Abstract
Nerve growth factor (NGF ) can influence mast cell development and function in murine rodents by interacting with its receptors on mast cells. We now report the identification of mRNA transcripts of full-length tyrosine kinase-containing trkA, trkB, and trkC neurotrophin receptor genes in HMC-1 human mast cell leukemia cells. Although HMC-1 cells lacked p75 mRNA, they expressed transcripts for the exon-lacking splice variant of trkA (trkAI), truncated trkB (trkB.T1), and truncated trkC. By flow cytometry, HMC-1 cells exhibited expression of TrkA, TrkB, and TrkC receptor proteins containing full-length tyrosine kinase domains. NGF stimulation of HMC-1 cells induced tyrosine phosphorylation of TrkA protein, increased expression of the early response genes c-fos and NGF1-A, and activation of ERK-mitogen–activated protein (MAP) kinase, results which indicate that TrkA receptors in HMC-1 cells are fully functional. Highly purified populations of human lung mast cells expressed mRNAs for trkA, trkB and trkC, whereas preparations of human umbilical cord blood-derived mast cells expressed mRNAs for trkA and trkC, but not trkB. Moreover, preparations of human umbilical cord blood-derived immature mast cells not only expressed mRNA transcript and protein for TrkA, but exhibited significantly higher numbers of chymase-positive cells after the addition of NGF to their culture medium for 3 weeks. In addition, HMC-1 cells expressed mRNAs for NGF, brain-derived neurotrophic factor (BDNF ), and neurotrophin-3 (NT-3), the cognate ligands for TrkA, TrkB, and TrkC, whereas NGF and BDNF transcripts were detectable in human umbilical cord blood mast cell preparations. Taken together, our findings show that human mast cells express a functional TrkA receptor tyrosine kinase and indicate that NGF may be able to promote certain aspects of mast cell development and/or maturation in humans. Our studies also raise the possibility that human mast cells may represent a potential source for neurotrophins.
NERVE GROWTH FACTOR (NGF ) represents the prototype for a family of related neurotrophic factors known as the neurotrophins, which also includes brain-derived neurotrophic factor (BDNF ), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5).1 The neurotrophins are essential for the growth, differentiation, and survival of many neurons in the developing nervous system and for the maintenance of some neurons in the mature nervous system.2 Although neurotrophins are thought to exert their actions predominantly on neurons, some neurotrophins, particularly NGF, can also influence the development and activation of many hematopoietic cell types, including basophils and mast cells.3-6
Mast cells are derived from hematopoietic precursors and represent critical effector cells in allergic diseases and other IgE-dependent responses.7-9 Several lines of evidence indicate that NGF can influence rat and mouse mast cell development and function. In neonatal rats, subcutaneous injection of NGF can induce mast cell hyperplasia and hypertrophy in many tissues and organs,6,10,11 and mast cell numbers can be greatly decreased in rats by antibodies to NGF.11 In the presence of IL-3, NGF can promote colony formation and induce maturation of mouse bone marrow-derived cultured mast cells in vitro.12 NGF can also promote the survival of rat peritoneal mast cells (PMCs) by suppressing apoptosis.13,14 Moreover, in vitro studies have indicated that NGF can induce degranulation and mediator release from rat PMCs.15-18 Finally, a recent report has demonstrated that rat PMCs can synthesize, store, and spontaneously release biologically active NGF.19 Taken together, these findings indicate that rat mast cells represent a potential source of NGF, and that neurotrophins may mediate autocrine and/or paracrine effects on rat and mouse mast cell development and function.
All neurotrophins can interact with two distinct types of cell surface receptors to exert their biologic effects. The low-affinity neurotrophin receptor, known as p75, binds all neurotrophins and appears to mediate a number of diverse functions in neurons, such as increasing the high-affinity binding of NGF, promoting the retrograde transport of neurotrophins, increasing the discrimination of ligand, and regulating NGF-dependent apoptosis.20 By contrast, the high-affinity neurotrophin receptors have been identified as the Trk family of receptor tyrosine kinases. NGF, BDNF, and NT-3 are primary ligands for TrkA, TrkB, and TrkC, respectively, whereas TrkA and TrkB also serve as receptors for NT-3 and NT-4/5.21
Mature rat mast cells, such as PMCs, express TrkA, but not p75.14,18 The TrkA detected in these cells is functional and appears to interact specifically with NGF to initiate relevant signaling responses, such as tyrosine autophosphorylation and the induction of expression of early response genes.13,14,18 On the other hand, cultured mouse mast cells maintained in interleukin-3 (IL-3) express gene transcripts for both TrkA and p75,22 suggesting that the pattern of mRNA expression of these two types of NGF receptors can differ among distinct mast cell preparations, or according to species.
To begin to characterize the pattern of expression of neurotrophin receptors in human mast cells, we analyzed the human mast cell line, HMC-1. HMC-1 cells, which are derived from a patient with mast cell leukemia,23 express many of the features of immature mast cells, and in some respects, resemble the mouse “pro-mastocyte.”24 We found that HMC-1 mast cells expressed the gene transcripts and the proteins of TrkA, TrkB, and TrkC receptors, but lacked expression of p75 mRNA. Moreover, the TrkA receptors present in HMC-1 cells could function to initiate signaling responses. In addition, we detected mRNA transcripts for trkA, trkB, and trkC in highly purified human lung mast cells. We also demonstrated that human umbilical cord blood-derived mast cells can express the transcript and protein for TrkA receptor, and showed that treatment with NGF in vitro can significantly increase the number of chymase-positive cells in such mast cell population. Finally, we found that HMC-1 cells expressed mRNAs for NGF, BDNF, and NT-3, and that human umbilical cord blood-derived mast cell preparations expressed mRNAs for NGF and BDNF. Some of our results have been previously reported in abstract form.25
MATERIALS AND METHODS
Culture of human cell lines and sources of animal tissues.The human mast cell line HMC-1 was originally established from the peripheral blood of a patient with mast cell leukemia.23 HMC-1 cells were grown in RPMI medium supplemented with 10% fetal calf serum (FCS) and L-glutamine. K-562, a human chronic myelogenous leukemia cell line, and Hs294T, a human melanoma cell line, were obtained from American Type Culture Collection (ATCC) (Rockville, MD). K-562 cells were grown in RPMI medium supplemented with 10% FCS, whereas Hs294T cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) with 4.5 g/L glucose supplemented with 10% FCS. The fetal monkey mesencephalon was dissected from coronal sections of a brain removed from an African green monkey fetus (St Kitts Biochemical Research Foundation, St Kitts) at 150 days of gestation.26
Culture of human umbilical cord blood-derived mast cells.Normal human mast cells were generated as previously described by Saito et al27 with slight modifications. Briefly, mononucleated cells were isolated from human umbilical cord blood (Advanced Biotechnologies, Columbia, MD) by centrifugation over Histopaque-1077 (Sigma Chemical, St Louis, MO), and were then cultured in Iscove's modified Dulbecco's medium (Sigma) supplemented with 10% heat-inactivated FCS (Hyclone, Logan, UT), E coli–derived recombinant human stem cell factor (SCF, at 80 ng/mL, generously provided by Dr Keith E. Langley, AMGEN Inc, Thousand Oaks, CA), recombinant human IL-6 (50 ng/mL, AMGEN), and prostaglandin E2 (PGE2) (1 μmol/L; Cayman Chemical, Ann Arbor, MI). The culture medium was changed weekly, and the cells were maintained for 12 to 23 weeks. For sensitizing cells with IgE, cells were cultured in 24-well culture plate with human myeloma IgE (Biodesign International, Kennebunk, ME) at a cell density of 2 × 105/mL.
Preparation of 99% pure human lung mast cells.Lung mast cell purification was performed using gradient centrifugation and magnetic cell separation systems.28 Briefly, lung mast cells were dispersed from ∼12.0 g of macroscopically normal human lung tissue obtained from patients undergoing partial pneumonectomy for lung cancer. The lung tissue was cut into strips with scissors, washed extensively in calcium- and magnesium-free Tyrode's buffer, and then incubated in 0.5 mg/mL collagenase (type I, Sigma) in the same buffer for 45 minutes at 37°C. Cells dispersed by this procedure were separated from undissociated tissue by subsequent filtration through 800 μm and 80 μm pore-sized nylon gauze and washed twice with the buffer by centrifugation at 500g for 10 minutes at room temperature. The numbers and percentage of mast cells were assessed by counting in a hemocytometer after metachromatic staining with Kimura stain.29 In a typical experiment, mast cells obtained at this stage were ∼1.58 × 106/g of wet lung tissue and the purity was 9.7%. Mast cells were enriched by centrifugation through a Percoll gradient. The dispersed lung cell suspension was overlaid onto 30%/80% isotonic Percoll layers and was centrifugated at 500g, 4°C for 20 minutes. The interface was harvested and washed with four to five times volume excess of calcium- and magnesium-free Hanks' Balanced Salt Solution (HBSS) supplemented with 2% FCS. Mast cells at this step represented a purity of 30.9% and 1.08 × 106 cells/g. Cells were cultured overnight in RPMI 1640 containing 10% FCS, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
The next day, cells were washed twice and were resuspended in 2 mL of phosphate-buffered saline (PBS) containing 5 mmol/L EDTA and 0.5% bovine serum albumin (BSA) (PBS/BSA), 8% rat serum, 50 μg/mL human IgG for 15 minutes at 4°C (to help reduce any nonspecific binding of antibodies). The cells were then pelleted and resuspended in 15 μg/mL SR-1 monoclonal mouse IgG2a antibody against human c-kit (provided by Dr Keith Langley) for 15 minutes at 4°C. After being washed, cells were incubated with 15 μg/mL of rat-antimouse-IgG2a + b antibody conjugated with colloidal microbeads (Miltenyi Biotec, Sunnyvale, CA) in PBS/BSA for 30 minutes at 4°C. The c-kit-positive cells were recovered using MACS cell separation system, according to the manufacturer's specifications (Miltenyi Biotec), yielding preparations of >99% mast cells according to staining with Kimura stain or with a mouse monoclonal antibody against human mast cell tryptase (see below).
Stimulation of human mast cells with anti-IgE.Cells were sensitized overnight with 1 μg/mL of human myeloma IgE (Biodesign International). They were then washed twice and stimulated with goat antihuman IgE (1 μg/mL, Sigma) or with vehicle alone for 2 hours at 37°C (1.5 × 105 cells in 500 μL) in HBSS supplemented with 0.4 mmol/L MgCl2 , 1.0 mmol/L CaCl2 , and 0.1% BSA. The specimens were washed with ice-cold HBSS/0.1% BSA and resuspended in an adequate amount of buffer. Cells were pelleted again and stored at −80°C until RNA was extracted.
Immunocytochemical analysis of human cord blood-derived mast cells.Immunocytochemical identification of human mast cell tryptase and chymase was performed as reported by Saito et al with slight modifications.27 Briefly, cytospin preparations were fixed with 4% paraformaldehyde in PBS for 15 minutes at room temperature, and washed in Tris-buffered saline (TBS). After incubation with 5% rabbit serum in TBS for 20 minutes, cells were incubated with a mouse monoclonal IgG1κ antibody against either human mast cell tryptase or human mast cell chymase (Chemicon International, Temecula, CA), or a control mouse IgG1κ antibody (MOPC21, Sigma) at 2 μg/mL for 1 hour at room temperature. Slides were then washed and incubated with rabbit antimouse IgG (APAAP kit, Dako, Carpinteria, CA) for 1 hour. After washing, slides were incubated with alkaline phosphatase-antimouse alkaline phosphatase complex (APAAP) (Dako) for 1 hour. Cells were then reacted with red substrate (Fast Red TR/Naphthol AS-MX with 1 mmol/L levamisole; Sigma) for 5 minutes, counterstained with hematoxylin, and covered with CrystalMount (Biomeda, Foster City, CA). From each slide, 500 cells were analyzed for chymase using light microscopy by an observer who was unaware of the identity of the individual slides, and the strength of chymase positivity was graded from negative (−) to strongly positive (+++). The distribution of mast cells exhibiting various intensities of chymase positivity (− to +++) in individual specimens was compared using the χ2 test.
Cytokines and antibodies.Purified mouse 2.5S nerve growth factor was purchased from Upstate Biotechnology (Lake Placid, NY). For flow cytometry and Western blotting experiments, specific affinity-purified rabbit anti-TrkA (Product No. 763), anti-TrkB (794), and anti-TrkC (798) polyclonal antibodies and their respective control peptides were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). These three Trk antibodies were raised against three different polypeptides, each comprised of 15 nonhomologous amino acids present in the tyrosine kinase domain of each of the three Trk receptors. These antibodies recently have been used to characterize Trk receptors in human transformed B lymphocytes by flow cytometry30; this study also showed that these antibodies could identify TrkA, TrkB, and TrkC proteins in PC12 cells and rat embryonic dorsal root ganglia by Western blotting and in human dorsal root ganglia and sural nerve biopsy specimens using immunohistochemistry.30
Reverse transcriptase-polymerase chain reaction (RT-PCR) and Southern analysis.Total RNA was extracted from cultured cells and from fetal monkey midbrain using the RNAzolB method according to the manufacturer's specifications (Biotecx Laboratories, Houston, TX). Human substanta nigra poly A+ mRNA was purchased from Clontech Laboratories (Palo Alto, CA). Approximately 5 μg of total RNA, or ∼0.1-0.5 μg of heparinase-treated total RNA (for cultured and purified human mast cells),31 or 0.5 μg of poly A+ mRNA, was reversed transcribed with random hexamers using the StrataScript RNaseH− reverse transcriptase (Stratagene, La Jolla, CA) at 37°C for 60 minutes. The cDNA synthesized was subsequently amplified in a 100-μL volume reaction mixture by a DNA thermal cycler for 30 seconds at 96°C, 2 minutes at 55°C, and 3 minutes at 72°C for 38 thermocycles followed by an additional 7 minutes at 72°C using 2.5 U of AmpliTaq DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT). PCR products were analyzed on 1.5% agarose gels, and then transferred onto Zetabind nylon membrane (Cuno, Meriden, CT). Blots were hybridized at 42°C and washed at 58°C to a final stringency of 0.1× SSC.
Full-length isoforms of trk transcripts.To amplify simultaneously trkA, trkB, and trkC cDNAs that contain the full-length tyrosine kinase domains, we used two degenerate oligo primers (A and B) corresponding to the highly conserved regions of the extracellular domain [PTH(Y,M,V)NNG(N,D)YTL] and of the tyrosine kinase domain (EGAFGKVFLAEC) shared among human TrkA, mouse TrkB, and porcine TrkC receptor proteins.32-34 The sequences of the primers are: Primer A, 5′-ATGGATCCCAC(CT)CA(CT)(TGA) -(AT)( C G )A A ( C T ) A A ( C T ) G G ( C A ) ( A G ) A C T A C A C ( G A C )C T-3;pr -(sense); Primer B, 5′-TCTCGAGCACTC(GCA)GC(CTA)AGGAA(GA)AC(CT)TTCCCAAAGGC(TG)CC(CT)TC-3′ (antisense). To characterize the different splice variants of trkA gene transcripts, PCR was performed using a pair of primers (C and D) flanking the putative alternative splicing region of human trkA mRNA.18 Primer C: 5′-ATGGACAACCCTTTCGAGTTCAAC-3′ (5′ corresponding to residue 1219); Primer D: 5′-GACCCCAAAAGGTGTTTCGTCC-3′ (3′ corresponding to residue 1299).
Truncated isoforms of trkB and trkC transcripts.To amplify cDNAs encoding human truncated TrkB (TrkB.T1) by PCR, primers E and F, which are specific for human truncated trkB.T1 sequence, were used.35 Primer E: 5′-ATGGGCTGGCCTGGAATTGACGAT-3′ (5′ corresponding to residue 1249); Primer F: 5′-CATCAACCAACAAGCACCACAGCC-3′ (3′ corresponding to residue 1567 of human trkB.T1). To amplify cDNAs encoding human truncated TrkC by PCR, specific human trkC primers G and H were used.35 Primer G: 5′-AGATGCCATGGTTAAGAGGCTTGG-3′ (5′ corresponding to residue 1162); Primer H: 5′-CCACTGGGCACAGCCAACCAGACC-3′ (3′ corresponding to residue 1751 of human truncated trkC).
p75.To detect human p75 mRNA transcripts by PCR, primers R and S, which are specific for human p75 cDNA sequence, were used.36 Primer R: 5′-ACAGGCCTGTACACACACAGC-3′ (5′ corresponding to residue 213); Primer S: 5′-GCCAGGGATCTCCTCGCACTC-3′ (3′ corresponding to residue 672).
NGF, BDNF, and NT-3.Human NGF, BDNF, and NT-3 genes lack any introns.37 Accordingly, it was crucial for valid RT-PCR analysis of the corresponding transcripts that the total RNA isolated from the HMC-1 cells and human umbilical cord blood-derived mast cells be treated with DNase I (RQ1 RNase-free DNase) (Promega, Madison, WI) at 37°C for 30 minutes, at 1 U/10 μg total RNA, to eliminate any contamination by genomic DNA. The DNase I-treated RNA was subsequently reverse transcribed as described above (human umbilical cord blood-derived mast cell RNA was subjected to an additional treatment with heparinase I before the reverse transcription procedure), and the resulting cDNA was amplified by PCR using native Pfu DNA polymerase (Stratagene) with three different sets of gene-specific primers as listed in the following37-39: NGF, Primer T: 5′-TCATCATCCCATCCCATCTTCCAC-3′ (5′ corresponding to residue 1825); Primer U: 5′-CACAGCCTTCCTGCTGAGCACACA-3′ (3′ corresponding to residue 2144). BDNF, Primer V: 5′-GACCCTGCCCGCCGAGGGGAG-3′ (5′ corresponding to residue 466); Primer W: 5′-AGTGTCTATCCTTATGAATCGCC-3′ (3′ corresponding to residue 764). NT-3, Primer X: 5′-CGGTACGCGGAGCATAAGAGTCAC-3′ (5′ corresponding to residue 487); Primer Y: 5′-TCCGATTTTTCTCGACAAGGCACA-3′ (3′ corresponding to residue 817).
cDNA probes.The full-length human trkA (ATCC# 41055) and mouse zif/268 cDNA (ATCC# 63027) clones were obtained from ATCC. The c-fos probe is a 1.2-kb HindIII/EcoRI mouse cDNA fragment kindly provided by Dr Brent Cochran (Massachusetts Institute of Technology, Cambridge). The partial cDNA probes for monkey trkB, trkC, and p75 were generated by RT-PCR using fetal monkey midbrain total RNA and primers A and B (trkB and trkC) or primers R and S (p75) as previously described.26 The authenticity of the PCR-amplified monkey cDNAs was verified by subcloning the DNA fragments into pGEM-T Vector (Promega) and subsequent DNA sequencing analysis using Sequenase version 2.0 (U.S. Biochemical, Cleveland, OH).26 Similarly, the partial cDNA probes for human NGF and NT-3 were generated by RT-PCR of human brain poly A+ mRNA using Pfu DNA polymerase (Stratagene) and primers T and U (NGF ) or primers X and Y (NT-3). The human BDNF probe was generated by RT-PCR of DNase I-treated RNA from HMC-1 cells using Pfu DNA polymerase and primers V and W. The amplified DNA fragments of the predicted sizes were subcloned into pCR-Script SK(+) vector (Stratagene), and subsequent DNA sequencing showed that they contained authentic human cDNA sequences for NGF, BDNF, and NT-3. The cDNA probes were labeled with [α-32P] deoxycytidine triphosphate (New England Nuclear, Boston, MA) using the exo(−) Klenow fragment of DNA polymerase I with random nonamer primers (Stratagene).
Northern analysis.Total RNA (20 μg/lane) was isolated by the RNAzolB method, electrophoresed in 1% agarose-formaldehyde denaturing gel, and transferred onto Zetabind nylon membrane. RNA blots were hybridized at 42°C, and washed at 42°C to a final stringency of 0.2× SSC.
Flow cytometry.Approximately 1.0 × 106 HMC-1 cells, or human umbilical cord blood-derived mast cells, were fixed with a 1:1 mixture of methanol/ethanol for 30 minutes at 4°C and subsequently incubated with the primary anti-TrkA, anti-TrkB, or anti-TrkC antibody at 10 μg/mL for 30 minutes at 4°C. After three consecutive washes with PBS containing 1% BSA (pH 7.3) and 15% goat serum, cells were incubated with a fluorescein-conjugated goat F(ab′)2 antirabbit IgG (10 μg/mL) (Santa Cruz Biotechnology). Cells were resuspended in PBS and analyzed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Negative controls were cells incubated with a rabbit IgG preparation (10 μg/mL) (Sigma) instead of a specific primary antibody. We also assessed the specificity of staining by preincubation each anti-Trk antibody for 10 minutes before using it for flow cytometry analysis, with 0.02 to 20 μg/mL of either a peptide representing the appropriate Trk receptor or a peptide representing an inappropriate Trk receptor (Santa Cruz Biotechnology).
TrkA immunoprecipitation and Western blotting.Approximately 2.0 × 107 cells were lysed in 1.0 mL of ice cold RIPA buffer (PBS with 1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.1 mg/mL phenylmethylsulfonyl fluoride, 63 μg/mL aprotinin, 1 mmol/L sodium orthovanadate) by passage through 21-gauge needles. The lysate was cleared by centrifugation, and incubated with 2 μg of anti-TrkA antibody (763) for 3 hours at 4°C, and subsequently incubated with 20 μL of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) with gentle mixing overnight at 4°C. Immunoprecipitates were washed four times with RIPA buffer, subjected to 6% SDS-polyacrylamide gel electrophoresis, and then electroblotted onto a Hybond-ECL nitrocellulose membrane (Amersham, Arlington Heights, IL). Membranes were incubated in blocking buffer (0.01 mol/L Tris, pH 7.5, 0.1 mol/L NaCl, 0.1% Tween 20, 1% BSA) at 37°C for 20 minutes and then immunoblotted with a horseradish peroxidase-conjugated recombinant antiphosphotyrosine antibody RC20 (Transduction Laboratories, Lexington, KY) at 1:2,500 dilution at 37°C for 20 minutes. The phosphorylated proteins were detected using an enhanced chemiluminescent detection method (Amersham).
Mitogen-activated protein (MAP) kinase activity.Previous studies from our laboratory have shown that ERK1-pp44 and ERK2-pp42 MAP kinases are coordinately regulated in mouse mast cells that are stimulated with SCF or IgE and antigen.40 Therefore, only pp44 MAP kinase activity was measured in this study. ERK1-MAP kinase pp44 was partially purified from ERK1 ImmunoCruz System (Santa Cruz Biotechnology), which consists of an immunoaffinity column containing ERK1 rabbit polyclonal IgG antibody coupled to an agarose matrix, as described in the instruction manual supplied by the manufacturer. The eluted antigen was assayed for phosphorylation of myelin basic protein (Santa Cruz Biotechnology) using a substrate peptide kinase assay as previously described.41 Briefly, 30 μL of the eluted antigen was incubated with 25 mmol/L Tris-HCl (pH 7.5), 1 mmol/L MgCl2 , 50 μmol/L adenosine triphosphate (ATP), 2.5 μg of myelin basic protein, and 1 μCi of [γ-32P]ATP in a reaction volume of 50 μL. After incubation at 30°C for 15 minutes, 25 μL of the reaction was spotted onto a 1.5 × 1.5 cm P-81 cellulose phosphate paper (Life Technologies, Gaithersburg, MD) and washed twice with 1% phosphoric acid and then water before quantitation of radioactivity by liquid scintillation counting. The protein concentration for each eluted antigen sample was determined by Bio-Rad Protein Assay (Bio-Rad, Hercules, CA), and used to calculate specific activities (cpm/μg protein).
RESULTS
HMC-1 cells express mRNA transcripts for trkA, trkB, and trkC that contain the full-length tyrosine kinase domains.Recent studies have indicated that mRNA for trkA, but not trkB or trkC, is detectable in rat and mouse mast cells.18 22 To assess the pattern of expression of trk mRNAs in human mast cells, we first searched for the presence of mRNA transcripts encoding the tyrosine kinase-containing isoforms of different members of the Trk receptor family in HMC-1 mast cells.
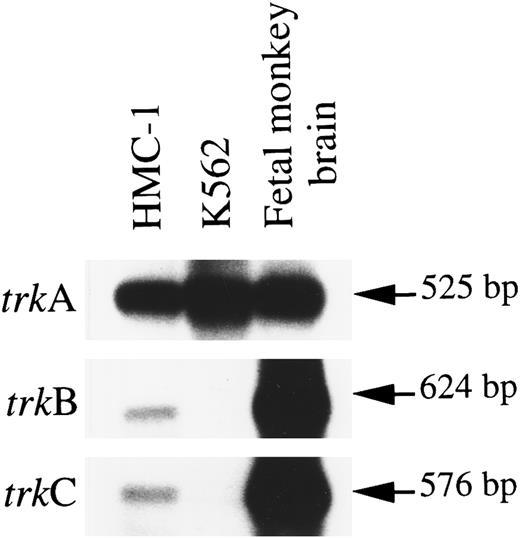
RT-PCR was performed on HMC-1 RNA with two degenerate oligo primers, A and B, which correspond to highly conserved regions of the extracellular domain and of the tyrosine kinase domain shared among human TrkA, mouse TrkB, and porcine TrkC receptor polypeptides.32-34 These two primers have previously been shown to be effective in simultaneously amplifying partial trkA, trkB, and trkC sequences by PCR with fetal monkey brain cDNAs.26 The partial monkey trkB and trkC sequences amplified are highly homologous to their human counterparts,26,35 and thus were used as cDNA probes in our subsequent Southern hybridization studies. Southern analysis of the PCR products using human trkA and monkey trkB and trkC cDNAs as probes detected strong signals for the mRNA expression of trkA (predicted size 525 bp) in HMC-1 cells and in fetal monkey brain (Fig 1). Moreover, we could detect weaker, but consistently reproducible, hybridization signals indicating the expression of mRNAs for trkB (predicted size 624 bp) and trkC (predicited size 576 bp) in HMC-1 cells. These hybridization signals did not appear to result from nonspecific cross-hybridization of different trk cDNA probes with the HMC-1 trk gene messages, as illustrated by the observation that the human K-562 cell line, which is known to express exclusively the TrkA receptor,32 exhibited no detectable expression of trkB and trkC mRNAs (Fig 1). Moreover, the trkB and trkC signals detected on the Southern blot exhibited the expected difference in their molecular weights (trkB >trkC) (Fig 1). The results shown in Fig 1 were obtained in at least four separate experiments.
Expression of mRNA transcripts encoding trkA, trkB, and trkC genes in the HMC-1 mast cell line, the K-562 chronic myelogenous leukemia cell line, and fetal monkey brain tissue. The cDNA was synthesized from total RNA by reverse transcriptase and amplified by PCR using two degenerate oligonucleotide primers A and B. The identity of the PCR products was confirmed by Southern blot analysis using 32P-labeled human trkA, monkey trkB, and monkey trkC probes.
Expression of mRNA transcripts encoding trkA, trkB, and trkC genes in the HMC-1 mast cell line, the K-562 chronic myelogenous leukemia cell line, and fetal monkey brain tissue. The cDNA was synthesized from total RNA by reverse transcriptase and amplified by PCR using two degenerate oligonucleotide primers A and B. The identity of the PCR products was confirmed by Southern blot analysis using 32P-labeled human trkA, monkey trkB, and monkey trkC probes.
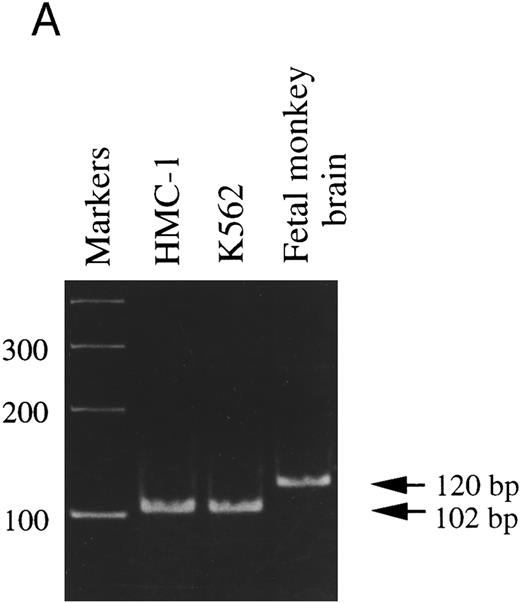
It has been reported that two different splice variants of trkA mRNA transcripts are expressed in PC12 cells and rat PMC (trkAI and trkAII).18,42 Moreover, transfection studies in PC12nnr5 cells show that, in contrast to TrkAI receptors, TrkAII receptors exhibit enhanced functional responses to stimulation by the presumably noncognate ligand, NT-3.43 To assess which of the two trkA transcripts was expressed in HMC-1 cells, PCR was performed using a pair of primers that flank the putative alternative splicing region of trkA mRNA.18 As shown in Fig 2A, the 120-bp band amplified from the monkey brain represents the exon-containing form (trkAII), whereas the HMC-1 cells and the K-562 cells appeared to express exclusively the exon-lacking form (trkAI), as represented by the 102-bp amplified fragment. To confirm the identities of these DNA fragments, DNA gel bands amplified from HMC-1 cells and fetal monkey brain were purified and subcloned into pGEM-T Vector. Our DNA sequencing analysis confirmed that the HMC-1 cells expressed the trkAI isoform, whereas the trkAII isoform was found in the specimen from fetal monkey brain.
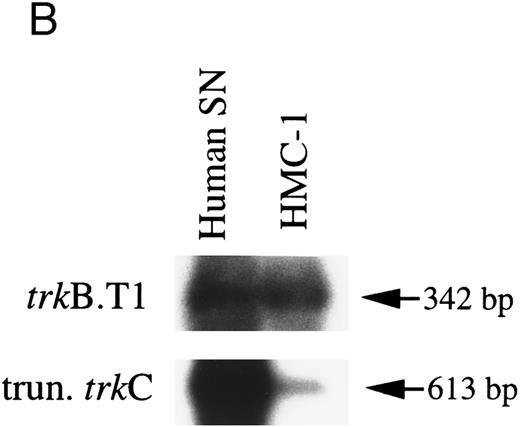
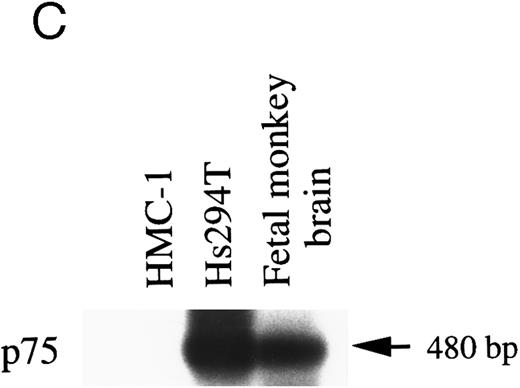
RT-PCR analysis to detect: (A) alternatively spliced trkA mRNA isoforms in HMC-1 cells, K-562 cells, and fetal monkey brain tissue, (B) the expression of truncated isoforms of trkB and trkC in human substanta nigra and HMC-1 cells, and (C) the expression of p75 mRNA transcripts in HMC-1 cells, Hs294T cells, and fetal monkey brain tissue. The cDNAs were synthesized from total RNA or poly A+ mRNA from each of the indicated cells or tissues by reverse transcriptase and amplified by PCR using primers C and D for trkA in (A), and primers E and F for trkB.T1 in (B), and primers G and H for truncated trkC in (B), and primers R and S for p75 in (C). The PCR products were analyzed on a 5% nondenaturing polyacrylamide gel in (A). The identity of PCR products in (B) and (C) was confirmed by Southern blot analysis using 32P-labeled monkey trkB and trkC cDNA probes in (B), and a monkey p75 cDNA probe in (C).
RT-PCR analysis to detect: (A) alternatively spliced trkA mRNA isoforms in HMC-1 cells, K-562 cells, and fetal monkey brain tissue, (B) the expression of truncated isoforms of trkB and trkC in human substanta nigra and HMC-1 cells, and (C) the expression of p75 mRNA transcripts in HMC-1 cells, Hs294T cells, and fetal monkey brain tissue. The cDNAs were synthesized from total RNA or poly A+ mRNA from each of the indicated cells or tissues by reverse transcriptase and amplified by PCR using primers C and D for trkA in (A), and primers E and F for trkB.T1 in (B), and primers G and H for truncated trkC in (B), and primers R and S for p75 in (C). The PCR products were analyzed on a 5% nondenaturing polyacrylamide gel in (A). The identity of PCR products in (B) and (C) was confirmed by Southern blot analysis using 32P-labeled monkey trkB and trkC cDNA probes in (B), and a monkey p75 cDNA probe in (C).
HMC-1 cells express truncated isoforms of trkB and trkC transcripts.Isoforms of trkB and trkC transcripts encoding truncated tyrosine kinase domains have been identified in rats and in humans.35,44,45 To assess if these isoforms were expressed in HMC-1 cells, RT-PCR was performed with PCR primers specific for the previously described human truncated trkB (trkB.T1) and trkC cDNAs.35 As shown in Fig 2B, which shows human substanta nigra mRNA as a positive control, Southern analysis using partially overlapping monkey trkB and trkC probes indicated that both truncated trkB (predicted size 342 bp) and trkC (predicted size 613 bp) transcripts were detectable in the HMC-1 cell line. These results were obtained in two separate experiments.
p75.It has been reported that rat PMCs lack expression of both mRNA and protein of the low-affinity neurotrophin receptor, p75,18 whereas p75 mRNA is detectable in mouse mast cells that have been derived from the spleen and maintained in IL-3 in vitro.22 To assess whether HMC-1 cells expressed the p75 gene transcript, RT-PCR was performed with two oligo primers specific for the human p75 cDNA sequence.36 As shown in Fig 2C, Southern analysis of the PCR products using monkey p75 cDNA as a probe showed no detectable signal of p75 message in HMC-1 cells. In contrast, positive controls such as the Hs294T cells and the fetal monkey brain exhibited strong signals of hybridization for p75 mRNA (predicted size 480 bp).
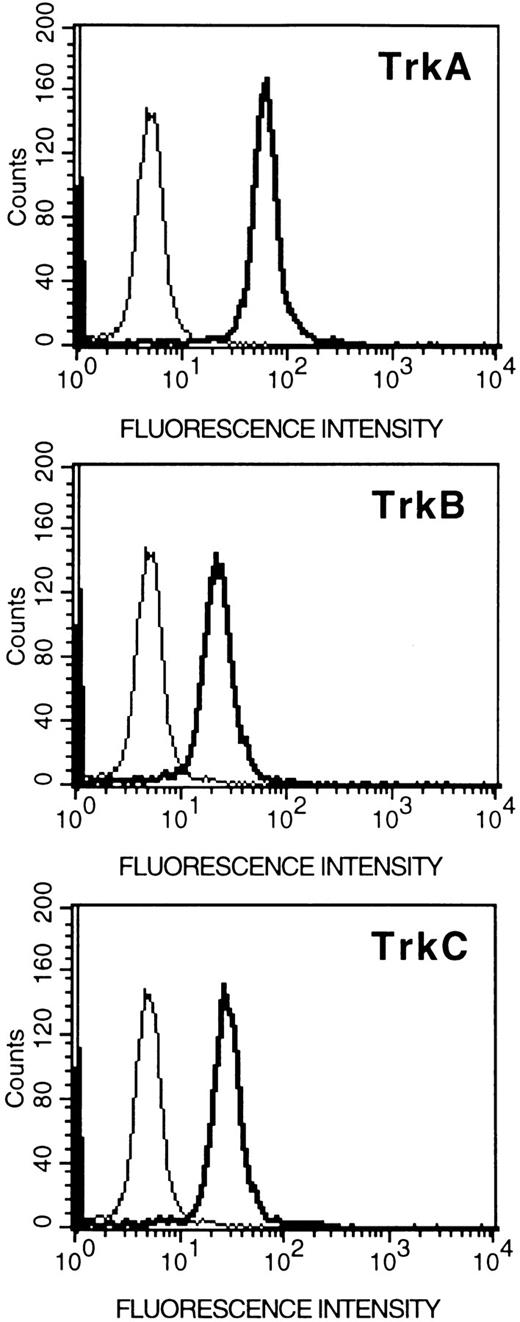
Expression of Trk proteins in HMC-1 cells.To assess whether the expression of trk gene transcripts detected in HMC-1 cells could be correlated with the expression of Trk receptor proteins, the cells were incubated with specific antibodies against various Trk peptides, and analyzed by flow cytometry. As assessed in six experiments, we found that the HMC-1 cells exhibited positive labeling with TrkA, TrkB, or TrkC antibodies, indicating that the HMC-1 cells express proteins for all three types of Trk receptors (Fig 3). The staining obtained with each antibody appeared to be specific, as it could be competitively blocked in a concentration-dependent manner by preincubation with the appropriate Trk receptor control peptides, but not with the irrelevant peptides (data not shown). Furthermore, because these antibodies were raised to recognize the respective tyrosine kinase domains in the three Trk receptor proteins, these results also support the conclusion that the Trk receptors (A, B, and C) expressed in HMC-1 cells are of the full-length isoforms.
Flow cytometric analysis (relative log fluorescence) of expression of Trk proteins in HMC-1 mast cells. HMC-1 cells were incubated with an anti-TrkA, anti-TrkB, or anti-TrkC antibody (as indicated in the figures), or with rabbit IgG (negative control, the thin line in the figures), and subsequently stained with fluorescein-conjugated goat antirabbit IgG and analyzed by flow cytometry. Data are representative of six separate experiments.
Flow cytometric analysis (relative log fluorescence) of expression of Trk proteins in HMC-1 mast cells. HMC-1 cells were incubated with an anti-TrkA, anti-TrkB, or anti-TrkC antibody (as indicated in the figures), or with rabbit IgG (negative control, the thin line in the figures), and subsequently stained with fluorescein-conjugated goat antirabbit IgG and analyzed by flow cytometry. Data are representative of six separate experiments.
Induction of signaling responses by NGF in HMC-1 cells.The predominant expression of trkA (as opposed to trkB or trkC) gene message and protein in HMC-1 cells is consistent with the possibility that the TrkA receptor tyrosine kinase may play a more significant role in mast cell development/function in humans than either the TrkB or TrkC receptors. However, normal human mast cells are usually available only in very small numbers, the amount of which would not be sufficient for functional analysis of TrkA at the cellular level. We, therefore, used HMC-1 cells to assess whether the TrkA receptors expressed by these cells are functional. Specifically, we examined the ability of NGF, the cognate ligand for TrkA, to initiate relevant intracellular signaling responses in these cells.
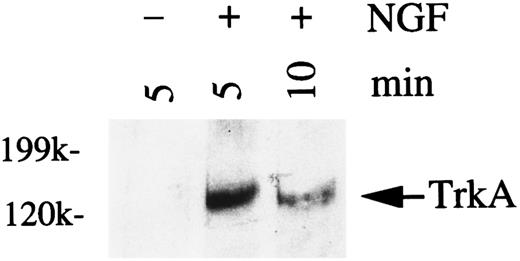
NGF stimulation of HMC-1 cells induces tyrosine phosphorylation of TrkA.In many different cell types that express the TrkA protein, including rat PMCs, exposure to NGF induces phosphorylation of the tyrosine residue on the TrkA receptor protein.14,18 21 To assess the phosphorylation of TrkA in response to NGF stimulation, HMC-1 cells were treated with NGF (200 ng/mL) for 5 or 10 minutes, and the cell lysates were immunoprecipitated with a specific anti-TrkA antibody. Immunoblot analysis of TrkA with a recombinant antiphosphotyrosine antibody showed that tyrosine phosphorylation of a protein of approximately 140 kD occurred 5 minutes after NGF treatment and that levels of the phosphorylated species decreased by 10 minutes after stimulation (Fig 4). The results shown in Fig 4 are representative of those obtained in four separate experiments. Similar results were also obtained for NGF-stimulated PC12 cells (50 ng/mL) using the same anti-TrkA antibody and identical immunoblotting procedures and reagents (data not shown).
Western analysis of tyrosine phosphorylation of TrkA in HMC-1 mast cells stimulated with NGF. HMC-1 cells were stimulated with NGF (200 ng/mL) for 5 or 10 minutes. TrkA was immunoprecipitated with specific anti-TrkA antibody from lysates of NGF-treated (+) or untreated (−) cells, and the immunoprecipitates were probed with the RC20 antiphosphotyrosine antibody.
Western analysis of tyrosine phosphorylation of TrkA in HMC-1 mast cells stimulated with NGF. HMC-1 cells were stimulated with NGF (200 ng/mL) for 5 or 10 minutes. TrkA was immunoprecipitated with specific anti-TrkA antibody from lysates of NGF-treated (+) or untreated (−) cells, and the immunoprecipitates were probed with the RC20 antiphosphotyrosine antibody.
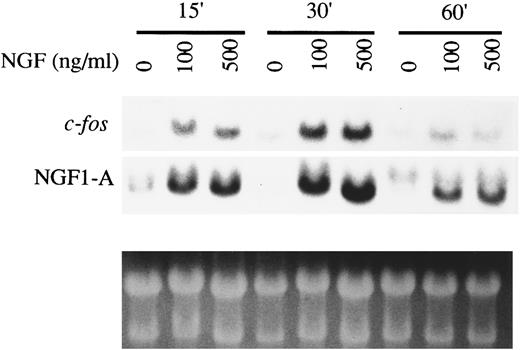
Induction of expression of early response genes in HMC-1 cells by NGF.The induction of expression of early response genes such as c-fos and NGF1-A (zif/268) represents a marker for NGF signal transduction via the TrkA receptor tyrosine kinase in many cell types, including rat PMCs.13,46 To determine whether the TrkA receptor expressed in HMC-1 cells could interact with NGF to initiate a similar response, we examined the effects of NGF on the expression of early response genes by Northern analysis. HMC-1 cells were starved in RPMI medium with 0.1% FCS for 16 hours before NGF stimulation. Figure 5, which is representative of three experiments that gave similar results, shows that NGF at 100 or 500 ng/mL induced increased expression of mRNAs for c-fos and NGF1-A at 15 and 30 minutes after stimulation, but the enhancement effects had diminished by 60 minutes after stimulation. The time course of this NGF effect in HMC-1 cells is reminiscent of that observed in rat PMCs stimulated with NGF.13 Lower doses of NGF (≤25 ng/mL) were found to be ineffective in eliciting a detectable early gene response in these HMC-1 cells (data not shown).
Induction of expression of early response genes by NGF in HMC-1 mast cells. HMC-1 cells were stimulated with NGF, at the concentrations indicated, for 15, 30, or 60 minutes. Total RNA was extracted and Northern blots were hybridized sequentially to mouse cDNAs of c-fos and zif/268 (NGF1-A). Ethidium bromide staining was used to assess loading of RNA.
Induction of expression of early response genes by NGF in HMC-1 mast cells. HMC-1 cells were stimulated with NGF, at the concentrations indicated, for 15, 30, or 60 minutes. Total RNA was extracted and Northern blots were hybridized sequentially to mouse cDNAs of c-fos and zif/268 (NGF1-A). Ethidium bromide staining was used to assess loading of RNA.
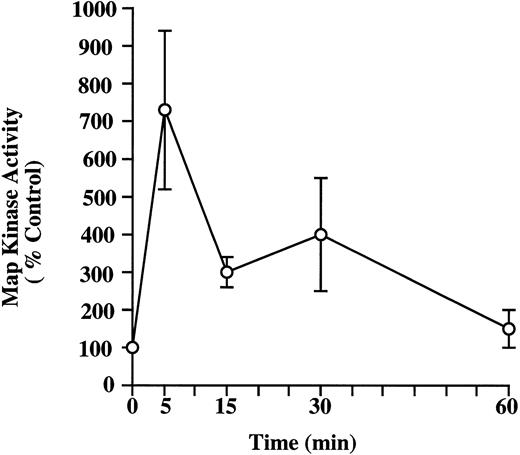
Activation of MAP kinase activity in HMC-1 cells by NGF.It has been shown in many cell types that the Ras/Raf/MAP kinase signaling pathway is activated in response to stimulation by many different growth factors, including NGF.47-49 To assess whether the activation of MAP kinases might represent part of the cascade of signal transduction initiated by NGF in mast cells, we examined the effects of NGF stimulation on MAP kinase activity in HMC-1 cells. The pp44 ERK-1 MAP kinase was partially purified from control and NGF-stimulated HMC-1 cells, and MAP kinase activity was quantified by a substrate peptide kinase assay using myelin basic protein as the substrate. Figure 6 shows the time course of NGF effects on MAP kinase activation in HMC-1 cells; the data shown were derived from four different experiments, all of which gave similar results. Stimulation of HMC-1 cells with NGF (100 ng/mL) significantly elevated pp44 MAP kinase activity (by threefold to sevenfold), as assessed at 5, 15, and 30 minutes after stimulation. However, by 60 minutes, the MAP kinase activity in HMC-1 cells had returned to basal levels.
Kinetics of induction of MAP kinase activity by NGF in HMC-1 mast cells. HMC-1 cells were stimulated with NGF (100 ng/mL) for 5, 15, 30, or 60 minutes. pp44ERK-1 MAP kinase was partially purified from HMC-1 cells using an immunoaffinity column. MAP kinase activity of the eluted antigen was quantified by a substrate peptide kinase assay using myelin basic protein as the substrate, and the specific activity was expressed as cpm/μg eluted protein. The results shown are the mean ± standard error of mean (SEM) of values obtained from four separate experiments.
Kinetics of induction of MAP kinase activity by NGF in HMC-1 mast cells. HMC-1 cells were stimulated with NGF (100 ng/mL) for 5, 15, 30, or 60 minutes. pp44ERK-1 MAP kinase was partially purified from HMC-1 cells using an immunoaffinity column. MAP kinase activity of the eluted antigen was quantified by a substrate peptide kinase assay using myelin basic protein as the substrate, and the specific activity was expressed as cpm/μg eluted protein. The results shown are the mean ± standard error of mean (SEM) of values obtained from four separate experiments.
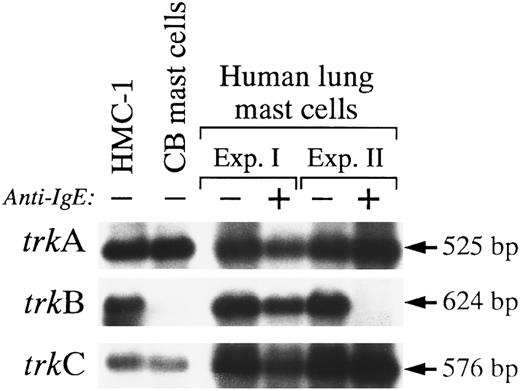
Expression of different trk mRNA transcripts in human cord blood-derived mast cells and in mast cells purified from human lungs.To assess the physiological relevance of Trk receptor expression in the HMC-1 mast cell line, we performed RT-PCR using primers A and B, followed by Southern blot analysis, to examine the patterns of mRNA expression of different trk genes in human cord blood-derived mast cells that had been cultured in SCF, IL-6, and PGE2 for 14 weeks (purity of 93% according to metachromatic staining with Kimura stain), and in human mast cells that had been purified from human lungs (purity of 99% according to metachromatic staining with Kimura stain or immunocytochemistry for tryptase).
In our initial studies, two rounds of PCR with nested primers were necessary to yield a detectable trkA signal on a Southern blot of unstimulated human umbilical cord blood-derived mast cells (data not shown). However, when these cells were sensitized and stimulated with anti-IgE, weak signals for trkA and trkC, but not trkB, could be detected in these cells after one round of PCR amplification (data not shown). We speculated that the weak signals detected by RT-PCR for trk mRNA expression in these populations of mast cells might have reflected the low amounts of RNA that were extracted from these samples. In a subsequent study, better RNA yield was obtained from a population of unstimulated human umbilical cord blood-derived mast cells (23 weeks, 99% purity). RT-PCR and Southern analysis showed strong signals for the expression of trkA and trkC, but not trkB, in these mast cells (Fig 7).
Expression of mRNA transcripts encoding trkA, trkB, and trkC in HMC-1 cells, human cord blood-derived mast cells, and mast cells purified from human lungs. The cDNA was synthesized from total RNA (HMC-1) or heparinase-treated total RNA (human umbilical cord blood-derived or lung mast cells) by reverse transcriptase and amplified by PCR using degenerate oligonucleotide primers A and B. The identity of the PCR products was confirmed by Southern blot analysis using 32P-labeled human trkA, monkey trkB, and monkey trkC cDNA probes. (+) denotes specimens from cells that were sensitized with human IgE and subsequently stimulated with antihuman IgE, whereas (−) denotes specimens from cells not stimulated with antihuman IgE (see Materials and Methods for details).
Expression of mRNA transcripts encoding trkA, trkB, and trkC in HMC-1 cells, human cord blood-derived mast cells, and mast cells purified from human lungs. The cDNA was synthesized from total RNA (HMC-1) or heparinase-treated total RNA (human umbilical cord blood-derived or lung mast cells) by reverse transcriptase and amplified by PCR using degenerate oligonucleotide primers A and B. The identity of the PCR products was confirmed by Southern blot analysis using 32P-labeled human trkA, monkey trkB, and monkey trkC cDNA probes. (+) denotes specimens from cells that were sensitized with human IgE and subsequently stimulated with antihuman IgE, whereas (−) denotes specimens from cells not stimulated with antihuman IgE (see Materials and Methods for details).
On the other hand, consistently good RNA yields were obtained from samples of unstimulated mast cells purified from human lungs. When such samples were analyzed by RT-PCR, we found that robust hybridization signals were readily detected for trkA, trkB, and trkC messages (Fig 7). The intensities of the hybridization signals were comparable to those observed with HMC-1 cells (the two Southern blots illustrated in Fig 7 showing trkB and trkC expression had been highly overexposed as compared with those shown in Fig 1, which used fetal monkey brain as the positive controls). Interestingly, in one of two experiments (Experiment II), anti-IgE stimulation appeared to downregulate the expression of mRNA transcript for trkB, but not trkA or trkC, in purified human mast cells (Fig 7). Indeed, prolonged exposure of the Southern blot did not show any detectable signal for trkB message (the lack of trkB signal did not appear to occur as a result of poor PCR amplification, as trkA and trkC mRNAs could be detected using the same Southern blot with the same PCR amplification reaction mixture) (Fig 7).
These experiments were performed primarily to determine whether transcripts for TrkA, TrkB, or TrkC receptor were detectable in purified preparations of normal human mast cells. However, our results also raise the possibility that anti-IgE stimulation of human lung mast cells can downregulate the cells' expression of trkB mRNA. Thus, the extent of specific histamine release (% release from anti-IgE-stimulated cells minus % release from cells without anti-IgE) detected in response to anti-IgE challenge was higher in Experiment II (∼18% specific release) than in Experiment I (no specific release). This result suggests that the level of mast cell activation achieved in the second experiment was substantially greater than that in Experiment I.
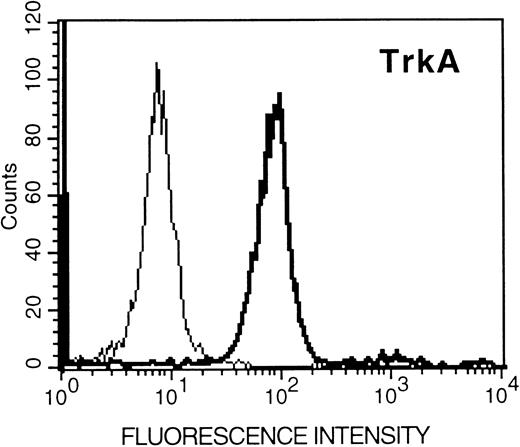
Human umbilical cord blood-derived mast cells express functional TrkA receptor protein and respond to NGF stimulation by exhibiting increased expression of chymase.To assess the physiologic relevance of the expression of functional TrkA receptor protein in HMC-1 cells, we examined the expression of TrkA protein in human umbilical cord blood-derived mast cells (of 90% purity by Kimura stain or immunocytochemistry for tryptase) using flow cytometry. As shown in Fig 8, specific staining of these human mast cells with the TrkA antibody could be detected, indicating that these cells indeed express TrkA protein.
Flow cytometric analysis (relative log fluorescence) of expression of TrkA protein in human umbilical cord blood-derived mast cells. Human umbilical cord blood-derived mast cells were incubated with an anti-TrkA and subsequently stained with fluorescein-conjugated goat antirabbit IgG and analyzed by flow cytometry. Data are representative of two experiments.
Flow cytometric analysis (relative log fluorescence) of expression of TrkA protein in human umbilical cord blood-derived mast cells. Human umbilical cord blood-derived mast cells were incubated with an anti-TrkA and subsequently stained with fluorescein-conjugated goat antirabbit IgG and analyzed by flow cytometry. Data are representative of two experiments.
To assess further whether these TrkA receptors were functional, we examined the ability of NGF, the cognate ligand of TrkA, to influence the expression of chymase in these mast cells. We performed this study because of two preliminary reports: one of them indicated that the tryptase activity in HMC-1 cells was significantly increased after these cells had been cultured in the presence of NGF,50 while the other report indicated that populations of human umbilical cord blood-derived mast cells, which had been cultured with NGF, contained more mast cells that expressed chymase, as well as tryptase, than did populations of control mast cells, which had been cultured without NGF.51 To evaluate whether NGF might affect chymase expression by human mast cells, we maintained human cord blood-derived mast cells that had been cultured in SCF, IL-6, and PGE2 for 19 to 20 weeks for an additional 3 weeks in medium containing SCF (80 ng/mL), IL-6 (50 ng/mL), and PGE2 (1 μmol/L) or SCF (80 ng/mL), PGE2 (1 μmol/L) and native or (as a control) boiled NGF at 500 ng/mL.
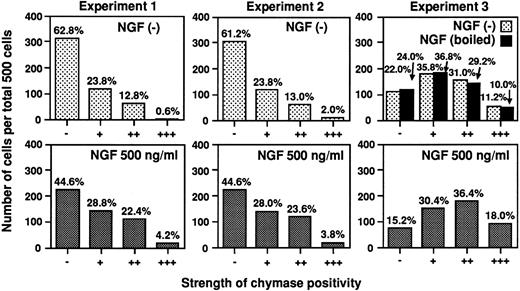
Figure 9 shows the data from three different experiments, all of which gave similar results. Photomicrographs depicting the immunohistochemistry results obtained in one of these experiments (Experiment 1 in Fig 9) are presented in Fig 10. As illustrated by data obtained from Experiment 1, NGF did not affect the total number of cells (1.2 × 106 cells in NGF (−) culture versus 1.1 × 106 cells in the NGF (+) culture) or the proportion of mast cells in the culture (% of tryptase-positive cells (ie, mast cells) in the NGF (−) culture was 99.8% versus 99.8% in the NGF (+) culture). However, immunocytochemical staining showed that the number of chymase-positive mast cells was increased by the NGF treatment (% of chymase-positive cells in the NGF (−) culture was 37.2% versus 55.4% in the NGF (+) culture) (Fig 9, Experiment 1). Moreover, in each of the three experiments, a significantly higher level of chymase expression was detected in the mast cells cultured with NGF, as opposed to those maintained in the absence of NGF (P < .05−.0001, χ2 test). However, NGF treatment did not appear to alter significantly the histamine content in these populations of human umbilical cord blood-derived mast cells [NGF(−) cultures: 12.2 ± 1.1 pg/cell, n = 3; NGF (+) cultures: 11.6 ± 3.3 pg/cell, n = 3].
Human umbilical cord blood-derived mast cells cultured with NGF exhibit increased expression of chymase. Human umbilical cord blood-derived mast cells were cultured in SCF, IL-6, and PGE2 for 19 or 20 weeks and were maintained for an additional 3 weeks in medium with or without NGF at 500 ng/mL (see text). Immunocytochemical analysis of the expression of chymase in these mast cells was performed as described in the Materials and Methods. The strength of chymase positivity was graded from negative (−) to strongly positive (+++) (as illustrated in Fig 10), and the distribution of mast cells exhibiting various intensities of chymase positivity in individual specimen was compared using the χ2 test. In each of the three independent experiments shown, the difference between the distribution of cells exhibiting various degrees of chymase positivity in cultures with or without exogenous NGF was significant (P < .0001, P = .0014, and P = .046 in Experiments 1, 2, and 3, respectively). In Experiment 3, we found that NGF which had been boiled (black bars in Experiment 3), when used at 500 ng/mL instead of native NGF in medium containing SCF and PGE2 , had no detectable effect on chymase immunoreactivity (P = .93 v values for cells cultured without NGF in medium containing SCF, IL-6 and PGE2 , P < .01 v values for cells cultured with native NGF ).
Human umbilical cord blood-derived mast cells cultured with NGF exhibit increased expression of chymase. Human umbilical cord blood-derived mast cells were cultured in SCF, IL-6, and PGE2 for 19 or 20 weeks and were maintained for an additional 3 weeks in medium with or without NGF at 500 ng/mL (see text). Immunocytochemical analysis of the expression of chymase in these mast cells was performed as described in the Materials and Methods. The strength of chymase positivity was graded from negative (−) to strongly positive (+++) (as illustrated in Fig 10), and the distribution of mast cells exhibiting various intensities of chymase positivity in individual specimen was compared using the χ2 test. In each of the three independent experiments shown, the difference between the distribution of cells exhibiting various degrees of chymase positivity in cultures with or without exogenous NGF was significant (P < .0001, P = .0014, and P = .046 in Experiments 1, 2, and 3, respectively). In Experiment 3, we found that NGF which had been boiled (black bars in Experiment 3), when used at 500 ng/mL instead of native NGF in medium containing SCF and PGE2 , had no detectable effect on chymase immunoreactivity (P = .93 v values for cells cultured without NGF in medium containing SCF, IL-6 and PGE2 , P < .01 v values for cells cultured with native NGF ).
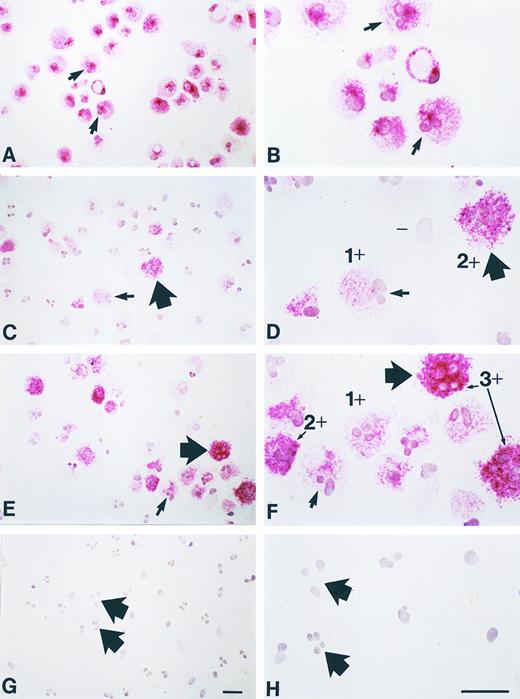
Long-term incubation with NGF enhances chymase immunoreactivity in human umbilical cord blood-derived mast cells. Human umbilical cord blood-derived mast cells (19 weeks after start of culture; < 99% purity by tryptase immunostaining) were maintained for an additional 3 weeks in culture with or without purified NGF at 500 ng/mL (see text). At the start of culture, tryptase immunoreactivity (red reaction product) is detectable in essentially all of the mast cells (A, B). (B) is a higher magnification view of some of the same cells shown in (A); the arrows in (A and B) depict the same two cells, both of which appear to have bilobed nuclei (a common finding in such in vitro-derived mast cells). (C and D) Mast cells that were maintained for an additional 3 weeks without NGF exhibit a range of chymase immunoreactivity (red reaction product), from none (eg, “-” in D), to 1 + or 2 + in intensity (examples depicted in D). (D) is a higher magnification view of some of the same cells shown in (C), some of which have bilobed (small arrow) or multilobed (thick arrow) configurations. (E and F ) Mast cells that were maintained for an additional 3 weeks with NGF exhibit increased chymase immunoreactivity (red reaction product), with some cells of 3 + staining intensity, as well as those of 1 + or 2 + intensity (examples indicated in F ). (F ) is a higher magnification view of some of the same cells as in (E), some of which have bilobed (small arrow) or multilobed (thick arrow) nuclear configurations. (G and H) Negative control (a mouse monoclonal IgG1K antibody of irrelevant antigen specificity, rather than either the mouse monoclonal IgG1K antitryptase or antichymase antibody, was used as the first antibody) showing lack of nonspecific immunoreactivity in these mast cells (these were aliquots of the same cell population depicted in C and D). (H) is a higher magnification view of some of the same cells shown in (G), some of which have multilobed nuclei (thick arrows), a common finding in such in vitro-derived mast cells. These cells are from Experiment 1 in Fig 9. Scale bars in G (for A, C, E and G) and H (for B, D, F, and H) represent 10 μm.
Long-term incubation with NGF enhances chymase immunoreactivity in human umbilical cord blood-derived mast cells. Human umbilical cord blood-derived mast cells (19 weeks after start of culture; < 99% purity by tryptase immunostaining) were maintained for an additional 3 weeks in culture with or without purified NGF at 500 ng/mL (see text). At the start of culture, tryptase immunoreactivity (red reaction product) is detectable in essentially all of the mast cells (A, B). (B) is a higher magnification view of some of the same cells shown in (A); the arrows in (A and B) depict the same two cells, both of which appear to have bilobed nuclei (a common finding in such in vitro-derived mast cells). (C and D) Mast cells that were maintained for an additional 3 weeks without NGF exhibit a range of chymase immunoreactivity (red reaction product), from none (eg, “-” in D), to 1 + or 2 + in intensity (examples depicted in D). (D) is a higher magnification view of some of the same cells shown in (C), some of which have bilobed (small arrow) or multilobed (thick arrow) configurations. (E and F ) Mast cells that were maintained for an additional 3 weeks with NGF exhibit increased chymase immunoreactivity (red reaction product), with some cells of 3 + staining intensity, as well as those of 1 + or 2 + intensity (examples indicated in F ). (F ) is a higher magnification view of some of the same cells as in (E), some of which have bilobed (small arrow) or multilobed (thick arrow) nuclear configurations. (G and H) Negative control (a mouse monoclonal IgG1K antibody of irrelevant antigen specificity, rather than either the mouse monoclonal IgG1K antitryptase or antichymase antibody, was used as the first antibody) showing lack of nonspecific immunoreactivity in these mast cells (these were aliquots of the same cell population depicted in C and D). (H) is a higher magnification view of some of the same cells shown in (G), some of which have multilobed nuclei (thick arrows), a common finding in such in vitro-derived mast cells. These cells are from Experiment 1 in Fig 9. Scale bars in G (for A, C, E and G) and H (for B, D, F, and H) represent 10 μm.
According to the manufacturer, the purified NGF used in our studies contained <0.0002 ng of lipopolysaccharide (LPS)/mg of NGF (Dr Lüsa Eisenlohr, Upstate Biotechnology, personal communication, January 1997), and our FCS preparations contained <0.005 ng of LPS/mL, according to a Limulus amebocyte assay (LAL assay; Whitaker Bioproducts, Inc, Walkersville, MD). Thus, when used at 500 ng/mL, these NGF preparations would have contributed little, if any, LPS to the culture medium. Accordingly, it is very unlikely that our results were affected by whatever small amounts of LPS might have been present in the NGF preparations. Nevertheless, in two experiments, we compared the expression of chymase immunoreactivity in mast cells cultured for 3 weeks in SCF, PGE2 , and either native NGF or NGF, which had been boiled for 1 hour (each at 500 ng/mL). We found that boiled NGF, in contrast to native NGF, had no detectable effect on the overall number of chymase-positive mast cells in cultures containing SCF and PGE2 or on the intensity of chymase expression in the mast cell population (see black bars in Experiment 3 in Fig 9). This experiment showed that essentially identical levels of chymase immunoreactivity were expressed by mast cells cultured for 3 weeks in SCF, IL-6, and PGE2 or SCF, PGE2 , and boiled NGF (P = .93). Similar results were obtained in the second experiment, in which mast cells that had been cultured for 3 weeks in SCF, PGE2 , and native NGF expressed more chymase immunoreactivity than did aliquots of the same mast cells that had been maintained for 3 weeks in SCF, PGE2 , and boiled NGF (P < .05 by χ2 test, data not shown).
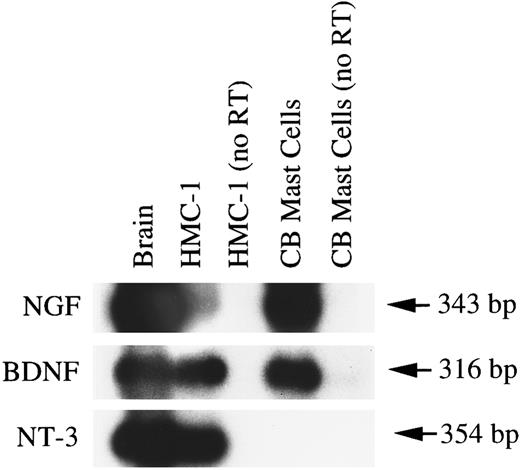
HMC-1 cells and human umbilical cord blood-derived mast cells express mRNA transcripts for NGF and other neurotrophins.As shown in Fig 9, different preparations of umbilical blood-derived human mast cells expressed, in the absence of treatment with exogenous NGF, variable proportions of cells that exhibited detectable immunoreactivity for chymase. A previous report showed the expression of mRNA for NGF in rat PMCs,19 raising the possibility that mast cells themselves might represent a potential source of NGF. To assess whether human mast cells could also express NGF mRNA and to determine whether these cells might express mRNAs for other members of the neurotrophin family, we performed RT-PCR and Southern analysis of DNase Itreated RNAs derived from HMC-1 cells and human cord blood-derived mast cells.
As shown in Fig 11 (representative of two experiments that gave similar results), positive hybridization signals of the predicted sizes for the mRNA expression of NGF (343 bp), BDNF (316 bp), and NT-3 (354 bp) could be detected in HMC-1 cells and in specimens of human brain. By contrast, human umbilical cord blood-derived mast cells appeared to express only the transcripts of NGF and BDNF, but not NT-3. However, when the DNase I-treated RNAs from HMC-1 cells and from human umbilical cord blood-derived mast cells were amplified by PCR without the reverse transcription step (as a negative control for genomic DNA contamination), no detectable hybridization signals could be detected for the three neurotrophins (Fig 11). Furthermore, the DNA fragment that was amplified by PCR using the HMC-1 RNA and the BDNF specific primers was subcloned and sequenced and shown to contain sequence identical to that reported for human BDNF cDNA.39
Expression of mRNA transcripts for NGF, BDNF, and NT-3 in human brain, HMC-1 cells, and human umbilical cord blood-derived mast cells (99% purity according to staining for tryptase). Poly A+ mRNA (human brain) or total RNA treated with DNase I (for both HMC-1 cells and human umbilical cord blood-derived mast cells) and heparinase I (for human umbilical cord blood-derived mast cells only) was reverse transcribed. The cDNAs were amplified using specific human NGF primers (T and U), BDNF primers (V and W), and NT-3 primers (X and Y). As a negative control for genomic DNA contamination, DNase I-treated RNAs from HMC-1 cells and from human umbilical cord blood-derived mast cells were amplified by PCR without the reverse transcription procedure (as shown in lanes labeled as “no RT”). The resulting PCR products were analyzed by Southern hybridization using 32P-labeled cDNA probes.
Expression of mRNA transcripts for NGF, BDNF, and NT-3 in human brain, HMC-1 cells, and human umbilical cord blood-derived mast cells (99% purity according to staining for tryptase). Poly A+ mRNA (human brain) or total RNA treated with DNase I (for both HMC-1 cells and human umbilical cord blood-derived mast cells) and heparinase I (for human umbilical cord blood-derived mast cells only) was reverse transcribed. The cDNAs were amplified using specific human NGF primers (T and U), BDNF primers (V and W), and NT-3 primers (X and Y). As a negative control for genomic DNA contamination, DNase I-treated RNAs from HMC-1 cells and from human umbilical cord blood-derived mast cells were amplified by PCR without the reverse transcription procedure (as shown in lanes labeled as “no RT”). The resulting PCR products were analyzed by Southern hybridization using 32P-labeled cDNA probes.
DISCUSSION
The HMC-1 cell line, which possesses many properties of immature mast cells,23,24 has proven to be a useful model for studying the molecular events underlying many cellular responses in human mast cells.52-54 In this study, we have characterized in detail, at the molecular level, the various types and isoforms of neurotrophin receptors expressed by HMC-1 cells. Our data show that HMC-1 cells express both the gene transcripts and the proteins for the TrkA, TrkB, and TrkC receptor tyrosine kinases, but lack detectable mRNA expression for the p75 low-affinity neurotrophin receptor. The TrkA receptors detected in HMC-1 cells are functional, as demonstrated by the ability of NGF, the cognate ligand of TrkA, to initiate appropriate cellular signaling responses in these cells. Thus, NGF stimulation of HMC-1 cells was shown to induce tyrosine autophosphorylation of TrkA, increased expression of early response genes, and activation of MAP kinase activity.
It is currently quite difficult to obtain normal human mast cells in large numbers. Accordingly, the full extent to which our observations with HMC-1 cells are relevant to normal human mast cells can only be established by further studies. However, we found that highly purified populations of both cultured umbilical cord blood-derived human mast cells and mast cells isolated from adult human lung tissue predominantly express trkA mRNA, and that human umbilical cord blood-derived mast cells also express TrkA protein. Moreover, the TrkA receptors expressed in human umbilical cord blood-derived mast cells appear to mediate at least one potentially physiological response to NGF stimulation, the increased expression of chymase.
In contrast to mRNA for trkA, the expression of trkB and trkC mRNAs in HMC-1 cells (and in populations of purified human lung mast cells) was unexpected, particularly in light of recent studies indicating a lack of expression of these trk mRNAs in either rat PMCs or mouse mast cells derived in IL-3–containing media.13 22 Indeed, of all of the hematopoietic cells examined to date, human mast cells, including the HMC-1 cell line and populations of mature lung mast cells, appear to be the only cell type that can express all three trk gene transcripts. However, unlike preparations of human lung mast cells, populations of human umbilical cord blood-derived mast cells, which have features of immature mast cells, express transcripts for trkA and trkC, but not trkB. These findings indicate that populations of human mast cells can express all three Trk receptors, at least at the level of mRNA, but that the patterns of expression of the three receptors in individual human mast cell populations (eg, lung v in vitro–derived cord blood mast cells) may vary.
Furthermore, we found that HMC-1 cells express mRNAs encoding the truncated forms of human TrkB (TrkB.T1) and TrkC receptors, as well as mRNAs for the full-length, tyrosine kinase-containing, forms.35 While the exact function(s) of these truncated TrkB and TrkC receptors have not been fully determined, there is speculation that they may act as “scavenger receptors” to maintain high levels of neurotrophins at the cell surface, or may initiate distinct cellular responses through ligand-dependent interactions with downstream signaling elements.55
Our findings indicate that there may be significant differences in the expression of neurotrophin receptors by mast cells of different species, as well as by different mast cell populations in the same species. Thus, recent studies show that rat PMCs can express transcripts for both the TrkAI and TrkAII isoforms,18 whereas we report here that the HMC-1 cell line expresses solely transcripts for the shorter TrkAI isoform that lack the 18 nucleotide insert.42,43 In addition, the p75 transcript is detectable in cultured mouse mast cells,22 but in neither rat PMCs14,18 nor HMC-1 cells (this study). Taken together, these findings and all of our other data are consistent with the suggestion of Jippo et al22 that the patterns of expression of neurotrophin receptors by mast cells can be quite heterogeneous, depending both on the cells' stage of development and species of origin.
Despite the apparent heterogeneity of neurotrophin receptor expression in mouse and human mast cells, the interaction of NGF with its receptors on different populations of mast cells appears to be consistently of the low-affinity type. An earlier report indicated that NGF binds to its receptor on rat PMCs with a KD that is consistent with low-affinity binding (4.0 × 10−9 mol/L).56 Similarly, a recent study shows that the NGF binding detected in cultured mouse mast cells also exhibits a low-affinity (2.1 × 10−9 mol/L).22 The dose response curves for NGF effects on serotonin release and on cell survival in rat PMCs are also consistent with a low-affinity interaction,13,18 although one recent apparently conflicting report has indicated that NGF can promote survival of rat PMCs via a high-affinity NGF receptor.14 In accord with most of these observations, we found that the doses of NGF required to induce the expression of early response genes in HMC-1 cells are consistent with a low-affinity type of interaction between NGF and the NGF receptors expressed in these cells.
The TrkA receptor tyrosine kinase expressed by HMC-1 cells appears to be functional, as NGF stimulation of these cells elicited signaling responses that are characteristic of NGF/TrkA interaction in other NGF-responsive cell types. These responses include tyrosine phosphorylation of TrkA, the induction of early response gene expression, and the activation of MAP kinase activity. However, we found that the kinetics of MAP kinase activation and expression of early response genes in HMC-1 cells stimulated with NGF were more transient than those observed with other cell types. In HMC-1 cells, these two NGF-induced signaling responses appeared to dissipate completely by 1 hour after stimulation (Figs 5 and 6), whereas both MAP kinase activity and the expression of c-fos remained elevated 1 hour after NGF-stimulation in PC12 cells and human B lymphocytes.57,58 Very similar transient kinetics of MAP kinase activation and early response gene expression were exhibited in rat PMCs that were treated with NGF,13 as well as in mouse bone marrow-derived cultured mast cells, which had been activated via c-kit (with SCF ) or FcεRI (with IgE and specific antigen).40,59 These findings are consistent with the widely held view that distinct patterns or kinetics of activation of common signaling elements may be used by different cell types to achieve specificity in their cellular responses to ligand-dependent stimulation of Trk receptors.46 In particular, it has recently been suggested that variation in the duration of MAP kinase activation may represent a mechanism that can elicit differential cellular responses to stimulation via receptor tyrosine kinases.60
Previous studies have indicated that NGF can promote the maturation of mouse bone marrow-derived cultured mast cells in vitro in the presence of IL-3.12 We show in this study, in confirmation of results reported by Igarashi et al51 in abstract form that NGF can enhance the expression of the cytoplasmic granule-associated protease, chymase, in human umbilical cord blood-derived mast cells cultured in the presence of SCF. Previously, Furitsu et al61 reported that factors derived from mouse 3T3 fibroblasts can induce the mast cell precursors that are present in the human umbilical cord blood mononuclear cell preparations to develop predominantly into mast cells that contain both tryptase and chymase.61 Based on this characteristic, such in vitro-derived mast cells resemble the mature mast cells that are present in human dermis and certain other sites.62 On the other hand, the human cord blood-derived mast cells, which are generated in vitro in the absence of 3T3 cells, but in the presence of SCF, exhibit many phenotypic characteristics of immature mast cells, including a predominantly tryptase+ chymase− or low phenotype.27 The ability of NGF to enhance the expression of chymase in umbilical cord blood-derived human mast cells thus provides evidence that NGF may represent one of the microenvironmental factors, which can synergize with SCF in promoting at least one of the phenotypic changes (ie, increased chymase expression) that is associated with the maturation/differentiation of certain populations of human mast cells in vivo. On the other hand, treatment with NGF did not significantly increase the cells' histamine content. This result suggests that NGF may have different effects on different aspects of human mast cell phenotype.
It has been proposed that there are complex and bidirectional, developmental, and functional interactions between mast cells and the nervous system.4-6 Our finding that various populations of human mast cells can express TrkA and TrkC, or Trk A, TrkB and TrkC, offers further support for this hypothesis. Moreover, a recent report showed that rat PMCs, which express TrkA receptors, can synthesize, store, and spontaneously release biologically active NGF.19 In the present study, we found that HMC-1 mast cells and cultured human umbilical cord blood-derived mast cells can express the gene transcript for BDNF, as well as NGF mRNA. Moreover, HMC-1 cells also express mRNA for NT-3. While the extent to which these findings pertain to other types of normal human mast cells and the extent to which human mast cells can produce and secrete neurotrophins, remain to be determined, our results raise the possibility that mast cell-derived neurotrophins in addition to NGF may mediate autocrine and/or paracrine effects on mast cell development and function.
ACKNOWLEDGMENT
We thank Drs Claudia Cabral and Chris S. Lantz for assistance with the flow cytometry, Dr Jürg Boesiger for analysis of the histamine content of human umbilical cord blood-derived mast cells, and Dr Keith Langley of AMGEN Inc for recombinant human SCF and IL-6 and the SR-1 antibody.
Supported in part by United States Public Health Service Grants No. CA/AI-72074, AI/CA-23990, and HL-56383 (Project 4) (to S.J.G.) and by the Beth Israel Hospital Pathology Foundation (Boston, MA).
Address reprint requests to See-Ying Tam, PhD, Department of Pathology, Research North, Beth Israel Deaconess Medical Center-East, 330 Brookline Ave, Boston, MA 02215.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal