphoid organs to present these Ag to specific T cells. In contrast to other types of APC, DC are potent activators of naive T cells and are, therefore, regarded as important initiators of primary specific immune responses.
The differential development of naive T helper (Th) cells into functionally distinct memory Th cells, such as Th type 1 (Th1) and Th2 cells, depends upon microenvironmental factors that are present at the time of T-cell activation. A powerful factor in this respect is interleukin-12 (IL-12), that strongly augments interferon-γ (IFN-γ) production by activated naive T cells and, therefore, skews their development toward the memory Th1 phenotype.2-4 Recently, it was shown that spleen- and skin-derived DC, as well as in vitro matured DC all have the capacity to produce IL-125-8 and it was, therefore, proposed that DC preferentially induce Th1 responses.5,6 8
It is well-documented that Th1 cells are required to generate protective, cell-mediated inflammatory responses against invading microorganisms, such as Leishmania major, Mycobacterium leprae, or Toxoplasma gondii.9-11 In other cases, however, Th1 cell-mediated responses may be disadvantageous, for instance in case of infection with certain nematode species of which the expelling requires strong Th2 responses.12 Moreover, Th1 cells may even play a pathogenic role, for instance in certain autoimmune diseases.13 Therefore, the concept that DC preferentially promote Th1 responses is rather unsatisfying. Because it is important that the development of Th1 responses does not occur via a default differentiation pathway, but can be selected when necessary, DC should produce physiologically significant IL-12 levels only when Th1 cell-mediated responses are beneficial.
In the present in vitro study, we examined the conditions under which human DC produce IL-12 and, consequently, skew naive Th cell responses toward the Th1 phenotype. For this purpose, we used CD1+ DC derived from peripheral blood precursors. As described previously,14 15 such cells fit the definition of DC based on (1) their typical dendritic morphology, (2) their high surface expression of CD1, major histocompatibility complex (MHC) II, and costimulatory molecules such as CD80 (B7.1) and CD86 (B7.2), (3) their high stimulatory capacity for naive T cells and, as mentioned before, (4) their potency to produce IL-12.
Here, we show that DC do not exert a Th1-promoting activity during the process of naive Th cell activation, because naive Th cells are poor inducers of IL-12 production. Only if DC are activated by exogenous IL-12-inducing factors such as bacteria or their constituents, they direct Th1 development through the release of IL-12.
MATERIALS AND METHODS
Antibodies and cytokines.T cells were stimulated using CD3 monoclonal antibody (MoAb) (CLB-T3/3) and CD28 MoAb (CLB-CD28/1), all obtained from the Central Laboratory of The Netherlands Red Cross Blood Transfusion Service (CLB) (Amsterdam, The Netherlands). Fluorescence-activated cell sorting (FACS) analysis was performed using MoAbs against the following surface markers: CD1a (OKT6; Ortho Diagnostic Systems, Beerse, Belgium), CD4 (OKT4; Ortho), CD14 (Leu M3; Becton Dickinson, San Jose, CA), HLA-DR (L243; Becton Dickinson), CD80 (B7-24),16 and CD86 (1G10), both kindly provided by Innogenetics N.V., Ghent, Belgium, CD40 (EA-5; a gift from Dr T. LeBien, University of Minnesota, Minneapolis, MN), CD45RA (2H4; Coulter, Hialeah, FL) and CD45RO (UCHL-1; a gift from Dr P. Beverly, London, UK). Human rIL-12 (sp. act. 170 × 106 U/mg), neutralizing IL-12 goat antibodies and the IL-12 p70-specific MoAb 20C2 were gifts from Dr M.K. Gately (Hoffmann-La Roche, Nutley, NJ). The IL-12 p40-specific MoAb C8.6 was a gift from Dr G. Trinchieri (The Wistar Institute, Philadelphia, PA). Human rIL-2 was a gift from from Cetus Corp (Emeryville, CA; sp.act. 3 × 106 U/mg). Human rIL-4 (sp. act. 107 U/mg) was a gift from Dr J. E. de Vries (DNAX Research Institute, Palo Alto, CA). Human rIFN-γ (sp. act. 8 × 107 U/mg) was a gift from Dr. P. H. van der Meide (Biomedical Primate Research Center, Rijswijk, The Netherlands). Human rGM-CSF (sp. act. 11.1 × 106 U/mg) was obtained from Sandoz Pharma LTD (Basel, Switzerland).
Generation of dendritic cells and macrophages from peripheral blood (PB).Peripheral blood mononuclear cells (PBMC) from healthy donors were isolated from freshly drawn PB by density centrifugation on Lymphoprep (Nycomed, Torshov, Norway). Subsequently, PBMC were centrifuged on a Percoll (Pharmacia, Uppsala, Sweden) gradient, consisting of three density layers (1.076, 1.059 and 1.045 g/mL). The light density fraction, containing predominantly monocytes, was seeded in 24 well-culture plates (Costar, Cambridge, MA) at a density of 0.5 × 106 cells/mL. After a 1-hour incubation at 37°C, nonadherent cells were removed and adherent cells were cultured in Iscove's modified Dulbecco's medium (IMDM; Life Technologies Ltd, Paisley, UK) and 10% fetal calf serum (FCS; Hyclone, Logan, UT), supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF ) (500 U/mL) to obtain macrophages (Mφ) or supplemented with GM-CSF and IL-4 (250 U/mL) to obtain DC.13 14 Every 2 days the media including the supplements were refreshed. After 6 days of culture, DC and Mφ were procured and extensively washed before use.
Isolation of CD4+CD45RA+ naive and CD4+CD45R0+ memory T cells.To obtain CD4+ T cells, peripheral blood lymphocytes (PBL) were procured from the lower interface of the Percoll gradient and were incubated with anti-CD4 coated dynabeads (Dynal AS, Oslo, Norway), followed by treatment with Detachabeads (Dynal AS), according to the manufacturer's instructions. Subsequently, CD4+ T cells were incubated at 4°C in petri dishes either coated with UCHL-1 (anti-CD45RO) or 2H4 (anti-CD45RA). After 2 hours, nonbound naive or memory T cells, respectively, were removed by gentle pipetting. This method yielded highly purified (>98%) CD4+CD45RA+ or CD4+CD45R0+ T cells as determined by FACS analysis (data not shown).
Stimulation of DC.DC (40 × 103/well) were stimulated in 96-well flat-bottom culture plates (Costar) in IMDM containing 10% FCS in a final volume of 200 μL. The following stimuli were used: fixed Staphylococcus aureus Cowan strain I (SAC; 75 μg/mL; Calbiochem, San Diego, CA), lipopolysaccharide (LPS; 0.1 μg/mL; Difco, Detroit, MI), and soluble rCD40L containing a modified leucine zipper sequence17 (1 μg/mL; kindly provided by Immunex Research and Development Corp, Seattle, WA). This concentration of rCD40L was optimal for upregulation of adhesion molecules and IL-6 production by freshly isolated monocytes (data not shown). Furthermore, DC were incubated with 1 × 105 naive or memory Th cells, in the absence or presence of the superantigen staphylococcal enterotoxin A (SEA; 1 ng/mL; Serva, Heidelberg, Germany). All modes of stimulation were performed in the absence or presence of IFN-γ (1,000 U/mL). Supernatants were procured after 24 hours and IL-12 p70 levels were analyzed. Furthermore, supernatants of SAC-activated DC were used to study the effects of DC-derived soluble factors on the development of naive Th cells.
Mixed leukocyte reaction.All T-cell cultures were performed in IMDM supplemented with 10% FCS, human transferrin (Behring-Werke, Marburg, Germany; 35 μg/mL) and human insulin (Novo Nordish A/S, Bagsvaerd, Denmark; 0.175 IU/mL). For mixed leucocyte reaction experiments, allogenic naive Th cells (6 × 104/well) were cocultured with different numbers of 3,000 rad irradiated DC, Mφ, or freshly isolated monocytes in 96-well flat-bottom culture plates in a total volume of 200 μL. Proliferation was measured after 5 to 6 days by [3H]TdR incorporation, [3H]TdR being present the last 16 hours of culture (13 kBq/well; Radiochemical Center, Amersham, UK).
DC-dependent maturation of naive Th cells.Because the precursor frequency of conventional Ag-specific Th cells is low in the naive Th cell population, naive Th cells (2 × 104/200 μL) were primed with the superantigen SEA, presented by irradiated (3,000 rad) autologous DC (4 × 104/200 μL). This resulted in a strong proliferative response, whereas in the absence of either DC or SEA no activation of T cells could be observed (data not shown). Both SAC-activated and nonactivated DC were used for priming, in the absence or presence of neutralizing polyclonal IL-12 antibodies (5 μg/mL). SAC activation of DC did not affect the proliferative response of the naive Th cells (data not shown). After 6 to 7 days rIL-2 (10 U/mL) was added and Th cells were further expanded for another 6 to 7 days. Within the culture period of 14 days, Th cells acquired the CD45RO memory phenotype, and the number of Th cells primed by DC under all different experimental conditions increased 200- to 500-fold (data not shown). Resting memory Th cells were procured, washed, and restimulated with immobilized CD3 MoAb and soluble CD28 MoAb. Supernatants were procured after 24 hours and levels of IFN-γ and IL-4 were analyzed.
DC-independent maturation of naive Th cells.To study the functional maturation of naive Th cells in the absence of DC we used a culture system as described previously.18 Naive Th cells (2 × 104/200 μL) were stimulated with immobilized CD3 MoAb and soluble CD28 MoAb in the presence of rIL-2 (10 U/mL). These conditions resulted in optimal proliferation of the naive Th cells and resulted in the onset of production of both IL-4 and IFN-γ.18 Control experiments indicated that addition of SAC alone did not affect the cytokine profile of the maturing naive Th cells (data not shown). DC supernatants were added (final dilution 1:2), either or not in the presence of neutralizing anti-IL-12 (5 μg/mL). After 12 to 14 days of culturing, resting cells were procured, washed, and restimulated as described above.
Cytokine measurements.Measurements of IFN-γ and IL-4 levels in the T-cell supernatants and IL-12 p70 in the DC supernatants were performed by specific solid-phase sandwich enzyme-linked immunosorbent assay (ELISA), as described previously.19-21 The limits of detection of these ELISAs are: IFN-γ: 100 pg/mL, IL-4: 50 pg/mL, IL-12 p70: 3 pg/mL.
RESULTS
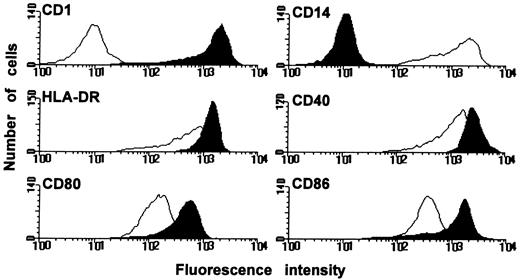
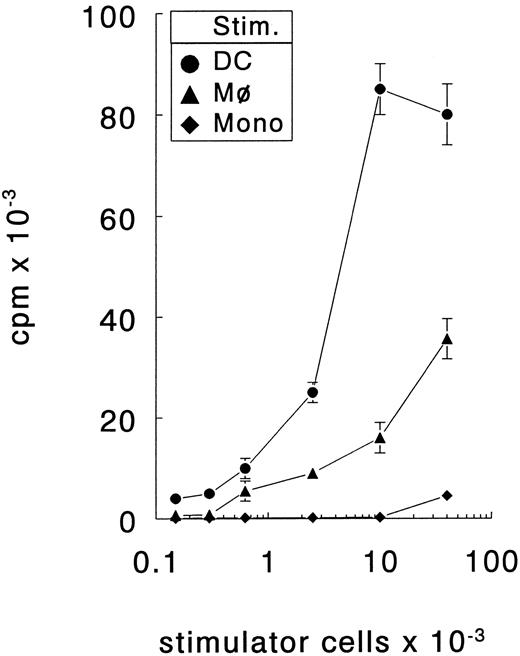
Generation of DC from PB precursors.The unique cell surface phenotype and the T-cell stimulatory capacity of the in vitro cultured DC were determined using in vitro cultured macrophages (Mφ) or freshly isolated monocytes as control cells. DC expressed high levels CD1a but not CD14, whereas Mφ expressed CD14 but not CD1a (Fig 1). Both APC types expressed HLA-DR, CD40, CD80, and CD86. However, DC expressed considerably higher levels of these surface molecules. The capacity of DC to stimulate naive T cells was determined by coculturing increasing numbers of DC, Mφ, or monocytes with allogenic naive (CD45RA+CD45RO−) CD4+ T cells. These mixed leucocyte reactions demonstrated that DC were more potent stimulators of naive Th cells than Mφ or monocytes (Fig 2). Only DC were capable of inducing proliferation by alloreactive naive Th cells at low cell numbers (150 cells/well) and, additionally, they induced the highest proliferative response at high cell numbers. Freshly isolated monocytes were even less efficient stimulator cells than Mφ; only at the highest cell number tested (40 × 103 cells/well) a low proliferative T-cell response could be observed. Regarding the surface phenotype and potent capacity to stimulate naive Th cells, the in vitro cultured DC that we have used here resemble the previously described in vivo and in vitro DC types.14,15,22 23
Surface phenotype of DC and Mφ. DC and Mφ were generated by culturing PB monocytes for 6 days in the presence of GM-CSF with or without IL-4, respectively. Subsequently, the expression of several surface molecules was determined by FACS analysis; DC black histograms; Mφ white histograms. Results are representative of eight independent experiments.
Surface phenotype of DC and Mφ. DC and Mφ were generated by culturing PB monocytes for 6 days in the presence of GM-CSF with or without IL-4, respectively. Subsequently, the expression of several surface molecules was determined by FACS analysis; DC black histograms; Mφ white histograms. Results are representative of eight independent experiments.
DC are more efficient stimulators of naive Th cells than Mφ or freshly isolated monocytes. Different number of stimulator cells (stim.) all obtained from the same donor were cocultured with allogenic naive Th cells. The proliferative response was determined at day 6. The results are presented as mean counts per minute ± standard error of the mean (SEM) of triplicate cultures. Results are representative of three independent experiments.
DC are more efficient stimulators of naive Th cells than Mφ or freshly isolated monocytes. Different number of stimulator cells (stim.) all obtained from the same donor were cocultured with allogenic naive Th cells. The proliferative response was determined at day 6. The results are presented as mean counts per minute ± standard error of the mean (SEM) of triplicate cultures. Results are representative of three independent experiments.
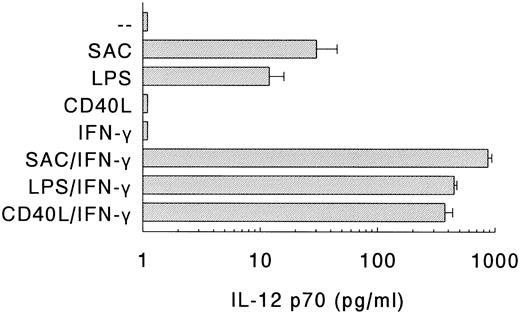
Production of IL-12 by DC in response to different stimuli.Next, the production of IL-12 p70 by DC on different modes of stimulation was determined (Fig 3). Stimulation of DC with SAC or LPS, which are potent inducers of IL-12 production in PBMC,24 resulted in the production of substantial amounts of IL-12. Recently, it was shown that CD40 ligand (CD40L), expressed on activated T cells, can induce IL-12 production in monocytes via interaction with CD40.25 However, despite the expression of high levels of CD40 (Fig 1), DC did not produce IL-12 on ligation of CD40 with soluble rCD40L (Fig 3).
Production of IL-12 p70 by DC. DC were stimulated with SAC, LPS, or soluble rCD40L in the absence or presence of IFN-γ. Supernatants were procured after 24 hours and levels of IL-12 p70 were analyzed. Results are presented as mean production ± SEM of four independent experiments.
Production of IL-12 p70 by DC. DC were stimulated with SAC, LPS, or soluble rCD40L in the absence or presence of IFN-γ. Supernatants were procured after 24 hours and levels of IL-12 p70 were analyzed. Results are presented as mean production ± SEM of four independent experiments.
As much as IFN-γ is a potent enhancer of IL-12 production by human monocytes,21,26,27 and IL-12 production by murine macrophages depends on the presence of IFN-γ,28 we also determined the IL-12 levels of DC that were stimulated in the presence of IFN-γ (Fig 3). IFN-γ, which by itself did not induce IL-12 production, strongly upregulated the production of IL-12 in response to either SAC or LPS. Notably, in the presence of IFN-γ, rCD40L also induced the production of large quantities of IL-12. In general, the IL-12 levels of the DC populations were comparable to, or even higher than, those of Mφ. For instance, in response to SAC/IFN-γ, DC produced 870 ± 67 pg/mL IL-12 (n = 4), whereas Mφ produced 365 ± 188 pg/mL IL-12 (n = 4), and in response to LPS/IFN-γ, DC produced 447 ± 25 pg/mL IL-12 (n = 4) and Mφ produced 501 ± 86 pg/mL IL-12 (n = 4).
Summarizing, DC produce substantial levels of IL-12 in response to bacteria (SAC) or bacterial compounds (LPS), and these levels can be strongly upregulated by IFN-γ. In contrast, the induction of IL-12 via CD40-CD40L interaction in DC requires IFN-γ as an obligatory, complementary signal.
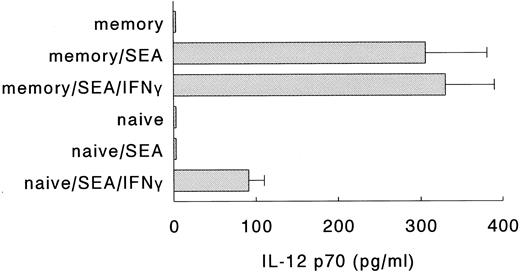
Naive Th cells require exogenous IFN-γ to induce IL-12 production in DC.In a next series of experiments, we examined whether the interaction between DC and naive or memory Th cells resulted in the production of IL-12. Both naive and memory Th cells express CD40L rapidly on activation,29 but the production of IFN-γ by naive Th cells is relatively delayed, as compared to memory Th cells.18,30 31 Since the induction of IL-12 through CD40 ligation requires the complementary action of IFN-γ, we anticipated that activated memory, but not activated naive Th cells would be able to induce IL-12 production. To test this hypothesis, DC were incubated with either naive or memory Th cells, in the absence or presence of SEA and/or exogenous IFN-γ. Figure 4 clearly shows that the induction of IL-12 p70 production in DC required both the activation of naive Th cells with superantigen and the presence of exogenous IFN-γ. Activated memory cells, on the other hand, were capable of inducing the production of IL-12 p70 in DC in the absence of exogenous IFN-γ, probably because the endogenous IFN-γ levels in these cultures were sufficient to allow for the induction of IL-12 production. Interestingly, even in the absence of exogenous IFN-γ, activated naive Th cells induced the production of the p40 subunit of IL-12 by DC (data not shown). Levels of IL-12 p40 were similar to the p40 levels induced by activated memory Th cells or SAC (± 3 ng/mL). Similar results were obtained when the naive and memory Th cells were preactivated for 16 hours with stimulatory MoAbs directed against CD3 and CD28 (data not shown). Furthermore, control experiments showed that SEA and/or IFN-γ without T cells did not induce the production of IL-12 p70 (data not shown). These results demonstrate that in contrast to activated memory Th cells, activated naive Th cells are poor inducers of IL-12 production by DC.
Naive Th cells require exogenous IFN-γ to induce IL-12 production in DC. Naive or memory Th cells were incubated with autologous DC, in the absence or presence of SEA to activate the Th cells and rIFN-γ. Supernatants were procured after 24 hours and levels of IL-12 p70 were analyzed. Result are presented as mean production IL-12 p70 ± SEM of triplicate cultures. Data are representative of three independent experiments.
Naive Th cells require exogenous IFN-γ to induce IL-12 production in DC. Naive or memory Th cells were incubated with autologous DC, in the absence or presence of SEA to activate the Th cells and rIFN-γ. Supernatants were procured after 24 hours and levels of IL-12 p70 were analyzed. Result are presented as mean production IL-12 p70 ± SEM of triplicate cultures. Data are representative of three independent experiments.
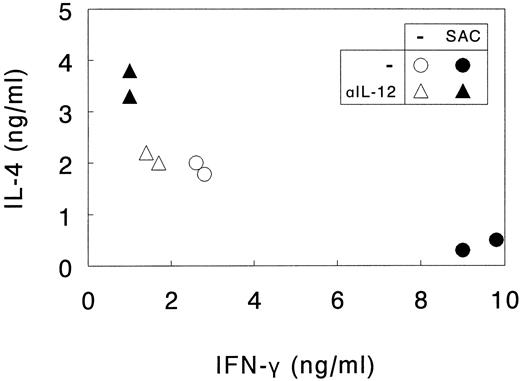
Induction of Th1 development by DC requires an exogenous IL-12–inducing factor.Subsequently, the role of DC in skewing the cytokine profile of naive Th cells was studied, focusing on the role of DC-derived IL-12. For this purpose, naive Th cells were activated by SEA presented by autologous DC that were or were not activated with SAC. Endogenous IL-12 production was neutralized with anti-IL-12. Similar high proliferative responses of the naive Th cells were observed under all conditions (data not shown). After addition of rIL-2 at day 6 to 7, Th cells were cultured for another 6 to 7 days until they had reached a resting state, and their cytokine profiles were analyzed after restimulation with CD3 and CD28 MoAbs.
As shown in Fig 5, priming of naive Th cells with nonactivated DC promoted the development of memory Th0 cells producing comparable amounts of the type 1 cytokine IFN-γ and the type 2 cytokine IL-4. Neutralization of endogenous IL-12 decreased IFN-γ levels only to a limited extent, indicating that under these conditions IL-12 does not play a major skewing role, as could be expected from the observation that naive Th cells are poor inducers of IL-12 production in DC (Fig 4).
SAC-activated DC direct Th1 development from naive Th cells through the release of IL-12. Naive Th cells were activated by SEA presented by autologous DC. DC were or were not activated with SAC and endogenous IL-12 production was or was not neutralized with anti-IL-12 (αIL-12). Th cells were cultured for 14 days, the last 7 days in the presence of rIL-2 and restimulated with CD3 and CD28 MoAb. Supernatants were procured after 24 hours and levels of IL-4 and IFN-γ were analyzed. Results are shown as duplicate points representing two independent primary cultures treated under identical conditions in the same experiment. Data are representative of three independent experiments.
SAC-activated DC direct Th1 development from naive Th cells through the release of IL-12. Naive Th cells were activated by SEA presented by autologous DC. DC were or were not activated with SAC and endogenous IL-12 production was or was not neutralized with anti-IL-12 (αIL-12). Th cells were cultured for 14 days, the last 7 days in the presence of rIL-2 and restimulated with CD3 and CD28 MoAb. Supernatants were procured after 24 hours and levels of IL-4 and IFN-γ were analyzed. Results are shown as duplicate points representing two independent primary cultures treated under identical conditions in the same experiment. Data are representative of three independent experiments.
In contrast, priming of the naive Th cells with SAC-activated DC always resulted in the development of Th1-like cells, producing high quantities of IFN-γ and low quantities of IL-4. In these cultures Th1 development was clearly mediated by DC-derived IL-12, because addition of neutralizing IL-12 antibodies completely abolished the increased IFN-γ production and, additionally, resulted in enhanced IL-4 production. Irrelevant isotype-matched control antibodies did not exert these effects (data not shown).
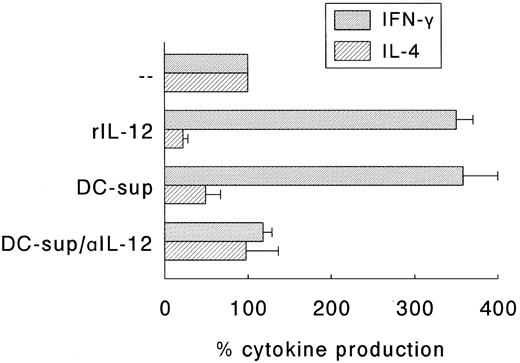
Next, we determined whether the SAC-induced Th1 development was indeed solely due to the SAC-induced production of IL-12 or that membrane molecules expressed by DC played a role in this respect. For this purpose, an accessory cell-independent T-cell maturation model was applied.18 In this model naive Th cells are activated by a combination of CD3 and CD28 MoAbs in the presence of rIL-2. The effect of DC-derived IL-12 was established by adding supernatants of 24 hours SAC-activated DC with or without neutralizing IL-12 antibodies. The production of IFN-γ and IL-4 by these Th cell cultures on restimulation with CD3 and CD28 MoAb were determined and compared to Th cell cultures that matured in the presence of rIL-12 (200 U/mL). Figure 6 shows that Th cells cultured in the presence of either rIL-12 or supernatants of SAC-activated DC produced higher levels of IFN-γ and, simultaneously, lower levels of IL-4 than control cultures, and that neutralizing anti-IL-12 antibodies completely abolished the regulatory effects of DC-supernatants on the onset of cytokine production. Control experiments indicated that irrelevant isotype-matched antibodies and supernatants of unactivated DC had no effect (data not shown).
IL-12 is the major Th1-skewing factor in supernatants of SAC-activated DC. Naive Th cells were stimulated with CD3 MoAb and CD28 MoAb in the presence of rIL-2. rIL-12 (200 U/mL) or supernatants of 24-hour activated DC (DC-sup; final dilution 1:2) were added, the latter in the absence or presence of neutralizing anti-IL-12 (αIL-12). Th cells were cultured for 14 days and restimulated with CD3 and CD28 MoAb. Supernatants were procured after 24 hours and levels of IFN-γ and IL-4 were analyzed. Results are expressed as the percentage (mean ± SEM of three independent experiments) of the cytokine production of Th cells that were cultured in the absence of rIL-12, DC-sup, and αIL-12.
IL-12 is the major Th1-skewing factor in supernatants of SAC-activated DC. Naive Th cells were stimulated with CD3 MoAb and CD28 MoAb in the presence of rIL-2. rIL-12 (200 U/mL) or supernatants of 24-hour activated DC (DC-sup; final dilution 1:2) were added, the latter in the absence or presence of neutralizing anti-IL-12 (αIL-12). Th cells were cultured for 14 days and restimulated with CD3 and CD28 MoAb. Supernatants were procured after 24 hours and levels of IFN-γ and IL-4 were analyzed. Results are expressed as the percentage (mean ± SEM of three independent experiments) of the cytokine production of Th cells that were cultured in the absence of rIL-12, DC-sup, and αIL-12.
Together, these data show that DC induce the development of naive Th cell populations into Th0 cell populations, producing both IFN-γ and IL-4, because the interaction between DC and naive Th cells does not facilitate the induction of IL-12 production. Therefore, the induction of Th1 development through DC-derived IL-12 requires the action of an exogenous IL-12–inducing factor.
DISCUSSION
The present study shows that human DC are potent producers of IL-12. The induction of the development of Th1 cells from naive Th cells through DC-derived IL-12, however, requires the action of exogenous IL-12–inducing factors, because the interaction between DC and naive Th cells does not facilitate the induction of IL-12 production in DC. In the absence of IL-12–inducing factors, DC efficiently promote the production of both IL-4 and IFN-γ in maturing naive Th cell populations.
The DC populations used in this study were clearly superior to Mφ with respect to the expression of MHC class II and costimulatory molecules, and the capacity to stimulate naive Th cells. Like Mφ and monocytes, these DC produce IL-12 in response to well-known IL-12–inducers such as SAC and LPS and, additionally, their IL-12 levels can be strongly upregulated by IFN-γ.21,26 27 Although the amounts of IL-12 p70 induced by SAC-activation alone appeared to be relatively low, our functional data show that these low amounts are sufficient to induce Th1 development. In fact, supernatants of SAC-activated DC (containing ± 30 pg/mL IL-12) were equally effective in this respect as an excess of rIL-12.
The finding that IL-12 production by in vitro cultured human DC can be induced by stimuli such as SAC or LPS are in line with the results from other groups6,32 but appear to be in contrast with another, recently published study.8 However, these different results may be explained by the relatively low sensitivity of the IL-12 p70 ELISA (50 pg/mL) applied in the latter study.
Another IL-12–inducing pathway is the interaction between CD40L expressed by activated T cells and CD40 expressed by APC.25,33 Here we have shown that IL-12 production by DC after ligation of CD40 requires the complementary action of IFN-γ. This action of IFN-γ cannot merely be attributed to its upregulatory effect on CD40 expression, since DC already express high levels of CD40 in the absence of IFN-γ. Another, more likely, explanation may be that the expression of the IL-12 p35 and p40 subunits is differentially regulated on activation via CD40 or IFN-γ. CD40-CD40L interaction stimulates the mRNA accumulation of the p40 subunit, but not of the p35 subunit (33 and our own unpublished observation, June 1996), whereas IFN-γ directly induces transcription of the p35 gene.34
Apparently in contrast to our results, Cella et al8 reported that ligation of CD40 on DC is sufficient for the induction of high IL-12 levels. However, since they used CD40L-transfected J588L cells instead of rCD40L, it cannot be excluded that these cells release soluble factor(s) that, like IFN-γ, can complement the action of CD40 ligation by directly inducing the transcription of the p35 gene and/or can strongly enhance IL-12 production. The fact that in their experiments exogenous IFN-γ exerted only marginal upregulatory effects on the production of IL-12 actually supports this possibility.
The observation that CD40-CD40L interaction by itself is not sufficient for the induction of IL-12 production, explains our finding that DC-induced Th1 development from naive Th cells does not occur via a default differentiation pathway, but specifically requires an exogenous IL-12–inducing factor. Although CD40L on naive Th cells is expressed within 6 hours of activation,29 secretion of IFN-γ by activated naive Th cells is only detectable after 3 to 4 days.18,30 31 Thus, under these conditions the IFN-γ–requirement of DC to produce IL-12 on ligation of CD40 is not fulfilled. Indeed, here we have shown that in contrast to activated memory Th cells, naive Th cells are poor inducers of IL-12 production in DC, unless exogenous IFN-γ is present. Consequently, DC will not direct the development of naive Th cells toward the Th1 phenotype. Therefore,it may be anticipated that in the presence of exogenous IFN-γ during activation of naive Th cells by DC, the development of Th1 cells is facilitated through the induction of IL-12 production. Indeed, addition of rIFN-γ during the superantigen-priming of naive Th cells by DC resulted in the development of Th1 cells, an effect that was clearly dependent of DC, since no skewing effect of rIFN-γ was observed during the DC-independent maturation of naive Th cells (data not shown). Thus, it may be argued that, in addition to potent IL-12-inducers such as SAC, microenvironmental IFN-γ instructs DC to skew naive Th cells toward the Th1 phenotype.
Murine in vitro studies have indicated that DC preferentially promote the development of Th1 cells.5,6 In the study of Macatonia et al,5 however, it was shown that only in the absence of endogenous IL-4 during the primary activation of Th cells, Th1 responses could be driven by DC through the production of IL-12. In our culture system, on the other hand, endogenous IL-4 did not appear to play a major role in this respect, since IL-4 was never detected in primary cultures, nor was the skewing potential of DC affected by the addition of neutralizing anti-IL-4 (data not shown). The other mouse model study6 showed that polarized Th1 cytokine profiles were obtained after repetitive cycles of stimulation with DC. Under these conditions, Th1 development probably follows from CD40L-induced IL-12 production facilitated by the production of IFN-γ by matured Th cells, which occurs relatively rapidly after stimulation.
The observation that in the absence of IL-12–inducers human DC induce both IL-4 and IFN-γ production in maturing Th cells is in keeping with data from several murine in vivo studies. Ronchese et al35 showed that DC pulsed with protein Ag efficiently prime IL-4– and IFN-γ–producing Ag-specific T cells. Furthermore, evaluation of specific immune responses after epicutaneously application of Ag revealed that IL-12–inducing chemical haptens promoted a Th1 response,36 whereas protein Ag resulted in a Th2/Th0 or even a Th2 response, depending on the time course of sensitization.37
The capacity of DC to prime the production of IL-4 may be partly attributed to their high surface expression of CD80/CD86 and/or CD40. It was reported by various groups that ligation of the counterparts of these molecules, ie, CD28 and CD40L, respectively, promotes the induction of IL-4 production in naive Th cells.18 38-41 However, these IL-4–inducing pathways are clearly dominated by the action of IL-12, which strongly inhibits the onset of IL-4 production in naive Th cells.
In conclusion, the present findings support the concept that the nature of the Th cell response is not fixed by the intrinsic properties of DC, but is highly influenced by the biological activities of the Ag and/or microenvironmental factors. Thus, by inducing IL-12 production in DC, bacteria, bacterial compounds, or exogenous IFN-γ, eg, derived from activated bystander memory T cells or NK cells, will promote the development of Th1 responses. Conversely, Th2/Th0 responses will be facilitated by microenvironmental factors that inhibit the production of IL-12 by DC, such as IL-1042 and prostaglandin E2.43 The impact of these and other factors on the cytokine profile of DC and the consequences for the development of naive Th cells are currently under investigation.
ACKNOWLEDGMENT
The authors thank J. Schuitemaker for excellent technical assistance.
Supported by a grant from the Netherlands Organization for Scientific Research (The Hague) (900-505-251 to C.M.U.H.).
Address reprint requests to Catharien M.U. Hilkens, PhD, Academic Medical Center, University of Amsterdam, Department of Cell Biology & Histology, Meibergdreef 15, 1105 AZ, Amsterdam, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal