Abstract
The mechanisms that regulate circulating levels of thrombopoietin (Tpo) are incompletely understood. According to one favored model, the rate of Tpo synthesis is constant, whereas the serum concentration of free Tpo is modulated through binding to c-Mpl receptor expressed on blood platelets. Additionally, a role for c-Mpl expressed on megakaryocytes is suggested, particularly by the observation that serum Tpo levels are not elevated in human immune thrombocytopenic purpura. Whereas direct binding of Tpo to platelets has been demonstrated in vitro and in vivo, the role of megakaryocytes in modulating serum Tpo levels has not been addressed experimentally. The profoundly thrombocytopenic mice lacking transcription factor p45 NF-E2 do not show the predicted increase in serum Tpo concentration. To evaluate the fate of the ligand in these animals, we injected 125I-Tpo intravenously into mutant and control mice. In contrast to normal littermates, NF-E2 knockout mice show negligible association of radioactivity with blood cellular components, consistent with an absence of platelets. There is no corresponding increase in plasma-associated radioactivity to suggest persistence in the circulation. However, a greater fraction of the radioligand is bound to hematopoietic tissues. In the bone marrow this is detected virtually exclusively in association with megakaryocytes, whereas in the spleen it is associated with megakaryocytes and small, abnormal, platelet-like particles or megakaryocyte fragments that are found within or in close contact with macrophages. These findings implicate the combination of megakaryocytes and the latter particles as a sink for circulating Tpo in NF-E2 knockout mice, and provide an explanation for the lack of elevated serum Tpo levels in this unique animal model of thrombocytopenia.
AN INVERSE CORRELATION between the platelet count and circulating thrombopoietin (Tpo) levels is apparent in many experimental models,1-6 and has led to the hypothesis that the serum concentration of Tpo is controlled by a feedback mechanism dependent on platelet mass. One model to explain these findings is that Tpo synthesis by producer cells is constant, whereas the circulating, free fraction is titrated by c-Mpl (receptor)-mediated binding to cell surfaces7; thus, the concentration of free Tpo may simply represent the result of mass action between the ligand and its receptor. Several considerations support such a model. First, Tpo mRNA levels do not change appreciably in response to thrombocytopenia or thrombocytosis.8-11 Second, the physiologic regulation of macrophage colony-stimulating factor levels provides a precedent for this mechanism among hematopoietic cells and cytokines.12 Third, it avoids the need for a mechanism for accurate and constant monitoring of the platelet mass. And finally, in thrombocytopenic humans, serum Tpo levels correlate with the combined platelet and megakaryocyte mass rather than with platelet counts per se.13 Nevertheless, direct experimental evidence for the basis of Tpo regulation is lacking, and a combination of mechanisms may operate in vivo.
Mice lacking the transcription factor p45 NF-E2 exhibit megakaryocytosis and profound thrombocytopenia; these findings reflect an arrest in megakaryocyte cytoplasmic maturation, characterized by decreased granule biosynthesis and a failure to demarcate platelet fields.14 Paradoxically, serum levels of Tpo in these animals are not elevated. We have also shown that mRNA levels of both Tpo and c-Mpl in the fetal liver are the same as in littermate controls.14 These findings suggest that regulation of Tpo levels is more complex than previously appreciated, and possibly point to megakaryocytes as a component of the regulatory mechanism. The independent observation of normal Tpo levels in humans with thrombocytopenia associated with a normal or increased number of megakaryocytes, including patients with immune thrombocytopenic purpura,13 15 is consistent with this hypothesis.
Here, we show that binding of radiolabeled Tpo by the cellular fraction in the blood is markedly and predictably decreased in thrombocytopenic NF-E2-null mice relative to controls. Thus, sequestration of Tpo by a circulating cellular element is unlikely in these animals. However, binding of the radioligand is increased in hematopoietic tissues, where it is detected in association with the large, abnormal, and abundant megakaryocytes, as well as with small, platelet-like particles found within or close to macrophages. These findings provide an explanation for the low Tpo levels in NF-E2 knockout mice, and are consistent with a model in which constitutively synthesized Tpo is sequestered through binding to c-Mpl on the surface of platelets and megakaryocytes.
MATERIALS AND METHODS
Knockout mice and histologic analysis.Mice lacking p45 NF-E2 or c-Mpl were generated and maintained as previously described.6 14 Control (wild-type) mice were littermates in each case. Spleen and bone marrow were harvested from adult mice, preserved in neutral buffered 10% Formalin, processed for routine histologic analysis with 10-μm sections, and stained with hematoxylin-eosin.
Measurement of serum Tpo levels.This was performed as described previously, using Tpo-dependent modified BaF/3 cells.16
Detection of c-Mpl protein in platelet-rich plasma.Whole mouse blood collected in EDTA was first centrifuged at 300g to pellet red blood cells and leukocytes. The supernatant was then centrifuged at 1,000g, and the resulting platelet pellet was washed twice in EHS (150 mmol/L NaCl, 1 mmol/L EDTA, and 10 mmol/L HEPES, pH 7.6). The pellet was then lysed in sodium dodecyl sulfate (SDS) sample buffer, and its protein components were resolved by SDS–polyacrylamide gel electrophoresis followed by blotting onto nitrocellulose. Western analysis was performed by standard techniques using a hamster anti-mouse c-Mpl monoclonal antibody and horseradish peroxidase–conjugated goat anti-hamster IgG (Jackson ImmunoResearch Labs, West Grove, PA). The reaction was detected using an ECL kit (Amersham Inc, Arlington Heights, IL) according to the manufacturer's recommended protocol, followed by autoradiographic exposure for 15 seconds.
In vivo distribution of 125I-Tpo.Recombinant murine (rm)Tpo was iodinated by the indirect iodogen method,17 purified by size-exclusion chromatography, and formulated in 10 mmol/L Tris, 0.15 mmol/L NaCl, and 0.01% Tween-20, pH 7.4. Because of the poor survival rate of NF-E2-null mice, we were unable to obtain enough adult mice at one time; therefore, two separate groups of mice were used for independent experiments performed at different times using different 125I-rmTpo preparations (each experiment with two wild-type and two NF-E2-null adult mice). Mice were injected intravenously with 0.1 mL of the radioligand formulation (∼3.21 μCi) via a lateral tail vein and killed 3 hours later, when the following tissues were collected: experiment 1, blood (in 0.38% citrate), spleen, and sternum; and experiment 2, blood (in 0.38% citrate), spleen, sternum, lung, femur, liver, and kidney. Tissues from all animals were sectioned, rinsed in phosphate-buffered saline, blotted dry, weighed, and stored on ice until aliquots were counted for 1 minute in a Wallac (Turku, Finland) 1470 gamma counter. Values were normalized for tissue weight (cpm per gram) and expressed as the percent of injected dose per gram tissue.10 Sternums and spleens were cut into 2-mm pieces, fixed in Karnovsky's fixative, and processed for electron microscopic autoradiography.18
RESULTS
Serum Tpo levels.Tpo-dependent cultured cell lines provide a reproducible bioassay for detection and relative quantitation of Tpo in murine serum.16,19 In our assay using c-Mpl–transfected BaF/3 cells,16 the Tpo concentration in normal mouse serum is not detectable, whereas elevated levels associated with thrombocytopenia provide a readily measurable signal. Although the minimum amount of murine Tpo detectable in this assay (ie, its sensitivity) is unknown, the effective range for human Tpo in the assay is 7 to 114 pg/mL. As previously reported,6 Tpo levels in thrombocytopenic c-Mpl–deficient mice are consistently elevated over control levels (Fig 1), and the assay can detect a 300-fold dilution of serum from these mice. In contrast, adult mice homozygous for a disrupted p45 NF-E2 allele14 do not have high circulating Tpo levels, despite apparently more severe thrombocytopenia. Because Tpo levels in surviving adult NF-E2-null mice may reflect a steady state established through unknown physiologic mechanisms, we also examined sera from newborn mice with clinical signs of hemorrhage and noted no increase in the Tpo concentration (Fig 1). These data provide independent confirmation of our previous report that free Tpo is undetectable in the serum of NF-E2 knockout mice by this bioassay.14
Relative serum concentrations of Tpo from wild-type, NF-E2-null, and c-Mpl knockout (−/−) mice at various ages as determined in the bioassay using Tpo-dependent BaF/3 cells. Results are expressed as incorporated 3H-thymidine counts per well after 2 days of culture in medium supplemented with the mouse serum. rTPO, recombinant Tpo (saturating concentration, 10 ng/mL) used as an internal standard.
Relative serum concentrations of Tpo from wild-type, NF-E2-null, and c-Mpl knockout (−/−) mice at various ages as determined in the bioassay using Tpo-dependent BaF/3 cells. Results are expressed as incorporated 3H-thymidine counts per well after 2 days of culture in medium supplemented with the mouse serum. rTPO, recombinant Tpo (saturating concentration, 10 ng/mL) used as an internal standard.
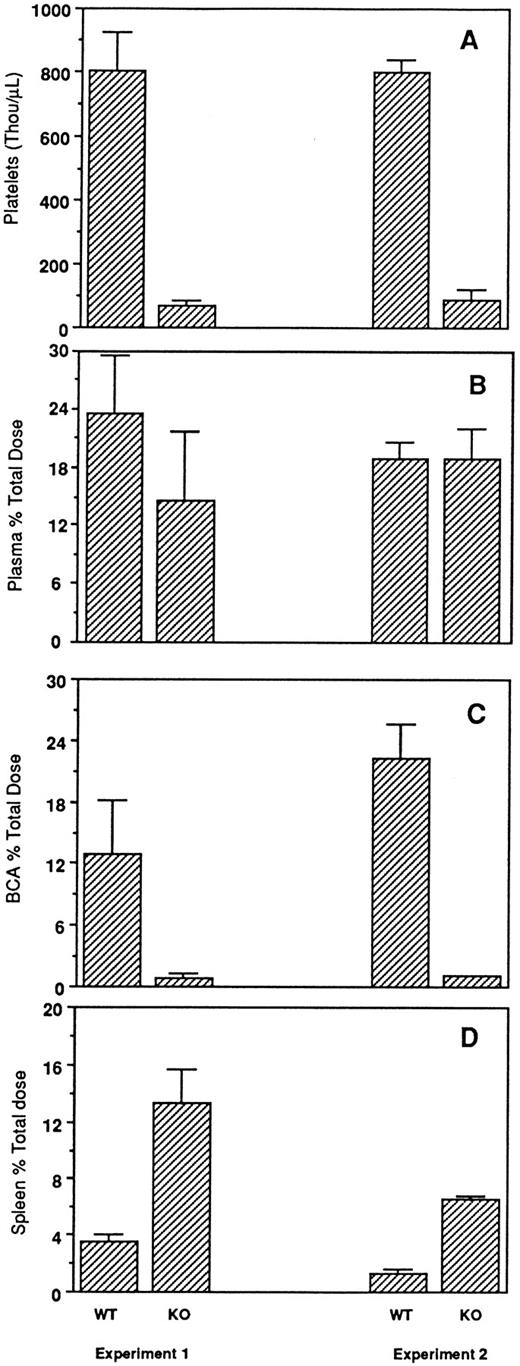
Fate of radiolabeled Tpo in vivo.Intravenous injection of radioiodinated Tpo in normal mice results in rapid binding to circulating platelets, followed by clearance of the radiolabel and degradation of the polypeptide; these events occur either more slowly or not at all in mice lacking c-Mpl.10 Thus, c-Mpl protein expressed on the surface of circulating platelets appears to be an important regulator of serum Tpo levels. Figure 2 and Table 1 show the results of simultaneous administration of 125I-Tpo to NF-E2-null and wild-type mice. In contrast to the normal number of platelets in wild-type mice, dramatic thrombocytopenia is evident in mice lacking NF-E2 (Fig 2A) and is confirmed by the absence of recognizable platelets on blood smears14 (and data not shown). This paucity of circulating platelets correlates with the almost complete lack of radioactivity associated with the cellular fraction of whole blood (Fig 2C) or with lung tissue (Table 1) compared with control mice. Thus, in the absence of a platelet source of c-Mpl, a negligible fraction of the radioligand (background counts only) associates with cellular blood components. The blood cell fraction assayed in these experiments includes circulating platelets in normal mice, as evidenced by Tpo binding in control animals (Fig 2C) and as previously described.10 In contrast, spleens from NF-E2−/− mice contain significantly more radioactivity than those from control mice (Fig 2D), which suggests that they remove much more 125I-Tpo from the circulation than the latter.
In vivo distribution of 125I-rTpo within the blood and spleen of NF-E2 knockout (KO) and wild-type (WT) mice. (A) Platelet levels (thousands per microliter) measured before injection. Mice were injected intravenously with 125I-rTpo and killed 3 hours later, and radioactivity associated with the plasma fraction (B), blood cells (C), and spleen (D) was measured. Data are presented as the mean ± SD from two separate experiments. BCA, blood cell–associated.
In vivo distribution of 125I-rTpo within the blood and spleen of NF-E2 knockout (KO) and wild-type (WT) mice. (A) Platelet levels (thousands per microliter) measured before injection. Mice were injected intravenously with 125I-rTpo and killed 3 hours later, and radioactivity associated with the plasma fraction (B), blood cells (C), and spleen (D) was measured. Data are presented as the mean ± SD from two separate experiments. BCA, blood cell–associated.
Comparison of Tissue-Associated Radioactivity in Wild-Type and NF-E2-Null Mice
| Tissue . | Wild-Type . | NF-E2-Null . |
|---|---|---|
| Blood | 40.0 ± 0.64 | 19.9 ± 2.97 |
| Spleen | 1.31 ± 0.33 | 6.64 ± 0.21 |
| Femur | 0.38 ± 0.09 | 0.58 ± 0.07 |
| Sternum | 0.33 ± 0.22 | 0.34 ± 0.03 |
| Lung | 4.24 ± 0.85 | 0.94 ± 0.04 |
| Kidney | 2.31 ± 0.19 | 1.23 ± 0.31 |
| Liver | 3.69 ± 0.47 | 3.66 ± 0.53 |
| Tissue . | Wild-Type . | NF-E2-Null . |
|---|---|---|
| Blood | 40.0 ± 0.64 | 19.9 ± 2.97 |
| Spleen | 1.31 ± 0.33 | 6.64 ± 0.21 |
| Femur | 0.38 ± 0.09 | 0.58 ± 0.07 |
| Sternum | 0.33 ± 0.22 | 0.34 ± 0.03 |
| Lung | 4.24 ± 0.85 | 0.94 ± 0.04 |
| Kidney | 2.31 ± 0.19 | 1.23 ± 0.31 |
| Liver | 3.69 ± 0.47 | 3.66 ± 0.53 |
Data (mean ± SD) are presented as a % of the total dose per organ; n = 2.
One potential reason for the normal level of free Tpo in NF-E2 knockout mice is that the ligand is bound to soluble receptors or circulating megakaryocyte fragments that do not precipitate with the blood cell fraction in the experiment. However, plasma-associated radioactivity in the mutant animals is not increased (Fig 2B). When we subject the pellet resulting from high-speed centrifugation of platelet-rich plasma to immunoblot analysis to assess c-Mpl protein levels, control samples generate a strong signal, whereas equal amounts of the identical fraction from NF-E2−/− plasma yield a substantially weaker one (Fig 3). The absence of recognizable platelet forms in the peripheral blood of mutant animals suggests that the source of this small amount of intact c-Mpl protein within an insoluble plasma fraction may be circulating megakaryocyte fragments. Moreover, immunoreactive c-Mpl is undetectable in the soluble plasma fraction of control or NF-E2−/− adult mice (data not shown). Taken together, these results make it unlikely that free Tpo in NF-E2 knockout mice is cleared largely through mechanisms operating within the circulation.
Immunoblot analysis of the insoluble fraction corresponding to platelet-rich plasma from adult wild-type (WT) and NF-E2 knockout (KO) mice using anti–c-Mpl monoclonal antibody. The only reactive species noted on the blot migrates as predicted for native c-Mpl protein.
Immunoblot analysis of the insoluble fraction corresponding to platelet-rich plasma from adult wild-type (WT) and NF-E2 knockout (KO) mice using anti–c-Mpl monoclonal antibody. The only reactive species noted on the blot migrates as predicted for native c-Mpl protein.
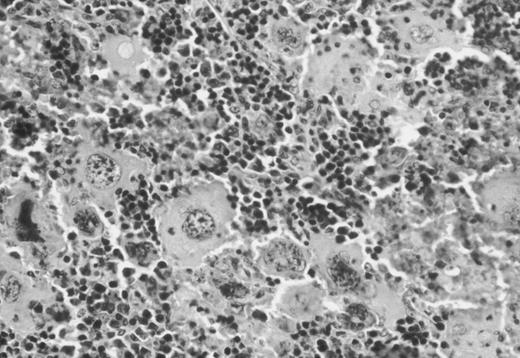
Binding of 125I-Tpo to NF-E2−/− megakaryocytes and megakaryocyte fragments in vivo.Although blood platelets, which carry approximately 200 c-Mpl molecules per cell in mice,10 are likely to be the major source of this receptor, it is also expressed on megakaryocytes. Loss of NF-E2 in vivo is accompanied by megakaryocytosis,14 marrow hypercellularity, and splenomegaly.20 Indeed, prior studies may have underestimated the degree of megakaryocyte hyperplasia in the steady state; extensive review of spleen and bone marrow histologic sections reveals considerable expansion of the megakaryocyte population in adult mice (Fig 4). Thus, a substantial amount of c-Mpl may be available on NF-E2−/− megakaryocytes to sequester free Tpo from the circulation.
Hematoxylin-eosin–stained representative histologic section of the spleen of a 12-week-old NF-E2-null male mouse showing the dramatic increase in the number of megakaryocytes.
Hematoxylin-eosin–stained representative histologic section of the spleen of a 12-week-old NF-E2-null male mouse showing the dramatic increase in the number of megakaryocytes.
Figure 2C and Table 1 show that a greater fraction of 125I-Tpo localizes to the spleen and femur of mutant mice compared with littermate controls. Autoradiography of electron micrographs of sternum samples further shows that 125I-Tpo associates virtually exclusively with the characteristically large, abnormal megakaryocytes and not with other marrow cellular elements; samples from control mice also show radioligand bound only to megakaryocytes (Fig 5A and C). This demonstration of direct binding of 125I-Tpo to megakaryocytes in vivo is consistent with the notion that these cells may participate in the clearance of free Tpo from the circulation in normal and NF-E2 knockout mice. Although megakaryocyte maturation is clearly abnormal in the sternum of NF-E2-null mice, we observe the rare presence of platelet-like structures that are also capable of binding the radioligand (Fig 5B).
Cellular localization of 125I-rTpo within the sternum of NF-E2-null and control mice analyzed by electron microscopic autoradiography. Autoradiographic silver grains associated with a large abnormal megakaryocyte from a mouse lacking p45 NF-E2 (A) or a megakaryocyte from a control mouse (C) are circled. (B) Release of an anucleate platelet-like structure, capable of binding 125I-rTpo (circle around the autoradiographic silver grain), into the vascular space of an NF-E2-null mouse. Bar, 2 μm.
Cellular localization of 125I-rTpo within the sternum of NF-E2-null and control mice analyzed by electron microscopic autoradiography. Autoradiographic silver grains associated with a large abnormal megakaryocyte from a mouse lacking p45 NF-E2 (A) or a megakaryocyte from a control mouse (C) are circled. (B) Release of an anucleate platelet-like structure, capable of binding 125I-rTpo (circle around the autoradiographic silver grain), into the vascular space of an NF-E2-null mouse. Bar, 2 μm.
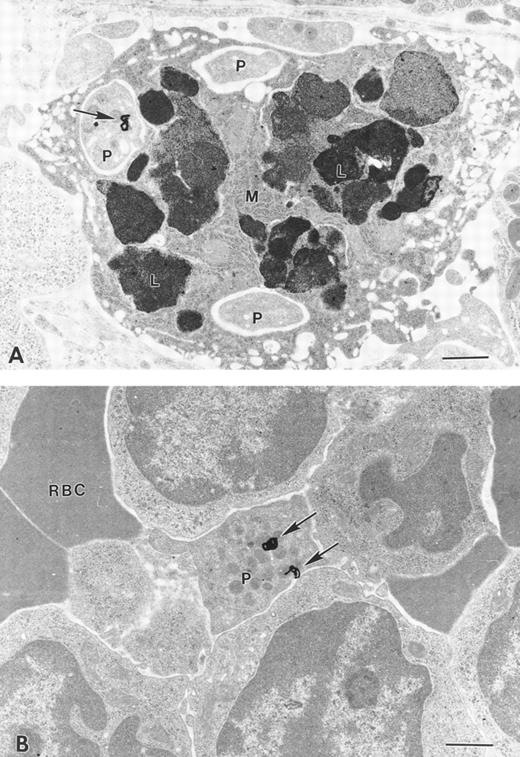
Electron microscopic autoradiography of spleens from normal mice indicates that the majority of 125I-Tpo is bound to platelets (Fig 6B). Surprisingly, we also observe binding of the radioligand to previously undetected platelet-like particles in the spleen of NF-E2−/− adult mice; most of these are small, found within or in close contact with macrophages, and contain few or no granules (Fig 6A). However, as in the bone marrow, numerous silver grains are also seen in association with megakaryocytes within NF-E2-null and control spleens (data not shown). Taken together, these data suggest that NF-E2−/− megakaryocytes release abnormal fragments or platelet-like particles that do not circulate in appreciable numbers but are cleared rapidly by splenic macrophages. The presence of these particles is consistent with the previously noted abnormal megakaryocyte maturation and the sublethal bleeding in rare NF-E2−/− mice that survive beyond the neonatal period. Binding of Tpo to these particles and to megakaryocytes provides an explanation for the absence of increased levels of free Tpo in this animal model of thrombocytopenia.
Cellular localization of 125I-rTpo within the spleen of NF-E2-null and control mice analyzed by electron microscopic autoradiography. Silver grains (arrows) associated with (A) megakaryocyte fragments/platelet-like structures (P) found within or in close contact with macrophages (M) in NF-E2−/− mice and (B) with a mature platelet (P) from a control mouse. Bar, 2 μm.
Cellular localization of 125I-rTpo within the spleen of NF-E2-null and control mice analyzed by electron microscopic autoradiography. Silver grains (arrows) associated with (A) megakaryocyte fragments/platelet-like structures (P) found within or in close contact with macrophages (M) in NF-E2−/− mice and (B) with a mature platelet (P) from a control mouse. Bar, 2 μm.
DISCUSSION
Whereas the relative roles of various cytokines in megakaryocytopoiesis continue to be investigated, Tpo is clearly the major physiologic regulator of platelet production in vivo.21,22 Therefore, it is important to understand how the serum concentration of Tpo is controlled. Experimental manipulation of the platelet mass in animals has provided invaluable insights; immune or chemically induced thrombocytopenia results in a detectable increase in serum Tpo activity, which can be abrogated by transfusion of platelets in vivo3,10,23 or absorbed by incubation with platelets in vitro.9,23 This suggests that platelets are the primary regulators of the concentration of free Tpo. A likely similar role for megakaryocytes has been inferred,11,13,15 but has not received direct experimental support. Megakaryocytes have access to the circulation by virtue of a parasinusoidal location in hematopoietic tissues and their intravascular cytoplasmic processes.24,25 In addition, each megakaryocyte presents a large surface area, which is comprised not only of the plasma membrane but also of that fraction of the demarcation membrane system known to be in communication with the cell exterior.26 These considerations add plausibility to a role for megakaryocyte-associated c-Mpl in sequestering Tpo from the circulation.
To explore the reasons for apparently normal (ie, undetectable in murine bioassays) serum levels of Tpo in the profoundly thrombocytopenic mice lacking transcription factor NF-E2, we followed the fate of radiolabeled Tpo administered in vivo. In contrast to control mice, in which a significant fraction of the radioligand associates with platelets in the cellular fraction of whole blood, only a background level of radioactivity is detected in the same fraction from NF-E2 knockout mice. Additionally, a much lower level of radioactivity is associated with the lungs of these mutant mice compared with controls, and we find little 125I-Tpo in the plasma. In hematopoietic tissues, the radioligand binds directly to megakaryocytes from normal or mutant animals, consistent with a role for these Mpl-expressing cells in modulating free Tpo levels, as previously suggested.11,13 15
Surprisingly, in the spleen of mutant mice, we also note binding of 125I-Tpo to small, previously undetected particles that resemble megakaryocyte fragments or poorly developed platelets and are usually present within or in close association with macrophages. These particles can thus sequester free Tpo from the serum and, together with the abundant megakaryocytes, likely contribute to the paradoxically low concentration of free Tpo in the serum of NF-E2 knockout mice. Although the precise nature of these structures remains to be elucidated, they are not present in the blood in appreciable numbers, as judged by the very small amount of immunoreactive c-Mpl protein in the insoluble plasma fraction and our inability to detect them visually in the blood. Rather, the sum of our observations suggests that in vivo these particles are recognized as being abnormal and are likely cleared rapidly by splenic macrophages.
Although the unique model of thrombocytopenia in mice lacking transcription factor NF-E2 provides a useful molecular inroad to megakaryocyte development and platelet biogenesis, it also exposes some apparent paradoxes. First, it reveals an apparent concordance rather than the usual inverse correlation between platelet numbers and serum Tpo levels. Second, it raises the question of alternate hemostatic mechanisms that permit survival, albeit with chronic bleeding, in a small fraction of mutant mice. Our present findings address each of these issues. We have shown that free Tpo is cleared in these knockout mice by cells in the bone marrow and spleen, including megakaryocytes and small, platelet-like particles or megakaryocyte fragments. The latter structures, which in turn appear to be cleared rapidly by macrophages, likely also provide some degree of hemostasis to avoid hemorrhagic death in a few mutant mice. Further studies should clarify the nature of these particles and also provide a grasp of the molecular processes that prevent normal thrombocytopoiesis in the absence of NF-E2.
ACKNOWLEDGMENT
We thank Bethany Swencki, Eric Stefanich, and Ramon Widmer for expert technical assistance.
Supported in part by grants from the National Institutes of Health (R.A.S. and S.H.O.). S.H.O. is an Investigator of the Howard Hughes Medical Institute.
Address reprint requests to Ramesh A. Shivdasani, MD, PhD, Dana-Farber Cancer Institute, 44 Binney Boston, MA 02115.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal