Abstract
The mechanisms by which hematopoietic progenitor cells are normally anchored in stromal niches and yet can be mobilized by specific growth factors are poorly understood. It is likely, however, that integrins and their extracellular matrix (ECM) ligands play a key role in this process, and recent evidence suggests that integrin function is modulated by signals originating from activated growth factor receptors. We have now examined this further by studying the role of growth factors on α4β1 integrin-mediated adhesion of human CD34+ hematopoietic progenitor cells to specific recombinant fibronectin fragments coated onto tissue culture dishes. Cells were prepared from cord blood and peripheral blood harvests. During a 30-minute adhesion assay a mean of 74% of CD34 cells attached to the so-called H120 fragment of fibronectin, which contains the strongest α4β1 integrin-binding sequence. The level of cell adhesion was significantly reduced by low concentrations of interleukin-3 (IL-3) (2.5 to 10 ng/mL), whereas stem cell factor (SCF ) and granulocyte colony-stimulating factor (G-CSF ) at these concentrations did not affect adherence of the cells. Migratory behavior of CD34 cells was examined using fibronectin fragments adsorbed onto a Transwell filter. The H120 fragment supported much higher levels of cell migration than the H0 fragment of fibronectin, which contains a weak α4β1 integrin binding sequence. Over a 16-hour assay, migration of peripheral blood progenitor cells was increased slightly by SCF and by G-CSF. However, a marked stimulation was observed with IL-3, which significantly increased migration. Similar effects were noted with cord blood cells, although a small proportion of cells were able to migrate in the absence of growth factors. These results indicate that there is a highly selective and functional link between the α4β1 integrin and IL-3/IL-3–receptor that could affect the position of stem and progenitor cells in the marrow stroma and influence their growth and development.
INTERACTIONS BETWEEN hematopoietic progenitor cells and the bone marrow stroma in which they reside are recognized to be of physiologic importance not only for their normal proliferation and differentiation, but also for maintenance of the hematopoietic stem cell.1,2 These interactions are dynamic, but the mechanisms of cell adhesion to stroma are poorly understood and, likewise, there is little information on the underlying processes that govern cell migration and egress into the peripheral circulation. It is likely that highly regulated interactions between adhesion receptors on the progenitor cells and their extracellular matrix ligands play key roles. Foremost among adhesion receptors are the integrins that mediate attachment to specific matrix proteins. Recent evidence linking signaling pathways from integrins and growth factor receptors suggests that cooperation between the pathways is essential for normal cell development and function.3 The ability of integrins to transfer information in both directions between the extracellular matrix and the intracellular compartment adds complexity, but allows for the possibility of growth factors providing an inside-out signal, which could relay to integrins resulting in changes in their ligand binding activity.4,5 This would enable growth factors, which themselves may be sequestered in discrete stromal environments by extracellular matrix molecules such as heparan sulfate, to provide a level of control over the localization and movement of hematopoietic progenitor cells that would not be provided by adhesion receptors alone.6 This cross-talk between growth factors and integrins may play a role in the dynamics of stem cell mobilization and homing.
Both α4β1 (very late activation antigen-4 [VLA-4]) and α5β1 (VLA-5) integrins are present on CD34 hematopoietic progenitor cells, but α4βb1 is expressed at a higher level and is retained for longer periods during subsequent myeloid maturation.7,8 The role of α4β1 and α5β1 integrins in adhesion of hematopoietic progenitor cells has been described in several studies. Both α4β1 and α5β1 can mediate adhesion of CD34 progenitors to fibronectin and α4β1 can also bind to vascular cell adhesion molecule-1 (VCAM-1), present on the surface of activated endothelial cells.7,9 Long-term culture initiating cells and colony-forming unit (CFU)-mix progenitors adhere to the fibronectin COOH-terminal heparin-binding domain10 and primitive murine CFU-spleen (CFU-S) day 12 colony-forming cells adhere to the α4β1 binding sequence in fibronectin.11 The importance of α4β1 is also highlighted in a study that showed that the addition of anti-α4β1 antibodies to long-term bone marrow cultures abrogated lymphopoiesis and retarded myelopoiesis.12 Much less is known about how integrins are involved in promoting migration of hematopoietic progenitor cells, although α4β1 may have a role, as it is known to mediate lymphocyte migration on fibronectin and VCAM-113 and it has also been shown that α4β1 antibodies selectively mobilize hematopoietic progenitors into the blood.14 The general importance of β1 integrins in hematopoiesis is indicated by the finding that chimeric mice generated with β1 integrin-deficient embryonal stem cells lack β1 −/− cells in the blood, spleen, thymus, and marrow, as the cells were unable to migrate to these sites.15 The role of α4 integrins in migration is shown by a study in which the cytoskeletal linkages with the α4 cytoplasmic domain, rather than the equivalent domains in α2 and α5 , mediated cell migration.16
The integrin α4β1 binds to two main sites in the alternatively spliced IIICS region of fibronectin, which are represented by the CS1 sequence, the strongest α4β1 binding site, and the CS5 sequence, which is a weaker binding site.17-19 A third weak α4β1 binding site is in the adjacent Hep II domain and is represented by the peptide sequence HI.20 Minimal active peptide sequences have been defined for each of the three sites: LDV for CS1, REDV for CS5, and IDAPS for H1. Here we have used two different recombinant proteins from the IIICS region as ligands for α4β1 ; H120, which contains all three binding sites and HO, which contains H1 alone, thus enabling assessment of the effect of strong and weak α4β1 binding sequences on attachment and migration of CD34 cells.
Although hematopoietic progenitor cells expressing CD34 are heterogeneous and contain cells already committed to specific lineages, they represent the earliest population that is readily isolated in sufficient numbers for adhesion and migration assays.21,22 We have isolated CD34 cells from human umbilical cord blood or peripheral blood stem cell harvests for use in a short-term adhesion assay in which the cells attach in the presence or absence of growth factors to culture surfaces coated with H120 and H0. We have also determined the effect of growth factors on the ability of CD34 cells to migrate across filters coated with these recombinant proteins. The growth factors chosen for this study, stem cell factor (SCF ), interleukin-3 (IL-3), and granulocyte- colony stimulating factor (G-CSF ) have all been used clinically for their ability to mobilize hematopoietic progenitor cells.23-25 On the basis of our results, we propose that there is a functional link between the IL-3 receptor and the α4β1 integrin.
MATERIALS AND METHODS
Cloning and expression of recombinant HepII/IIICS variants.cDNA clones for the five different variants of the HepII/IIICS region of human fibronectin were synthesized using reverse transcriptase-polymerase chain reaction (RT-PCR) amplification of primary human skin fibroblast mRNA as described by Mould et al.26 The PCR products were ligated into pUC119 and transformed into E coli JM109. Individual clones containing each of the variants were identified by restriction analysis and sequenced. Inserts were subcloned into the EcoRI site of the pGEX-2T expression vector and used to transform JM109. Recombinant clones were expressed as glutathione-S–transferase fusion proteins. These were isolated by glutathione affinity chromatography, thrombin digestion, and elution of the recombinant HepII/IIICS proteins from a heparin-agarose affinity column. The H120 (containing H1, CS1, and CS5 sequences) and HO (containing the H1 sequence only) variants were used for adhesion and migration experiments.
Isolation and analysis of CD34 cells.Umbilical cord blood collected into heparin was layered over Ficoll (Lymphoprep; Nycomed, Birmingham, UK) and the interface mononuclear cells were collected following density gradient centrifugation. CD34 cells were isolated using a magnetic separation column according to the manufacturer's instructions (mini-Macs; Miltenyi Biotec, Bergisch Gladbach, Germany), which resulted in average purity of 82% CD34+ cells. Aliquots from peripheral blood progenitor cell harvests mobilized with cyclophosphamide and G-CSF were treated in the same way, but did not require density gradient centrifugation. For maximum purity in the migration experiments, CD34 cells isolated by mini-Macs were sorted from a Facs Vantage cell sorter (Becton Dickinson, San Jose, CA) using a second fluorochrome-conjugated CD34 antibody, anti-HPCA-2 (Becton Dickinson) and control IgG (Becton Dickinson). Antibodies were added to the cells in phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA) and left for 10 minutes at room temperature. The cells were then washed twice with PBS/0.5% BSA before sorting. All experiments with cord blood were performed using fresh cells, but aliquots from a frozen batch of peripheral progenitor cells were thawed and used as necessary. The viability of these cells was normal when stained with trypan blue. For three-color fluorescence-activated cell sorting (FACS) analysis, CD34 cells isolated from the mini-Macs column were resuspended in PBS/0.5% BSA and fluorochrome-conjugated antibodies were added; anti-CD34 fluorescein isothiocyanate (FITC) (Becton Dickinson), anti-VLA–4 (CD49d) phycoerythrin (PE) (Becton Dickinson), and anti-CD38 tricolor (TC) (CALTAG, San Francisco, CA). Control cells were labeled with the corresponding fluorochrome-conjugated IgG. The cells were washed twice with PBS/0.5% BSA and analyzed using the PC lysis program (Becton Dickinson).
Cells from the murine hematopoietic progenitor cell line, FDCP-mix, which had not been subjected to an antibody enrichment procedure, were also used in the adhesion assay after washing three times in serum-free medium.
Cell adhesion assay.Assays were performed in 96-well microtiter plates (Costar, Cambridge, MA). Wells were coated for 60 minutes at room temperature with 100 μL aliquots of recombinant H120 or HO proteins at 15 μg/mL in Dulbecco's PBS, and nonspecific binding sites blocked for 30 minutes at room temperature with 100 μL of 10 mg/mL heat-denatured BSA.17 In one experiment, human fibronectin (50 μg/mL) was used as the ligand (Sigma, Poole, Dorset, UK).
CD34 cells recovered from the mini-Macs were resuspended to between 106 to 2 × 106/mL in serum-free medium (Bio-Whittaker, Wokingham, UK; X-vivo 10). The following growth factors were added to the cell suspension separately or in combination: rhIL-3 (Sandoz, Basel, Switzerland) at 10 ng/mL, rhIL-6 (Sandoz) at 200 U/mL, rhG-CSF (Amgen, Thousand Oaks, CA) at 500 ng/mL, and rh SCF (Amgen) at 100 ng/mL.
Conditioned medium (2%) containing IL-3 was added to the FDCP-mix cells. Growth factors were added at room temperature 30 minutes before starting the attachment assay. One hundred microliter aliquots of cells were added to the wells in duplicate and incubated for 30 minutes at 37°C in a 5% CO2 incubator. In one experiment, cells were incubated for 5 minutes. Nonadherent cells were removed by shaking the plate and washing twice with 100 μL PBS and bound cells were removed by aspiration with PBS. Both fractions were counted with a hemocytometer and adhesion expressed as a percentage of bound cells/(unbound + bound cells). For inhibition of cell adhesion to the recombinant proteins, 10 μg of blocking anti-VLA–α4 (HP2/1) and anti-β1 (3S3) antibodies (Serotec, Oxford, UK) together with a control IgG were added to the CD34 cells at 4°C and left for 15 minutes before the adhesion assay.
Cell migration assay.Migration experiments were performed using filters of 5 μm diameter pore size in 6.5-mm Transwell chambers (Costar). The membranes were coated on both sides by incubation with the recombinant fibronectin fragments (coating solution concentration 15 μg/mL) H120 or HO in Dulbecco's PBS for 60 minutes and nonspecific binding sites were blocked with BSA as described for the adhesion assay. FACS sorted CD34 cells were resuspended to 5 × 105 to 106/mL in serum-free medium and 100 μL aliquots were placed in the upper chamber of the Transwells. Growth factors used separately or in combination as described for the adhesion assay were placed in the upper and lower chambers. The cells were incubated at 37°C in 5% CO2 for either 4 hours or 16 hours. Cells on the upper surface of the membrane were fixed with methanol and removed using a cotton-bud. The membranes were then washed and stained with 0.1% crystal violet. Cells on the underside of the membrane were counted visually using a low power field and those that had dropped into the lower chamber were counted with a hemocytometer. Migration was expressed as the total numbers of these cells. Blocking anti-α4 and anti-β1 antibodies used to inhibit migration were added to the cells at 4°C and left for 15 minutes before the migration assay. Additional antibodies were also added to the cells in the upper chamber after 2 hours.
RESULTS
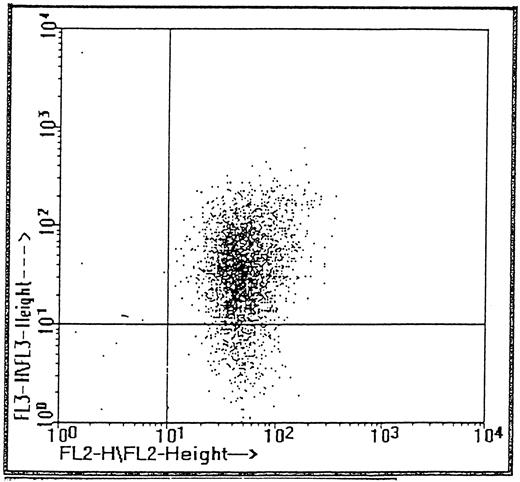
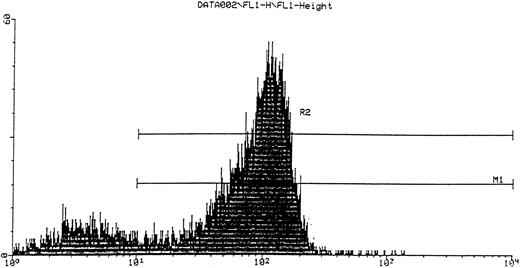
FACS analysis of CD34 cells.CD34 cells isolated from cord blood with the mini-Macs column were triple-labeled with fluorescent antibodies (see Materials and Methods) to CD34, VLA-4, and the CD38 antigen, which is a differentiation marker for more mature hematopoietic progenitor cells. Cells were gated by size on the lymphocyte population and by fluorescence on the CD34 cell population and the two additional fluorescence parameters (fl2 and fl3 ) were used to analyze subpopulations of α4 and CD38+ cells, respectively (Fig 1). Virtually all (99.8%) cells expressing CD34 also expressed α4 integrin including the CD38−, more immature phenotype. The proportion of CD34+CD38− cells shown in Fig 1 was 10.5%, a greater value than reported in some studies,21 22 but consistent with a mean of 9.7% ± 1.4%, which we obtained for three cord bloods. It can clearly be seen that all CD34 cells including those more obviously lacking the CD38 antigen are positive for α4 integrin. In CD34+ cells isolated from three peripheral blood stem cell harvests, a mean of 1.04% ± 0.35% of cells were negative for the CD38 antigen and the majority of this minor subpopulation (84%) also expressed α4 integrin.
Subpopulations of CD34 cells expressing α4 (PE, fl2) and CD38 (tricolor, fl3).
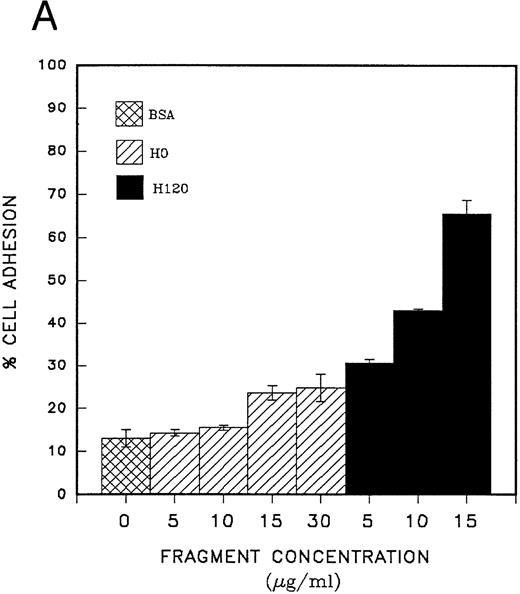
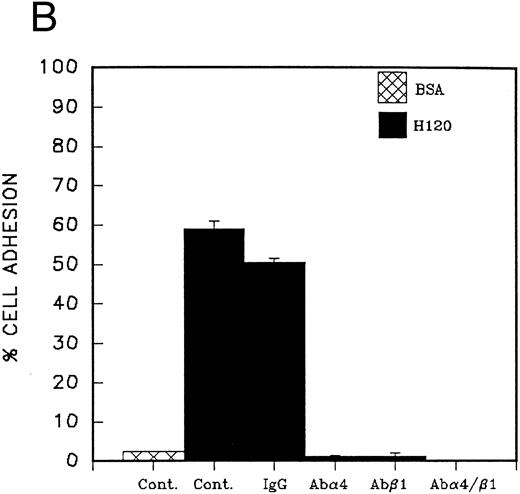
Adhesion of CD34 cells to α4β1 binding sequences of fibronectin; effect of growth factors.Cord blood CD34 cells suspended in serum-free medium attached efficiently to the H120 fragment used at concentrations from 5 to 15 μg/mL, and at the end of a 30-minute incubation at 37°C, a mean of 65% of cells were adherent at the highest concentration of H120 (Fig 2A). When whole fibronectin (50 μg/mL) was used as a ligand, the cells attached less efficiently (mean of 21% adherent cells) compared with the H120 fragment in the same experiment (mean of 70% adherent cells). Cells also attached less efficiently to the H0 fragment. Coating concentrations of 5 and 10 μg/mL of H0 were not significantly different from the BSA control (14% attached cells) and at 15 and 30 μg/mL, only 24% and 25% of cells were adherent (Fig 2A). The specificity of the interaction between α4β1 integrins and the H120 fragment was confirmed by almost complete inhibition of adhesion of CD34 cells that had been preincubated with blocking anti-α4 and anti-β1 antibodies, whereas adhesion in the presence of control antibodies was only marginally inhibited (Fig 2B).
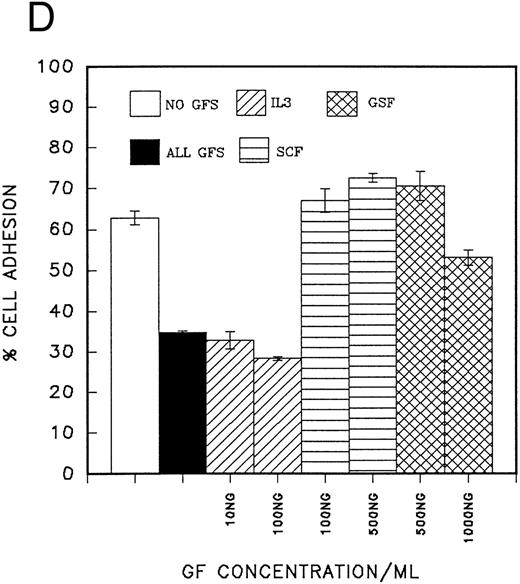
(A) Adhesion of CD34 cells isolated from mini-Macs to BSA and H0 and H120 fragments coated at different concentrations. All adhesion experiments were for 30 minutes at 37°C. All histograms in all figures for adhesion and migration represent the mean of duplicate wells ± SD. Student's t-test was used to calculate P values. (B) Adhesion of CD34 cells to H120 fragment coated at 15 μg/mL showing inhibition by blocking anti-α4 and anti-β1 antibodies, but not by IgG control. (C) Adhesion of CD34 cells to BSA and H0 and H120 fragments coated at 15 μg/mL with and without a mixture of growth factors; IL-3 (10 ng/mL), SCF (100 ng/mL), G-CSF (500 ng/mL), and IL-6 (200 U/mL). Reduction in adhesion to H120 induced by growth factors was significant (P < .05). (D) Adhesion of CD34 cells to H120 fragment coated at 15 μg/mL with IL-3 10 ng/mL, SCF 100 ng/mL, and G-CSF 500 ng/mL added together and separately at different concentrations. Changes in adhesion were significant for all growth factors (P < .005), IL-3 10 ng/mL (P < .01), IL-3 100 ng/mL (P < .005), and SCF (P < .05).
(A) Adhesion of CD34 cells isolated from mini-Macs to BSA and H0 and H120 fragments coated at different concentrations. All adhesion experiments were for 30 minutes at 37°C. All histograms in all figures for adhesion and migration represent the mean of duplicate wells ± SD. Student's t-test was used to calculate P values. (B) Adhesion of CD34 cells to H120 fragment coated at 15 μg/mL showing inhibition by blocking anti-α4 and anti-β1 antibodies, but not by IgG control. (C) Adhesion of CD34 cells to BSA and H0 and H120 fragments coated at 15 μg/mL with and without a mixture of growth factors; IL-3 (10 ng/mL), SCF (100 ng/mL), G-CSF (500 ng/mL), and IL-6 (200 U/mL). Reduction in adhesion to H120 induced by growth factors was significant (P < .05). (D) Adhesion of CD34 cells to H120 fragment coated at 15 μg/mL with IL-3 10 ng/mL, SCF 100 ng/mL, and G-CSF 500 ng/mL added together and separately at different concentrations. Changes in adhesion were significant for all growth factors (P < .005), IL-3 10 ng/mL (P < .01), IL-3 100 ng/mL (P < .005), and SCF (P < .05).
CD34 cells are, therefore, able to attach via the α4β1 to the H120 fragment in the absence of growth factors in serum-free medium in this short-term 30-minute assay. We then went on to determine the effects of growth factors on cell adhesion. In the initial experiments, CD34 cells from peripheral blood were used and the cells were incubated with the growth factor(s) for 30 minutes before starting the adhesion assay. Adhesion in the presence of the combination of IL-3 (10 ng/mL), SCF (100 ng/mL), G-CSF (500 ng/mL), and IL-6 (200 U/mL) was reduced by a mean of 26% on the H120 fragment and by 31% on the HO fragment. Cells showed no attachment to the BSA control (Fig 2C).
In a second series of experiments CD34 cells from peripheral blood were used and their adhesion to fibronectin fragments was measured in the presence of single growth factors or a growth factor combination. It was clear that IL-3 (10 ng/mL) was the only growth factor that significantly reduced cell adhesion (reduction = 48% P < .01), and this effect was not masked when SCF (100 ng/mL) or G-CSF (500 ng/mL) was added to the cells together with IL-3 (Fig 2D). At a high concentration (500 ng/mL), SCF increased the number of adherent cells by a mean of 16% (P < .05). No significant changes were observed with G-CSF (Fig 2D).
IL-3 alone was also found to strongly inhibit attachment to H120 of CD34 cells from cord blood (63% to 73% reduction, Table 1) and concentrations of IL-3 as low as 2.5 ng/mL produced a mean reduction in adhesion of 25% (not shown). Overall, in six separate experiments with CD34 cells from cord blood or peripheral blood, cell adhesion was consistently and significantly reduced by IL-3 (P < .001; Table 1). In one experiment with cord blood cells when the period of adhesion was reduced to 5 minutes, a mean of 25% of CD34 cells attached to H120 and the diminished response with IL-3 was already discernible, although statistically insignificant. In the same experiment, adhesion increased to a mean of 60% at 30 minutes without growth factors and was reduced by IL-3 to a mean of 42% (not shown).
Loss of Adhesion of CD34 Cells to H120 Due to IL-3
| . | % Adherent Cells . | % Adherent Cells With IL-3 . | % Reduction in . |
|---|---|---|---|
| . | in Serum-Free Medium . | . | Adhesion With IL-3 . |
| CB | 92.1 | 24.8 | 73 |
| CB | 68.4 | 22.7 | 66.8 |
| CB | 83.7 | 30.4 | 63.6 |
| PB | 62.8 | 32.8 | 47.7 |
| PB | 65.4 | 41.6 | 36.4 |
| PB | 71.6 | 43.7 | 38.9 |
| . | % Adherent Cells . | % Adherent Cells With IL-3 . | % Reduction in . |
|---|---|---|---|
| . | in Serum-Free Medium . | . | Adhesion With IL-3 . |
| CB | 92.1 | 24.8 | 73 |
| CB | 68.4 | 22.7 | 66.8 |
| CB | 83.7 | 30.4 | 63.6 |
| PB | 62.8 | 32.8 | 47.7 |
| PB | 65.4 | 41.6 | 36.4 |
| PB | 71.6 | 43.7 | 38.9 |
CD34 cells isolated from individual CB, and PB samples were preincubated in the presence or absence of IL-3 10 ng/mL for 30 minutes before attaching to the H120 fragment. IL-3 resulted in consistent loss of adhesion (P < .001).
Abbreviations: CB, cord blood; PB, peripheral blood.
Cells from the murine hematopoietic progenitor cell line FDCP-mix gave a similar value to CD34 cells for adhesion to H120 in serum-free medium after 30 minutes (mean of 67% attached cells) and showed a similar reduction in adhesion with IL-3 (mean of 17% attached cells).
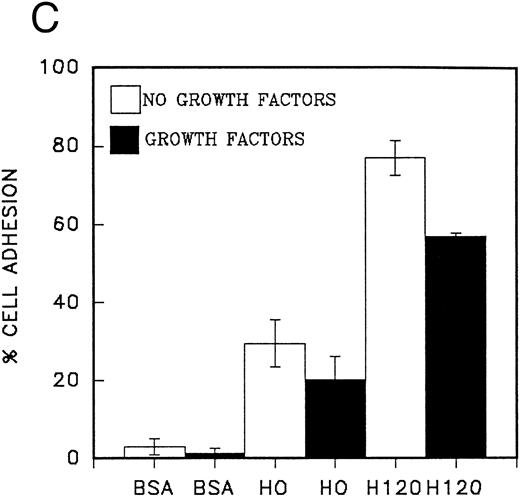
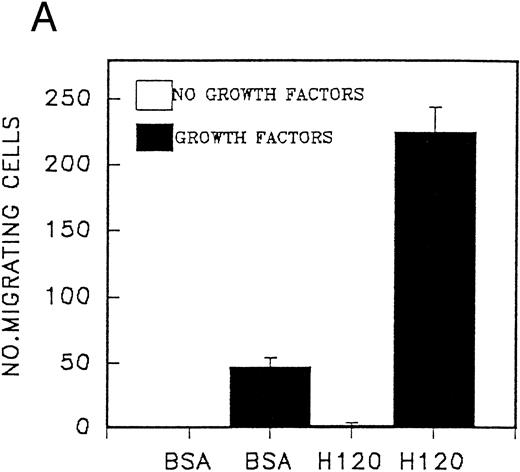
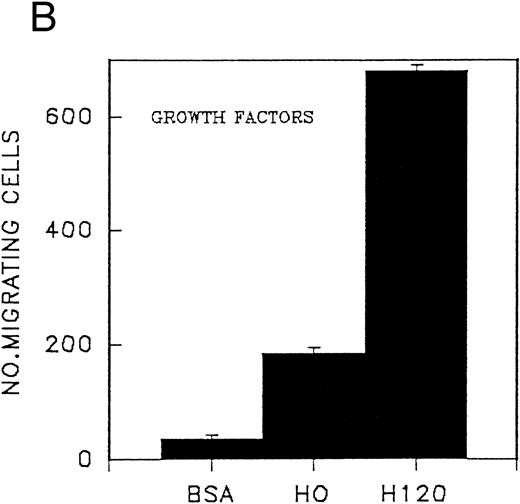
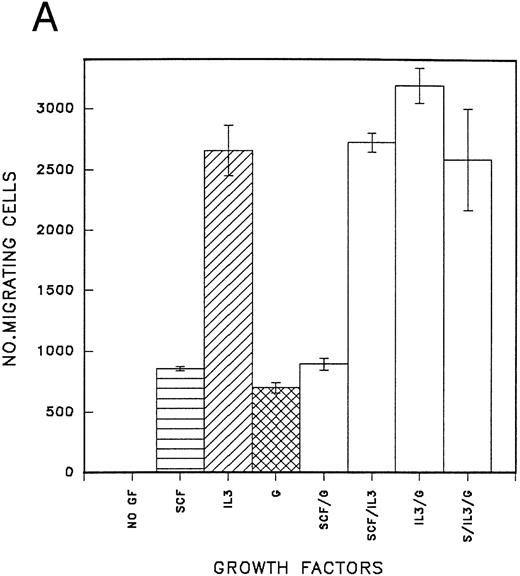
Migration of CD34 cells on α4β1 binding sequences; effect of growth factors.The different abilities of growth factors to modulate adhesion of CD34 cells to fibronectin fragments prompted us to examine their effect on cell migration through 5-μm Transwell filters coated with either the H120 or H0 fragments. Cells were FACS-sorted for the CD34 antigen following their initial isolation with the mini-Macs column so that all cells were CD34+ (Fig 3). In the first experiments, cells from peripheral blood were used and in the absence of growth factors, little or no cell migration was observed (Fig 4A). However, addition of a combination of growth factors resulted in a marked stimulation of migration through filters coated with the H120 fragment, which was approximately fivefold that occurring through filters coated with BSA (P = .01) (Fig 4A). In a separate experiment when growth factor-stimulated migration on BSA, H120, and H0 fragments was compared, again it was clear that H120 facilitated a growth factor induced migration to a much greater extent than either BSA (P < .001) and the H0 fragment, the latter supporting only 28% of the migration that occurred on H120 (P < .001) (Fig 4B). This is consistent with a predominant role for the stronger α4β1 binding sequences of H120 in promoting the migratory phenotype.
FACS sorting of CD34 cells (collected from R2 gate) for migration experiments.
(A) Migration of CD34 cells from a peripheral blood stem cell harvest over 16 hours at 37°C through filters coated with H120 15 μg/mL and BSA with and without a mixture of SCF 100 ng/mL, IL-3 10 ng/mL, G-CSF 500 ng/mL, and IL-6 200 U/mL. Migration was increased with H120 compared with BSA (P = .01) in the presence of growth factors. (B) Migration of CD34 cells over 16 hours through filters coated with BSA, H120, and H0 at 15 μg/mL with the mixture of growth factors used in (A). Migration was increased with H120 compared with BSA (P < .001) and H0 (P < .001).
(A) Migration of CD34 cells from a peripheral blood stem cell harvest over 16 hours at 37°C through filters coated with H120 15 μg/mL and BSA with and without a mixture of SCF 100 ng/mL, IL-3 10 ng/mL, G-CSF 500 ng/mL, and IL-6 200 U/mL. Migration was increased with H120 compared with BSA (P = .01) in the presence of growth factors. (B) Migration of CD34 cells over 16 hours through filters coated with BSA, H120, and H0 at 15 μg/mL with the mixture of growth factors used in (A). Migration was increased with H120 compared with BSA (P < .001) and H0 (P < .001).
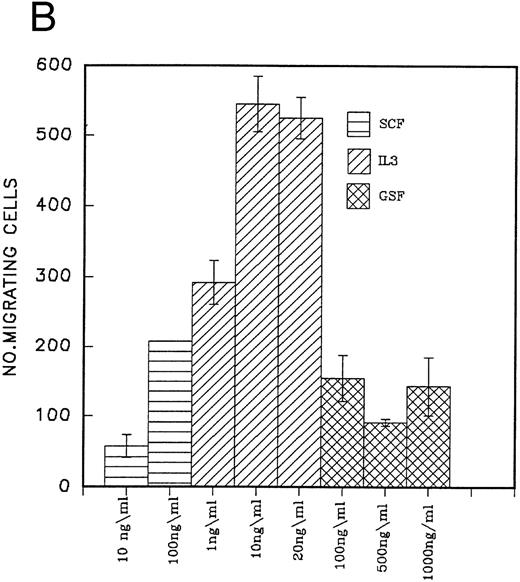
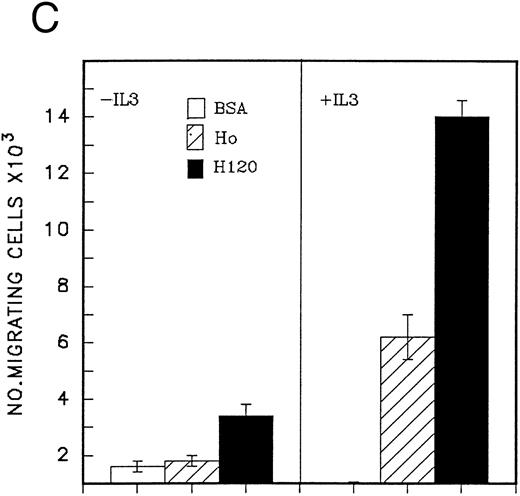
The effect of IL-3, G-CSF, and SCF used separately and in combination on the migration of peripheral blood CD34 cells on the H120 fragment is shown in Fig 5A. All growth factors stimulated cell migration, but IL-3 was clearly the most effective single growth factor, giving a threefold increase over SCF or G-CSF. Combinations of other growth factors with IL-3 did not provide an additional significant stimulus over that seen with IL-3 alone (Fig 5A). When different concentrations of growth factors were used, IL-3 was found to be active at 1 ng/mL, and its effects at this low concentration were greater than SCF at 100 ng/mL and G-CSF at 1,000 ng/mL (Fig 5B). Qualitatively similar effects to those shown in Fig 5A and B were seen in CD34 cells from fresh cord blood, although they showed a greatly increased migratory capacity on both H120 and H0 fragments (at least 10 times greater) compared with those from peripheral blood and included a minority of cells that could migrate on H120 in the absence of growth factors, which the peripheral blood cells were unable to do (Fig 5C).
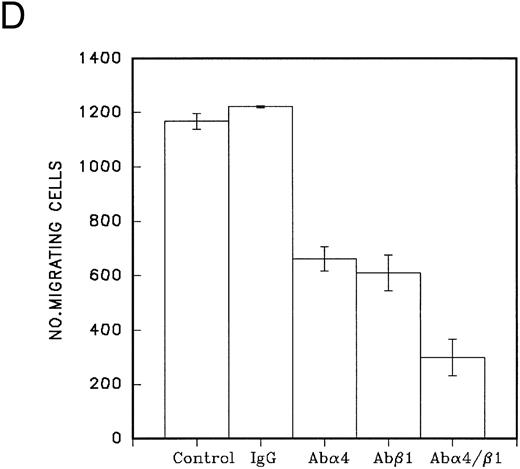
(A) Migration of CD34 cells over 16 hours at 37°C through filters coated with H120 at 15 μg/mL with IL-3 10 ng/mL, SCF 100 ng/mL, and G-CSF 500 ng/mL separately and in combination. IL-3 alone stimulated more migration than SCF (P = .01) and G-CSF (P = .01) alone. (B) Migration of CD34 cells over 16 hours at 37°C through filters coated with H120 at 15 μg/mL with different concentrations of SCF, IL-3, and G-CSF. A total of 1 ng/mL IL-3 stimulated more migration than 10 ng/mL SCF (P < .05). (C) Migration of CD34 cells from cord blood over 16 hours at 37°C through filters coated with BSA and H120 and H0 coated at 15 μg/mL with and without IL-3 10 ng/mL. Migration on H120 is increased with IL-3 (P < .005). (D) Migration of CD34 cells from cord blood over 4 hours at 37°C through filters coated with H120 at 15 μg/mL with IL-3 10 ng/mL. Effect of blocking anti-α4 and anti-β1 antibodies on IL-3 stimulated migration. Significant reduction occurred with anti-α4 (P < .01), anti-β1 (P = .01), and the combination (P = .001).
(A) Migration of CD34 cells over 16 hours at 37°C through filters coated with H120 at 15 μg/mL with IL-3 10 ng/mL, SCF 100 ng/mL, and G-CSF 500 ng/mL separately and in combination. IL-3 alone stimulated more migration than SCF (P = .01) and G-CSF (P = .01) alone. (B) Migration of CD34 cells over 16 hours at 37°C through filters coated with H120 at 15 μg/mL with different concentrations of SCF, IL-3, and G-CSF. A total of 1 ng/mL IL-3 stimulated more migration than 10 ng/mL SCF (P < .05). (C) Migration of CD34 cells from cord blood over 16 hours at 37°C through filters coated with BSA and H120 and H0 coated at 15 μg/mL with and without IL-3 10 ng/mL. Migration on H120 is increased with IL-3 (P < .005). (D) Migration of CD34 cells from cord blood over 4 hours at 37°C through filters coated with H120 at 15 μg/mL with IL-3 10 ng/mL. Effect of blocking anti-α4 and anti-β1 antibodies on IL-3 stimulated migration. Significant reduction occurred with anti-α4 (P < .01), anti-β1 (P = .01), and the combination (P = .001).
Blocking antibodies to α4 and β1 , added to cells at the start of a 4-hour migration assay, reduced the migration due to IL-3 by a mean of 74% (P = .001) when both antibodies were present (Fig 5D). After 16 hours, virtually no effect of the antibodies was apparent. To address this finding, the CD34 cell adhesion assay was used to test the inhibitory activity of fresh antibodies (blocking α4 and β1 as previously described) and the same antibodies left for 16 hours at 37°C. Adhesion to H120 was reduced by a mean of 88% with fresh antibodies, but only by a mean of 17% with the antibodies left for 16 hours. The loss of antibody activity during prolonged incubation would therefore account for the loss of inhibition of migration. The expression of VLA-4 on CD34 cells remained unchanged when analyzed by FACS using anti-VLA–4/PE immediately after enrichment (83% CD34+ VLA-4+, mean fluorescence of VLA-4 = 109) compared with cells left overnight at 37°C in serum-free medium (87% CD34+ VLA-4+, mean fluorescence of VLA-4 = 107).
The possibility that the effect of IL-3 is due to maintenance of cell viability rather than through a direct action on migration was excluded by confirming viability of all cells by trypan blue exclusion after 16 hours in serum-free medium without IL-3. Furthermore, after incubation of cells overnight in serum-free medium at 37°C followed by addition of IL-3 the next day, the cells were still able to migrate with the same efficiency (data not shown). Finally, there was no difference in cell numbers when cells were counted before and after incubation for 16 hours in serum-free medium containing IL-3, thereby excluding proliferation as the cause of the increased numbers of cells in the lower chamber in the presence of IL-3.
DISCUSSION
Cell adhesion and migration are essential processes in the control of growth and differentiation of hematopoietic stem and progenitor cells, and the increasing use of peripheral blood stem cells for transplantation emphasizes the potential practical benefits that may accrue from a deeper understanding of these fundamental cellular properties. The presence of integrins on the surface of hematopoietic cells and their significance in controlling cell attachment to the extracellular matrix has been highlighted in a number of publications,7-11 but to our knowledge, the present study is the first to examine the migration of CD34 hematopoietic progenitor cells in vitro on a single extracellular matrix ligand in response to growth factors. In one recent study CD34 cells were shown to migrate between endothelial cells grown on top of Transwell inserts, but the role of individual growth factors was not addressed.27 In a separate study, SCF and other growth factors were shown to have chemotactic and chemokinetic activity for murine hematopoietic progenitor cells, but this study did not incorporate a ligand such as fibronectin on which integrin-mediated adhesion and migration could occur.28
We have focused on the role of the strongly expressed α4β1 integrin on CD34 cells (Fig 1) using as substrate recombinant fibronectin fragments (H120 and H0) that bind only to this integrin and not to the α5β1 integrin, which is also expressed by these cells and binds to the RGD sequence in the central cell-binding domain of fibronectin. Fibronectin is a component of the marrow extracellular matrix,29 and we have found (unpublished data) that the alternatively spliced high-affinity CS1 sequence (present in H120) is expressed by human marrow stromal cells. Results shown in Fig 2 clearly demonstrate that CD34 cells from peripheral blood adhere to both H120 and H0 fragments and the use of specific antibodies indicated that attachment was mediated by the α4β1 integrin (Fig 2B). The H120 fragment, which contains the high-affinity LDV sequence, was the superior adhesive substrate enabling 65% of cells to attach in serum-free medium by comparison with only 24% attachment on H0 (Fig 2A). The combination of IL-3 with SCF and G-CSF caused a significant reduction in adhesion to H120 and the addition of single growth factors indicated that the inhibition was due to the action of IL-3 (Fig 2D). The effect was quite rapid, as the cells were preincubated at room temperature for only 30 minutes with IL-3 before the 30-minute adhesion assay.
Previous studies on adhesion of hematopoietic progenitor cells derived from bone marrow rather than peripheral blood or cord blood have suggested that cell surface integrins are activated by cytokines.30-32 In two of these studies,30 31 IL-3 was one of the cytokines that increased adhesion of CD34 cells to whole fibronectin. However, the low levels of initial adhesion in the absence of growth factors are difficult to compare with our results using H120 and our finding that whole fibronectin also gave low cell attachment values implies that adhesion to H120 is inherently stronger. Similar results obtained with FDCP-mix cells on H120 argue against the possibility of integrin activation by the CD34 cell-enrichment procedure, as does the reduced attachment of CD34 cells shown at 5 minutes. In addition, the cells in these studies were held at 4°C overnight in serum-free medium to ensure that they were completely free of any growth factors, whereas the procedures involved in cell preparation used in our work took about 4 hours and were performed at room temperature; cells were extensively washed during this time and were unlikely to have any significant contamination by growth factors. We have not yet looked at CD34 cells isolated directly from bone marrow, but we think it is unlikely that they would show qualitatively different responses.
We considered that because IL-3 affected cell adhesion in a distinctive manner from other growth factors, it may be influencing cell migration. A Transwell filter assay was established in which cells could migrate through 5-μm pore filters coated on both sides with 15 μg/mL of fibronectin fragments. Results indicated that the H120 fragment could not support migration of peripheral blood CD34 cells in the absence of growth factors (Fig 4A); cord blood CD34 cells were also nonmigratory on H0, but a small level of migration was observed on H120 (Fig 5C). Addition of a combination of growth factors provided a strong stimulus for both peripheral blood and cord blood CD34 cells to migrate on H120 and to a lesser degree on H0 (Figs 4B and 5C). When single growth factors were tested, IL-3 was clearly the most effective at inducing migration, giving an increase of approximately threefold over SCF or G-CSF (Fig 5A and B). IL-3 was effective at a concentration of 1 ng/mL with maximum activity occurring at 10 ng/mL (Fig 5B). Cells from cord blood were more responsive in the migration assay than those from peripheral blood, but in both cell populations, IL-3 was significantly more active than SCF and G-CSF. Migration in a short-term assay (4 hours) was strongly inhibited by combined use of antibodies to the α4 and β1 subunits indicating the importance of α4β1 integrin for migratory activity (Fig 5D).
The mechanism of IL-3–induced modulation of integrin function is unclear, but it would appear to be an example of inside-out signaling in which the IL-3 receptor elicits an intracellular signal (or signals) that modifies α4β1 integrin binding to its H120 (or H0) substrate. This signal is distinct in some way from signals resulting from activation of the SCF or G-CSF receptors. The IL-3 signal affects both cell adhesion, which is reduced, and cell migration, which is enhanced. We do not know if the activation state of α4β1 integrin is affected during the course of the adhesion and migration assays, although surface expression is unchanged over a period of 16 hours.
Reduction in adhesion may be a necessary first step in weakening cell attachment to the substratum leading to increased motility and migration. However, the relationship between adhesion and migration is complex. Adhesion allows cells to generate the traction essential for movement, but adhesion complexes must rapidly dissociate and reform if motility is to be maintained. Several recent reports have shown a link between integrin-mediated migration and growth factor pathways, eg, carcinoma cells expressing αvβ5 require prior cellular activation with growth factors for migration on vitronectin33 and CS1-melanoma cells expressing αvβ5 or a recombinant αvβ5 /β3 only migrate when exposed to insulin-like growth factor or insulin.4 A candidate signaling molecule for mediating the IL-3 effects in CD34 cells is the rac guanosine triphosphate (GTP)-binding protein, which induces lamellipodia and may play a role in migration of fibroblasts and can be activated by growth factors, eg, platelet-derived growth factor (PDGF ) or epidermal growth factor (EGF ).34
The capacity of IL-3 to reduce adhesion and promote migration of CD34 cells indicates a possible role for this cytokine in promoting movement of hematopoietic progenitor cells in vivo. However, despite the many effects of IL-3 on hematopoiesis, including stimulation of the survival and proliferation of multipotent stem and progenitor cells, its normal physiologic role is unclear. Unlike other hematopoietic cell growth factors, such as SCF, GM-CSF, G-CSF, M-CSF, and IL-6, which are all produced by marrow stromal cells, IL-3 is produced by T lymphocytes.35-37 Although some T cells are found in the bone marrow, IL-3 is difficult to detect except by RT-PCR techniques.36 IL-3 has been shown in a mouse model to cause migration of both pluripotent and committed progenitors from bone marrow to spleen,38 and it has chemotactic and chemokinetic activity for murine hematopoietic progenitor cells, which is similar to SCF.28 The mobilization of peripheral blood progenitor cells by G-CSF is enhanced by IL-3,23 as is the in vitro chemotaxis of CD34 cells in response to the chemokine SDF-1.39 Thus IL-3, either directly or indirectly, can clearly influence the local positioning of cells in the marrow stroma and also facilitate their migration. It is interesting to note, however, that although G-CSF is a weak activator of migration in our in vitro assay, it is much more potent than IL-3 in mobilizing hematopoietic progenitor cells from the marrow to the blood.24 This may indicate that mobilization of peripheral blood stem cells is a reflection not only of migration, but also of changes in adhesive properties of marrow stromal endothelial cells, which in turn may promote access of IL-3 bearing T lymphocytes.13
In summary, our findings show that by modulating the function of α4β1 integrin, IL-3 is a potent regulator of the adhesion and migration of CD34 cells. Its effects can be clearly distinguished from those of SCF and G-CSF. The mechanisms that support the effects of IL-3 are unknown, but it seems probable that there is a distinctive signal from the IL-3 receptor to the α4β1 integrin. The nature of this signal and the manner in which it regulates integrin function are now being investigated.
ACKNOWLEDGMENT
We thank Nydia Testa and Erica de Wynter for helpful technical advice and discussion of data. We also acknowledge the excellent assistance of Suzanne Bridge in the preparation of this manuscript.
K.P.S. is supported by a grant from the Cancer Research Campaign.
Address reprint requests to Karen P. Schofield, MD, CRC Department of Medical Oncology and Experimental Haematology, Paterson Institute for Cancer Research, Wilmslow Rd, Manchester, M20 4BX, UK.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal