Abstract
Interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF ) receptors share a common β chain (βc), and both cytokines enhance erythropoietin (Epo)-dependent in vitro erythropoiesis by primary hematopoietic progenitors and factor-dependent cells. These data suggest that the Epo receptor and βc may functionally interact. To determine whether such interactions can be documented, we studied a murine factor-dependent cell line (Ba/F3), which endogenously expresses IL-3R. First, Ba/F3 cells were transfected with murine EpoR, making them responsive to both IL-3 and Epo. Next, the EpoR expressing cells were transfected with murine βc. This resulted in an enhanced sensitivity of these cells to Epo, which was especially pronounced at low Epo concentrations. Ba/F3-EpoR were then treated with antisense oligodeoxynucleotides to the murine β. Control sense and nonsense had no effect on Epo-dependent growth, but the antisense markedly and specifically inhibited Epo-dependent growth. In contrast, the antisense did not affect β-globin message levels (another Epo-responsive effect in these cells) detectable by Northern blot. Finally, Western blot analysis of proteins immunoprecipitated from cells expressing both receptors with antibody against β and blotted with antibody against EpoR, or immunoprecipitated with antibody against EpoR and blotted with antibody against β, showed that EpoR and β coimmunoprecipitate. These data show that the β chain functionally and physically associates with the EpoR. This suggests that these cytokine receptors exist as a large supercomplex and offers the first molecular explanation for the synergistic effects of IL-3 and GM-CSF with Epo during erythropoiesis.
SEVERAL HEMATOPOIETIC growth factors are known to be important in the differentiation of stem cells into mature erythrocytes. During the early phases of their growth in vitro, erythroid progenitors require Steel Factor and interleukin-3 (IL-3) or granulocyte-macrophage colony-stimulating factor (GM-CSF ), while at later stages erythropoietin (Epo) becomes necessary for their proliferation and maturation into hemoglobin-containing cells.1-3 The Epo requirement of cells of the erythroid lineage correlates with the expression of Epo receptors on the cell surface.4
The GM-CSF, IL-3, and IL-5 receptors are all comprised of unique α chains5-8 that bind their specific ligands with low-affinity and a shared β chain (βc ) that alone does not bind ligand, but is essential for high-affinity binding.9 The cytoplasmic portion of β is believed to contain most of the domains responsible for ligand-induced proliferation.10,11 Additionally, in murine cells, a highly related protein (βIL-3 ) can form a functional receptor with the IL-3α subunit.12 Ligand binding is believed to activate the receptor by inducing the formation of a heterodimer. Unlike the IL-3 and GM-CSF receptors, the Epo receptor is believed to be composed of a single subunit that is activated on ligand-induced homodimerization.13-15 The cytoplasmic region of EpoR has significant amino acid homology to the intracellular portion of βc .16,17 Because IL-3 and GM-CSF are known to cooperate with Epo in erythropoiesis in vitro2,18,19 and both receptors activate common signal transduction pathways,20 we determined whether the β subunit is involved in Epo signaling. The results of that inquiry are presented here.
MATERIALS AND METHODS
Cell lines and cultures.Ba/F3 wild-type cells (Ba/F3 wt) and cDNA encoding the murine erythropoietin receptor (mEpoR) were obtained from A. D'Andrea (Dana-Farber Cancer Institute, Boston, MA).21 Ba/F3 cells were respectively electroporated with the mEpoR and murine βc (mβc) in the pDNA3 or pREP-4 vectors (Invitrogen, San Diego, CA); the latter carries the hygromycin resistance gene. The murine cell line is IL-3–dependent and was grown in serum-free and protein-free hybridoma medium supplemented with glutamine and 10% fetal calf serum (FCS) (Sigma, St Louis, MO). This medium was supplemented with purified recombinant mIL-3 (kindly provided by Immunex, Seattle, WA) for the Ba/F3 wt or 3 U/mL purified recombinant human Epo (Amgen, Thousand Oaks, CA) for Ba/F3-EpoR and Ba/F3-EpoR+ β c. In addition, the cells transfected with βc were maintained in 800 μg/mL hygromycin (Calbiochem, La Jolla, CA).
Thymidine incorporation.Ba/F3, Ba/F3-EpoR and Ba/F3-EpoR+ βc cell lines were washed three times and plated at 2 to 3 × 104 cells/well in triplicate in 160 μL of media containing 10% serum with the appropriate HGFs for 72 hours at 37°C. Three hours and 15 minutes before harvesting, 1 μCi of 3H thymidine (New England Nuclear, Boston, MA) was added to each well. Cells were harvested on filter paper by an automated harvester (Skatron, Tranby, Norway) and counted in a β counter (Wallac, Gaithersburg, MD).
Binding analysis.Human recombinant Epo was radio-iodinated to a specific activity of 8.6 × 104 cpm/ng (2.92 × 1018 dpm/mol) by the iodine monochloride method.22 A total of 3 to 5 × 106 cells was incubated with graded concentrations of 125I-Epo in RPMI/2% bovine serum albumin/20 mmol/L HEPES/0.2% Na azide for 90 minutes at room temperature to obtain equilibrium binding. Ligand-bound cells were separated by centrifugation through a 3:2 mixture of dibutyl phthalate (Sigma Chemical Co):bis phthalate (Eastman Kodak, Rochester, NY) oil, and cell-associated radioactivity of the pellets was counted in a gamma counter (Cobra Autogamma 5000; Packard Instrument Co, Meriden, CT). Specific binding was obtained by subtracting the radioactivity bound to transfected cells incubated in a 100-fold excess of unlabeled ligand (nonspecific binding) from the radioactivity bound to the transfectants (total binding). The binding data were subjected to Scatchard analysis and the affinity constants confirmed using the LIGAND program.23
Antisense.Ba/F3 and Ba/F3-EpoR cells were grown in media containg 1% albumin, lipids (GIBCO-BRL, Gaithersberg, MD), insulin, transferrin, selenium (all from Boehringer-Mannheim, Indianapolis, IN) with 20 mmol/L deprotected synthetic oligodeoxynucleotide (ODN) (Molecular Biology Core Facility, DFCI, or the Oligonucleotide Synthesis Facility, Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA), and the indicated concentrations of IL-3 or Epo. The ODN were designed from the coding sequence of the murine βc beginning at the first ATG initiation site. The ODN sequences were: antisense — GAGTGCCATTTGCTGGTCCAT; sense — ATGGACCAGCAAATGGCACTC; nonsense TGTCGAGTCACTTGCAGTGTC; positive control- polyT-21mer. An additional set of ODNs designed to start at the first internal ATG initiation site was also used. The cells were cultured for 5 days with the ODN, at which time RNA was extracted or cell proliferation measured by thymidine incorporation or direct counting.
Northern hybridization.RNA was prepared by the guanidinium/CsCl method.24 A total of 10 μg per lane was loaded on a 1% agarose-formaldehyde gel, and RNA was separated by electrophoresis at 150 V for 3 hours. RNA was transferred by capillary transfer overnight using 20× SSC to Duralon (Stratagene, La Jolla, CA) nylon membrane. RNA was cross-linked by UV light using a Stratalinker (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The β-globin DNA probes25 (obtained from Martin Carroll, Brigham and Women's Hospital, Boston, MA) were labeled with 32P using random primed labeling (Boehringer-Mannheim). Blots were prehybridized for 30 minutes at 68°C in a hybridization oven using a commercial hybridization solution (Quik-hyb, Stratagene). Hybridization was done for 2 hours at 68°C. Blots were washed in 2× SSC/0.1% sodium dodecyl sulfate (SDS) at room temperature followed by 0.1× SSC/0.1% SDS at 60°C, and exposed to film.
Immunoprecipitation.Parental Ba/F3 wild-type (wt) and transfected cells were placed on ice and incubated in media containing 2% bovine serum albumin and Epo (100 U/mL) for 1 hour. For Epo stimulation experiments, the cells were washed three times in phosphate-buffered saline (PBS), starved in RPMI/10% FCS for 12 hours, and then incubated for 15 minutes in Epo (50 U/mL). The cells were washed once with PBS, and the pellet solubilized in lysis buffer that contained 1% Triton X100 (Sigma), 150 mmol/L NaCl, 50 mmol/L Tris pH 8.2, with protease and phosphatase inhibitors (10 μg/mL leupeptin, 0.2 U/mL aprotinin, 10 μg/mL leupeptin, 2 mmol/L phenylmethylsulfonyl fluoride, 2 mmol/L Na fluoride, and 1 mmol/L Na orthovanadate). After centrifugation (168,000g for 15 minutes at 4°C), the supernatants were precleared with preimmune rabbit serum and sepharose beads for 1 hour and incubated with antibodies that react with either the n-terminus of the mEpoR26 (gift of A. D'Andrea and D. Barber), the murine βc and βIL-3 chain (antibody 21F2),27 or preimmune rabbit serum (Sigma) for 8 hours at 4°C. Protein A sepharose or protein G-plus agarose beads were then added (25 μL packed volume), and after 1.5 hours, the beads were washed three times with 1 mL lysis buffer at 4°C. The bound proteins were eluted from the beads by boiling in Laemmli sample buffer containing 5% β-mercaptoethanol and were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) (7% to 15% acrylamide gradient). The separated proteins were then transferred to nylon membrane (Hoefer, San Francisco, CA) and immunoblotted with a 1:50 dilution of rabbit polyclonal antibodies26 to the c-terminus of EpoR or a 1:100 dilution of a mixture of n- and c- terminus antibodies, or rabbit polyclonal antimouse IL-3/IL-5/GM-CSFR βc antibody (#K-17 Santa Cruz Biotechnology, Santa Cruz, CA). The membrane was washed and the bound antibodies detected with horseradish peroxidase secondary antibodies and enhanced chemiluminescence, as described by the manufacturers (ECL Western Blotting; Amersham, Arlington Heights, IL).
RESULTS
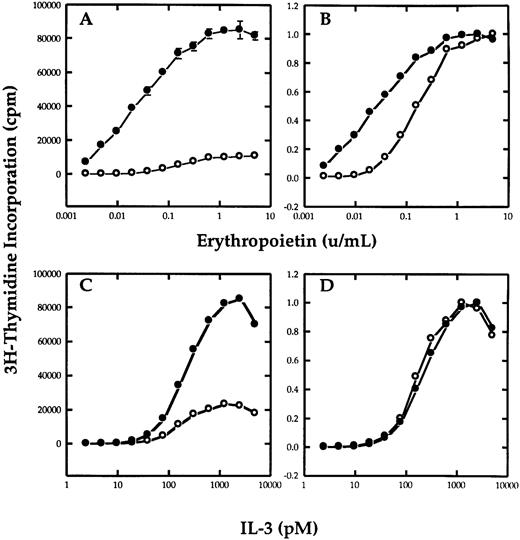
βc potentiates erythropoietin-dependent growth.To determine if the βc chain of the IL-3 receptor could functionally interact with EpoR in mediating a proliferative response, Ba/F3 cells that stably expressed the EpoR were transfected with a plasmid containing the hygromycin resistance gene with or without the cDNA of βc . After selecting the cells in hygromycin, the cells were cultured in the various concentrations of IL-3 or Epo and their growth was measured by thymidine incorporation. Figure 1A shows the effect that the transfection of βc has on the response of the cells to Epo. Figure 1B is the same plot normalized as a ratio of maximum proliferation to allow better comparision of the proliferative response to growth factor. Both Ba/F3-EpoR and Ba/F3-EpoR+ βc required Epo for survival and responded to Epo in a dose-dependent manner. However, the Ba/F3-EpoR+ βc had a greater sensitivity to Epo than Ba/F3-EpoR. The difference was most pronounced at the lowest Epo concentrations. In contrast, both cell lines had an identical proliferative response to IL-3 (Fig 1C and D), showing that the βc transfection did not effect the Epo dose response by nonspecifically enhancing the sensitivity of the cells to all growth factors. These results were obtained using nonclonal cells from three independent electroporation experiments.
Effect of βc on the proliferative response of Ba/F3-EpoR to Epo. Tritiated thymidine incorporation assay of Ba/F3-EpoR+ βc (•) or Ba/F3-EpoR (○) grown in various concentrations of Epo (A and B) or mIL-3 (C and D). (B and D), respectively, represent the data in (A and C) normalized to maximal proliferation. The upper panels show that cells transfected with βc (•) have a greater sensitivity to Epo than cells that were not (○). The lower panels show that the dose response of the same cells to IL-3 was not affected.
Effect of βc on the proliferative response of Ba/F3-EpoR to Epo. Tritiated thymidine incorporation assay of Ba/F3-EpoR+ βc (•) or Ba/F3-EpoR (○) grown in various concentrations of Epo (A and B) or mIL-3 (C and D). (B and D), respectively, represent the data in (A and C) normalized to maximal proliferation. The upper panels show that cells transfected with βc (•) have a greater sensitivity to Epo than cells that were not (○). The lower panels show that the dose response of the same cells to IL-3 was not affected.
To test the possibility that a significant difference in receptor number or ligand binding affinity could be responsible for the different Epo dose response seen in the Ba/F3-EpoR and EpoR+ βc cells, equilibrium binding assays using 125I Epo were performed. The results of these assays showed that EpoR expression and affinity in Ba/F3-EpoR (Kd = 374 pmol/L, 350 sites per cell) and Ba/F3-EpoR+ βc (Kd = 365 pmol/L, 200 to 500 sites per cell), were similar (data not shown).
Antisense to β mRNA inhibits erythropoietin-dependent growth.Because Ba/F3-EpoR+ βc appeared to be more sensitive to Epo than Ba/F3-EpoR, we needed to confirm the functional interaction between the two receptors by an independent method. Therefore, we decided to test whether antisense ODN to β could inhibit the Epo-dependent proliferation of Ba/F3 cells. Ba/F3-EpoR were cultured in media containing 20 mmol/L ODN and varying concentrations of Epo or IL-3. After 5 days in culture, tritiated thymidine incorporation was measured as shown in Fig 2 (data are representative of four experiments). Addition of β antisense ODN to the samples significantly inhibited the growth of Ba/F3-EpoR cultured in Epo (Fig 2A). Compared with either sense or nonsense, the samples with antisense ODN had approximately one half the number of cpm. This inhibitory effect was observed over the entire range of Epo concentrations tested and was even maintained at saturation levels of Epo. However, despite the significant reduction in the level of thymidine incorporation compared with the controls, the antisense ODN were not as inhibitory as equimolar concentrations of the positive control ODN, polyT. These results were independently confirmed by counting the cells after incubation in the different ODN (data not shown). Each of the individual ODN had similar effects when the cells were cultured in IL-3 (Fig 2B). The addition of antisense reduced IL-3–dependent growth at all concentrations of IL-3. An identical response was noted when another antisense oligonucleotide designed to hybridize with the β RNA beginning at the first internal ATG start site was tested under both conditions (data not shown). Despite its effect on Ba/F3-EpoR, β antisense oligonucleotide was unable to inhibit Ba/F3-EpoR+ βc at any concentration of IL-3 (data not shown).
Effect of oligodeoxynucleotides to β mRNA on the proliferation of Ba/F3-EpoR cells. Cells were cultured serum-free in the presence of 20 mmol/L antisense (•), sense (▪), nonsense (□) or positive control (○) oligonucleotides in the presence of Epo (A) or mIL-3 (B), and thymidine incorporation was measured on day 5. The results show that antisense oligonucleotides significantly reduces the growth of the cells compared with either sense or nonsense oligonucleotides.
Effect of oligodeoxynucleotides to β mRNA on the proliferation of Ba/F3-EpoR cells. Cells were cultured serum-free in the presence of 20 mmol/L antisense (•), sense (▪), nonsense (□) or positive control (○) oligonucleotides in the presence of Epo (A) or mIL-3 (B), and thymidine incorporation was measured on day 5. The results show that antisense oligonucleotides significantly reduces the growth of the cells compared with either sense or nonsense oligonucleotides.
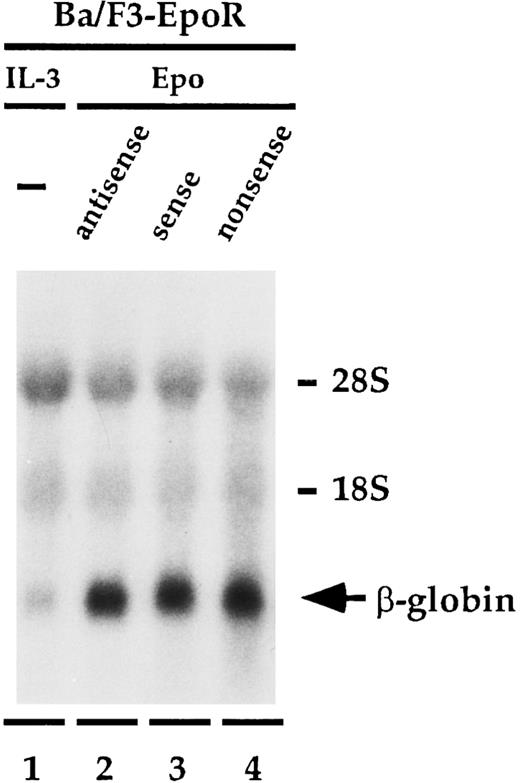
Antisense to β mRNA does not affect β globin expression.Having established that antisense to β mRNA specifically inhibits Epo-dependent proliferation of Ba/F3-EpoR, we wanted to determine if antisense to β could also influence the ability of Epo to increase the level of β-globin message found in the cells (Fig 3). Ba/F3-EpoR cells were treated with the oligonucleotides identically to the method used in the proliferation experiment except that on day 5, instead of adding thymidine, total cellular RNA was extracted and then analyzed by Northern blot. Cells that were cultured in mIL-3 expressed low levels of β-globin mRNA (Fig 3, lane 1). Incubating the cells in Epo (Fig 3, lanes 2 to 4) instead of IL-3 significantly enhanced the amount of β-globin message detected in these cells, as has been previously reported.25,28 29 However, in contrast to the significant effect that the antisense oligonucleotide had on Epo-dependent cell proliferation, the level of β-globin message in the presence of β antisense (Fig 3, lane 2) was similar to the level observed with both the sense (Fig 3, lane 3), and nonsense (Fig 3, lane 4) oligonucleotide controls. These findings were unchanged when the experiment was done at higher concentrations of Epo or if the mRNA was extracted on day 7. The upper part of Fig 3 shows the amount of ribosomal RNA present in each lane and shows that the total amount of RNA loaded in each lane is similar. This experiment was performed three times.
Effect of oligonucleotides to β mRNA on β-globin mRNA levels. Ba/F3-EpoR cells were cultured in IL-3 (lane 1) or with 20 mmol/L antisense (lane 2), sense (lane 3), or nonsense (lane 4) in the presence of Epo, and β-globin message levels were determined by northern blot analysis. The increased levels of β-globin mRNA induced by Epo were identical in each of the samples containing oligodeoxynucleotides.
Effect of oligonucleotides to β mRNA on β-globin mRNA levels. Ba/F3-EpoR cells were cultured in IL-3 (lane 1) or with 20 mmol/L antisense (lane 2), sense (lane 3), or nonsense (lane 4) in the presence of Epo, and β-globin message levels were determined by northern blot analysis. The increased levels of β-globin mRNA induced by Epo were identical in each of the samples containing oligodeoxynucleotides.
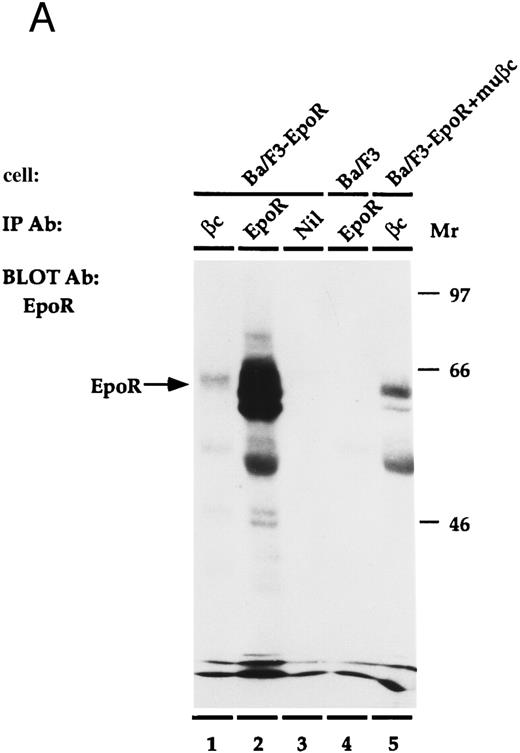
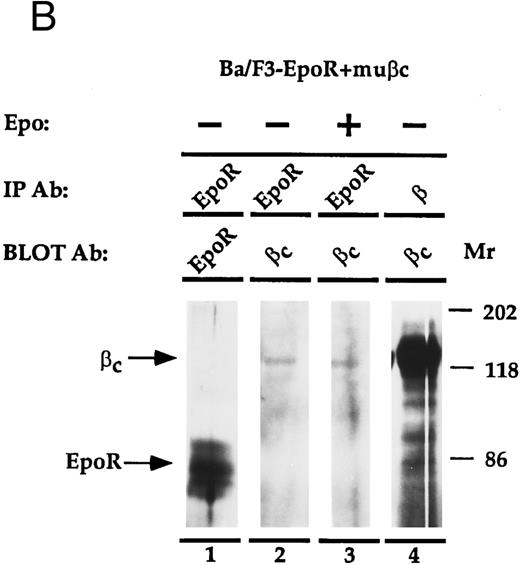
EpoR physically associates with β.The EpoR and β are proteins that bind ligand at the cell surface. Thus, it is possible that the two receptors associate to mediate their cooperative effects on proliferation. To investigate whether the proteins are physically associated, an immunoprecipitation experiment was performed (Fig 4). Ba/F3, or Ba/F3-EpoR+/-βc were incubated at 4°C with Epo and then were solubilized with Triton X100. Antibody against either β, the n-terminus of EpoR, or nonspecific control antibody was added to the soluble extract and this was followed by an incubation with protein G sepharose. The immunoprecipitated proteins were transferred to nitrocellulose after SDS-PAGE and were blotted with antibody reactive against the c-terminus of the Epo receptor. An autoradiograph of the results is shown in Fig 4A. As a positive control, the proteins of Ba/F3-EpoR cells were immunoprecipitated with antibody against the n-terminus of EpoR. A broad band corresponding to the EpoR was observed by Western blot (Fig 4A, lane 2). When the same cells were immunoprecipitated with monoclonal antibody against β and again blotted with antibody against c-terminal EpoR, a less intense band with slightly higher average molecular weight was seen (Fig 4A, lane 1). In contrast, the EpoR band was not present when Ba/F3-EpoR was precipitated with preimmune serum (Fig 4A, lane 3) or when parental (nontransfected) Ba/F3 cells were precipitated with the anti-n–terminal EpoR antibody (Fig 4A, lane 4). To test if the association between EpoR and βc correlated with the cooperative growth effects, the Ba/F3-EpoR+ βc used in the proliferation assays was also immunoprecipitated with antibody against β. A band identical in size to the one seen in Fig 4A, lane 1, but consistently more intense, was observed (Fig 4A, lane 5). These data are representative of four experiments.
(A) Coimmunoprecipitation of EpoR with β. Ba/F3, Ba/F3-EpoR, and Ba/F3-EpoR+ βc cells were incubated with Epo, and the solubilized proteins were immunoprecipitated with anti-EpoR (n-terminus), anti-β, or control antibody. After gel electrophoresis the immunoprecipitated proteins were transferred to nitrocellulose and blotted with antibody against EpoR c-terminus. Ba/F3-EpoR immunoprecipitated with antibody against β (lane 1), antibody against EpoR (lane 2), or control antibody (lane 3). Ba/F3 immunoprecipitated with anti-EpoR (lane 4). Ba/F3-EpoR+ βc immunoprecipitated with antibody against β(lane 5). Bands corresponding to EpoR were observed when EpoR expressing cells were immunoprecipitated with antibody against either EpoR or β. (B) Coimmunoprecipitation of β with EpoR. Ba/F3-EpoR+ βc cells were cultured without growth factor for 8 hours and then incubated in medium alone (lanes 1, 2, and 4) or medium containing Epo (50 U/mL) for 15 minutes (lane 3). The solubilized proteins were immunoprecipitated with either antibodies to the n-terminus of the EpoR (lanes 1 to 3) or monoclonal antibody to β (lane 4). After SDS-PAGE (7.5% acrylamide), the separated proteins were transferred to nylon membrane and immunoblotted with EpoR antibody (lane 1) or with polyclonal rabbit antibody against βc (lanes 2, 3, and 4). Bands corresponding to β were observed to similarly coimmunoprecipitate with EpoR in the presence or absence of Epo.
(A) Coimmunoprecipitation of EpoR with β. Ba/F3, Ba/F3-EpoR, and Ba/F3-EpoR+ βc cells were incubated with Epo, and the solubilized proteins were immunoprecipitated with anti-EpoR (n-terminus), anti-β, or control antibody. After gel electrophoresis the immunoprecipitated proteins were transferred to nitrocellulose and blotted with antibody against EpoR c-terminus. Ba/F3-EpoR immunoprecipitated with antibody against β (lane 1), antibody against EpoR (lane 2), or control antibody (lane 3). Ba/F3 immunoprecipitated with anti-EpoR (lane 4). Ba/F3-EpoR+ βc immunoprecipitated with antibody against β(lane 5). Bands corresponding to EpoR were observed when EpoR expressing cells were immunoprecipitated with antibody against either EpoR or β. (B) Coimmunoprecipitation of β with EpoR. Ba/F3-EpoR+ βc cells were cultured without growth factor for 8 hours and then incubated in medium alone (lanes 1, 2, and 4) or medium containing Epo (50 U/mL) for 15 minutes (lane 3). The solubilized proteins were immunoprecipitated with either antibodies to the n-terminus of the EpoR (lanes 1 to 3) or monoclonal antibody to β (lane 4). After SDS-PAGE (7.5% acrylamide), the separated proteins were transferred to nylon membrane and immunoblotted with EpoR antibody (lane 1) or with polyclonal rabbit antibody against βc (lanes 2, 3, and 4). Bands corresponding to β were observed to similarly coimmunoprecipitate with EpoR in the presence or absence of Epo.
To confirm the physical association between EpoR and β, the converse immunoprecipitation and Western blot analysis was performed (Fig 4B). In this experiment, Ba/F3-EpoR+ βc cells were cultured without Epo for 8 hours and then incubated with or without Epo (15 minutes) to determine if the coimmunoprecipitation is affected by the presence or absense of Epo. The solubilized cell extracts were immunoprecipitated with antibodies to the n-terminus of EpoR, or with antibody against β. After electrophoresis and transfer to nylon membrane, the immunoprecipitated proteins were blotted with antibodies to EpoR or βc (this antibody reacts specifically with βc). An appropriate sized band for the Epo receptor is seen when the Ba/F3-EpoR+ βc cells are immunoprecipitated with anti-EpoR and blotted with antibody against EpoR (Fig 4B, lane 1). When the proteins immunoprecipitated with antibody against EpoR were blotted with antibodies against βc (Fig 4B, lanes 2 and 3), 140 kD- sized bands corresponding to βc are visible. No difference in band intensity was observed when the immunoprecipitation was done in the absence (Fig 4B, lane 2) or presence (Fig 4B, lane 3) of Epo. These data substantiate the specificity of the EpoR-β association shown in Fig 4A and imply that the presence of Epo is not required for the interaction. The positive control, β immunoprecipitation followed by βc immunoblot (Fig 4B, lane 4), confirms the identity of βc . This band is also much more prominent than the corresponding band in lanes 2 and 3 (Fig 4B). One interpretation is that only a small portion of the total amount of βc found in cells is associated with the EpoR; another is that the immunoprecipitation is not efficient. Bands corresponding to EpoR or βc were not detected by immunoblot when preimmune sera or control antibody was used for the immunoprecipitation step (data not shown). This figure is one of two independent experiments.
DISCUSSION
Early studies showing that GM-CSF and IL-3 could enhance the effects of Epo on primary cells and cell lines first suggested the possibility that β and EpoR might interact.2,30,31 In this report, we show that transfection of βc into Ba/F3-EpoR cells significantly increases the growth sensitivity of the cells to Epo. The increase in proliferation is more pronounced at low Epo concentrations, suggesting that whatever the mechanism, the amount of β may be limiting. Confirming this observation, we find that antisense to β specifically inhibits Epo-stimulated cell proliferation. Antisense experiments have also suggested that the IL-3 receptor may interact with the thrombopoietin receptor (c-mpl).32 Thus, the most likely interpretation of these data is that β functionally influences the EpoR proliferative signaling pathway. However, this does not infer that β is required for Epo-induced cell growth. One possible explanation is that β helps to stabilize or maintain an active receptor complex. It could do so by associating with the EpoR to form binding sites for accessory molecules such as JAK2,33,34 by inhibiting negative regulatory molecules such as phosphatases,35,36 or by increasing receptor half-life. Recently, Nicola et al37 showed that βc is also an important component in the receptor transmodulation of the G-CSF and M-CSF receptors.
Despite inhibiting cell growth, β antisense did not affect β-globin mRNA levels. In an earlier study,38 we showed that human βc augmented β-globin message levels in cells that expressed a hybrid GM-CSF/EpoR receptor (GMER). We, therefore, expected that the antisense might reduce β-globin mRNA levels. One explanation may be that strong EpoR-βc heterodimers are more efficient than weak GMER dimers, but less efficient than strong EpoR homodimers in increasing β-globin mRNA; another is that changes in β-globin message levels may be less sensitive than growth to the contribution provided by βc . Finally, any noticable decrease in β-globin message could have been off-set by an increase in β globin level that is known to correlate with decreased cell cycling in Ba/F3-EpoR cells.25 In addition to their functional interaction, we show by coimmunoprecipitation experiments that β and EpoR also physically associate in an Epo-independent manner.
Other investigations have supported the possibility that these two proteins could functionally interact. Several groups have observed that the transfection of EpoR into IL-3–dependent cells permitted the cells to acquire Epo responsiveness, but the transfection of EpoR into cells that do not express endogenous βc did not result in Epo responsiveness39,40; the latter result, obtained in the CTLL-2 cell line, is however controversial.41 Hanazono et al42 showed that Epo stimulates tyrosine phosphorylation of βc in the UT-7 cell line. In contrast, IL-3 is not able to stimulate the phosphorylation of the EpoR.43 Analogously, Steel Factor has also been shown to induce serine/threonine phosphorylation of the IL-3R.44
Deletion of the β subunit by homologous recombination could provide confirmatory evidence that the EpoR and β interact. However, animals deficient in either βc or βIL-3 have not been reported to have impaired eythropoiesis,45,46 and recently similar results were reported for IL-3/βc double mutant mice that lack IL-3, GM-CSF, and IL-5 function.47 It is possible that a more detailed analysis such as testing the sensitivity of erythroid precursors to Epo in these animals might show a more subtle defect. It should be noted that all of these knockout animals have one β chain intact, and it is possible that Epo interacts with the nondisrupted β chain in these animals, providing an explanation for the absence of anemia. Therefore, mice deficient in both βc and βIL-3 will need to be assessed, as these two chains have functional redundancy. Such mice would be more analogous to our studies, as the antisense oligodeoxynucleotide we used would affect mRNA of both of these proteins because of sequence identity. Interestingly, our data, together with experiments by Wu et al48 who reported that the c-kit receptor also interacts with the EpoR, provide evidence that EpoR receptors may exist as a large complex of associated proteins.
In conclusion, we show here that the β has a role in the Epo-dependent proliferation of Ba/F3 cells that express EpoR, but has a more limited influence on β-globin message expression, another function ascribed to Epo in these cells. We also provide evidence that the cooperative effects of the two receptors on cell growth may be mediated by a direct physical association between the two proteins. The data provide an explanation for the synergistic effects of IL-3/GM-CSF and erythropoietin during in vitro erythropoiesis.
Supported by U.S. Public Health Service Award GM 13452 (Bethesda, MD), RO1 CA45559 (National Institutes of Health), and the Charles H. Hood Foundation (Boston, MA). P.T.J. is the recipient of a Clinical Oncology Career Development Award from the American Cancer Society (Atlanta, GA).
Address reprint requests to Paul T. Jubinsky, MD, PhD, Children's Hospital Medical Center, Hematology and Oncology, 3333 Burnet Ave, Cincinnati, OH 45229-3039.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal