Abstract
Several recent studies have shown that dendritic cells (DC) pulsed with soluble proteins can present peptide epitopes derived from these exogenous antigens on major histocompatability complex (MHC) class I molecules and induce an antigen-specific cytotoxic T lymphocyte (CTL) response. We provide evidence here that DC use macropinocytosis to capture soluble antigens that are then presented on MHC class I molecules. The presentation of an epitope derived from soluble ovalbumin was transporter associated with antigen presentation (TAP)-dependent, brefeldin A-sensitive, blocked by inhibitors of proteasomes, and resistant to chloroquine. These data suggest that exogenous antigens access the cytosol of DC and are proccessed for presentation via the same pathway described for conventional MHC class I-restricted cytosolic antigens. Proinflammatory mediators such as tumor necrosis factor-α (TNF-α) and lipopolysaccharide (LPS) reduced the efficiency of ovalbumin presentation via this pathway. This reduced presentation was not due to impaired expression of class I molecules because these substances upregulated the cell surface expression of Kb-molecules comparable to levels induced by interferon-γ (IFN-γ) treatment. The addition of IFN-γ increased ovalbumin presentation even in the presence of TNF-α or LPS. These results show that DC might be involved in the cross-priming phenomenon. This could offer the immune system an additional pathway for effective priming of cytotoxic T cells and provide the possibility to activate both CD4 and CD8 T-cell responses.
THE EXISTENCE OF separate processing pathways for presentation of exogenous and endogenous antigens provided a suitable model for understanding how major histocompatability complex (MHC) class II-restricted CD4+ helper T-cell responses are generated against extracellular antigens while MHC class I-restricted CD8+ cytotoxic T-cell responses are directed against cytosolic antigens.1,2 Exogenous antigens are internalized by antigen-presenting cells (APC), degraded in vesicular intracellular compartments, and loaded on MHC class II molecules in a post-Golgi compartment. In contrast, peptides derived from cytosolic antigens by the action of proteosomes are transported into the endoplasmic reticulum (ER) lumen by an adenosine triphosphate-dependent transporter associated with antigen presentation (TAP). In the ER lumen, a chaperone-mediated assembly generates a stable complex containing MHC class I heavy chain, β2-microglobulin, and an antigenic peptide. This complex trafficks to the cell surface, where it can be recognized by CD8+ T cells.1-4 Recently, this strict dichotomy was challenged by several studies that have shown that peptides generated from exogenous proteins can gain access to the cytosol and therefore be presented on class I MHC molecules.3-9
In vitro studies have shown that macrophages present class I-restricted peptides after endocytosis of particulate or soluble proteins by phagocytosis or macropinocytosis,5-9 but other bone marrow-derived APC (such as dendritic cells [DC]) may be involved in the cross-priming phenomenon seen in vivo.10-15DC are professional APC that can stimulate naive resting T cells and initiate primary T-cell responses when pulsed with antigenic peptides or proteins.16-27 They arise in bone marrow and migrate to peripheral tissues, where they can be found in an immature or unactivated form characterized by their ability to take up environmental antigens and present peptides from these antigens on MHC molecules. Activation of DC by inflammatory mediators results in reduction of endocytotic capacity and upregulation of MHC and costimulatory molecules. These activated DC are thought to migrate to the secondary lymphoid organs, where they present previously captured antigens and initiate immune responses.28-30
Therefore, DC may play an important role in cross-priming during induction of primary CD8-mediated responses against soluble antigens. It was recently shown that DC can take up exogenous proteins in vitro and induce an antigen-specific cytotoxic T lymphocyte (CTL) response.26 27 We analyzed the pathway by which DC take up, process, and present soluble antigens on MHC class I molecules and show that exogenous antigens access the cytosol of DC and are proccessed for presentation via the same pathway described for conventional MHC class I-restricted cytosolic antigens.
MATERIALS AND METHODS
Animals.Adult female C57BL/6 mice (H-2b) were obtained from The Jackson Laboratory (Bar Harbor, ME) and used at 6 to 8 weeks of age. TAP 1-deficient mice were obtained from Anton Berns (The Netherlands Cancer Institute, Amsterdam, The Netherlands).
Cell lines.EL-4 cells (C57BL/6, H-2b thymoma) were grown in RP10 media (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/L L-glutamine, and antibiotics). EG.7 is an EL-4 cell line transfected with the full-length ovalbumin cDNA.31 Transfectants were maintained in RP10 containing G418 at 0.4 mg/mL. B3 is a H-2Kb–restricted OVA 257-264 -peptide (SIINFEKL) specific CTL clone.32
Monoclonal antibodies.The following monoclonal antibodies were used: Y-3 (anti-H-2Kb; American Type Culture Collection, Rockville, MD), 3.168.8 (anti-CD8; PharMingen, San Diego, CA), H129.19 (anti-CD4; PharMingen), 145-2C11 (anti–CD3-ε; PharMingen), H9.2B8 (anti-CD51, αv-integrins; PharMingen), 1G10 (anti-CD80; PharMingen), GL1 (anti-CD86; PharMingen), RA3-6B2 (anti-CD45R/B220; PharMingen), Y-3P (anti-I-Ab; kindly provided by Dr Alexander Y. Rudensky, University of Washington, Seattle, WA), and N418 (anti-CD11c; which has been described previously33 ).
Peptides and reagents.The Kb-binding peptide OVA 257-264 (SIINFEKL) was synthesized using an Applied Biosystems Synergy peptide synthesizer (Foster City, CA) and analyzed by high-performance liquid chromatography. Peptide concentrations were determined using BCA assay (Pierce Chemical Co, Rockford, IL). Granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/mL), tumor necrosis factor-α (TNF-α; 50 ng/mL), interleukin-12 (IL-12; 50 ng/mL), IL-7 (50 ng/mL), IL-6 (100 ng/mL), and IL-4 (20 ng/mL) were purchased from R&D Systems (Minneapolis, MN). IFN-γ (100 U/mL) was from Genzyme (Cambridge, MA). All other reagents were obtained from Sigma (St Louis, MO). Lipopolysaccharide (LPS) was used at 10 μg/mL, brefeldin A was used at 5 μg/mL, and Chloroquine, LLnL (N-acetyl-L-leucinyl-L-leucinyl-L-norleucinal), and its methional analogue LLM were used at 50 μmol/L. Flt3 ligand (100 ng/mL) was provided by K. Brasel (Immunex Corp, Seattle, WA).
CTL assay.The standard 51Cr-release assay was performed as described.34 Target cells were pulsed with 1 μmol/L peptide for 1 hour or with 2 mg/mL ovalbumin overnight and labeled with [51Cr]-sodium chromate in RP10 for 1 hour at 37°C. Ten thousand cells were transferred to a well of a round-bottomed 96-well plate. Varying numbers of CTL were added to give a final volume of 200 μL and incubated for 4 hours at 37°C. At the end of the assay, the plates were centrifuged, and supernatants (100 μL/well) were harvested and counted in a gamma counter. The percentage of specific lysis was calculated as: 100 × (Experimental Release − Spontaneous Release/Maximal Release − Spontaneous Release). Spontaneous and maximal release were determined in the presence of either medium or 1% Triton X-100, respectively.
Generation of CTL in vivo.For the generation of primary polyclonal CD8+ CTL in vivo, C57BL/6 mice were immunized by intraperitoneal injection of 4 × 105 protein-pulsed DC in 200 μL saline on days 0 and 7. For each group, 5 mice were used. Seven days later, 50 × 106 pooled splenocytes were restimulated in vitro with 10 μmol/L OVA-peptide in RP10 media and tested for cytotoxicity after 5 days.
Preparation of DC and macrophages from spleen and bone marrow.Isolation of DC from spleen and bone marrow was performed as described previously. For enrichment of splenic DC (sDC) by plastic adherence, light-density cells were selected from spleen cell suspension by bovine serum albumin density centrifugation.35 After 3 hours of incubation, nonadherent cells were removed by gentle pipetting and the adherent cells were cultured overnight. Nonadherent cells were used as a source for DC. The remaining adherent cells were used as macrophages. Fluorescence-activated cell sorting analysis was performed to evaluate the purity of the DC fraction by staining the cells with anti-CD11c (N418). The enriched cells contained 74% to 86% DC, 6% to 12% CD3+, and 14% to 22% B220+ cells. Bone marrow DC (bmDC) were generated as reported by Inaba et al36 and used after 7 days. Seventy percent to 90% of the cells were CD11c+. Macrophages from bone marrow were grown as previously described.37
RESULTS
Presentation of soluble proteins via MHC class I is TAP dependent.To test the capacity of isolated DC and macrophages to present exogenous proteins, these cells were incubated with soluble ovalbumin (2 mg/mL) for 16 hours to allow antigen processing. The cells were washed and used as targets in a standard Cr-release assay. As shown in Fig 1, the ability of bmDC to capture and present soluble OVA was slightly more potent as compared with sDC. But isolated DC (splenic or bone marrow derived) were more eficient than macrophages. No differences in MHC class I presentation of soluble ovalbumin were observed between splenic and bone marrow macrophages.
Presentation of exogenous soluble ovalbumin on MHC class I molecules by DC and macrophages. DC (bmDC and sDC) and macrophages (bmM and sMac) isolated from bone marrow or spleens of C57BL/6 and TAP1° mice were incubated for 16 hours with 2 mg/mL ovalbumin or pulsed for 2 hours with synthetic OVA peptide (1 μmol/L) and used as targets in a standard 51Cr-release assay.
Presentation of exogenous soluble ovalbumin on MHC class I molecules by DC and macrophages. DC (bmDC and sDC) and macrophages (bmM and sMac) isolated from bone marrow or spleens of C57BL/6 and TAP1° mice were incubated for 16 hours with 2 mg/mL ovalbumin or pulsed for 2 hours with synthetic OVA peptide (1 μmol/L) and used as targets in a standard 51Cr-release assay.
To analyze whether a functional TAP transporter was required for loading of MHC class I molecules, macrophages and DC were isolated from the bone marrow of TAP1-deficient mice (TAP−/−). As described above, cells were pulsed overnight with soluble ovalbumin and the presentation of the OVA-peptide was analyzed in a 4-hour Cr-release assay. Macrophages and DC from TAP−/− mice were not lysed by an OVA-specific CTL clone B3 (Fig 1E and F ). However, these cells were able to present the synthetic OVA-peptide when it was added exogenously, showing that class I molecules are present on the surface of these cells. Together, these data sugest that a functional TAP is neccessary for presentation of peptides generated from soluble exogenous proteins.
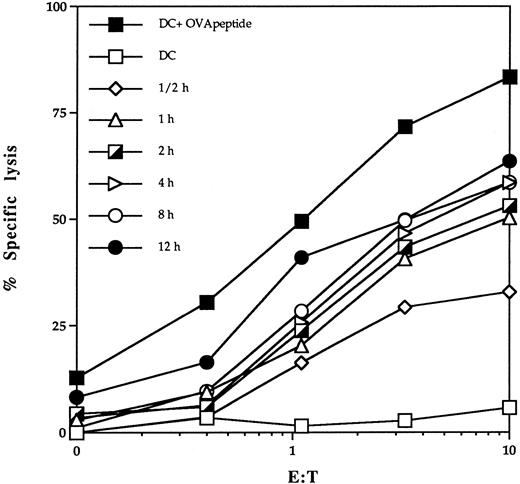
MHC class I pathway of soluble antigen presentation by DC.To analyze the pathway of MHC class I presentation of exogenous proteins, we used DC isolated from bone marrow of C57BL/6 mice. To determine the kinetics of ovalbumin presentation, bmDC were incubated with soluble protein for different lengths of time and used as targets in a standard Cr-release assay. Presentation of OVA-peptide on the surface of bmDC could be detected already within 30 minutes of incubation with whole ovalbumin (Fig 2).
Kinetics of delivery of antigenic peptides derived from exogenous antigens into class I presentation pathway of DC. bmDC were cultured in the presence of 2 mg/mL ovalbumin and 20 ng/mL GM-CSF for different time periods. Lysis of bmDC pulsed with OVA protein by the OVA-specific CTL clone B3 was assessed in a standard 51Cr-release assay. DC pulsed with 1 μmol/L OVA peptide (DC + OVA peptide) or untreated DC (DC) were included as a control.
Kinetics of delivery of antigenic peptides derived from exogenous antigens into class I presentation pathway of DC. bmDC were cultured in the presence of 2 mg/mL ovalbumin and 20 ng/mL GM-CSF for different time periods. Lysis of bmDC pulsed with OVA protein by the OVA-specific CTL clone B3 was assessed in a standard 51Cr-release assay. DC pulsed with 1 μmol/L OVA peptide (DC + OVA peptide) or untreated DC (DC) were included as a control.
We suspected that DC might capture soluble proteins by macropinocytosis. Macropinocytosis is dependent on membrane ruffling, which is inhibitable by amiloride.30 38 To test this possibility, bmDC cells were incubated with amiloride for 30 minutes before the addition of soluble ovalbumin to the media. Amiloride treatment blocked presentation of OVA to B3 CTL, consistent with a role for macropinocytosis in antigen uptake (Table 1).
Pathway of MHC Class I Presentation of Soluble Antigens by DC
| Treatment* . | Antigen . | % Specific Lysis at E:T . | ||
|---|---|---|---|---|
| . | . | 10 . | 3 . | 1 . |
| — | — | 5 | 2 | 1 |
| OVA-peptide | 76 | 56 | 32 | |
| OVA-protein | 48 | 28 | 14 | |
| Amiloride | — | 7 | 3 | 0 |
| OVA-peptide | 70 | 52 | 28 | |
| OVA-protein | 12 | 4 | 0 | |
| Brefeldin A | — | 4 | 5 | 0 |
| OVA-peptide | 73 | 54 | 26 | |
| OVA-protein | 10 | 4 | 2 | |
| LLnL | — | 7 | 1 | 4 |
| OVA-peptide | 72 | 52 | 31 | |
| OVA-protein | 6 | 5 | 3 | |
| LLM | — | 6 | 1 | 0 |
| OVA-peptide | 78 | 54 | 29 | |
| OVA-protein | 31 | 15 | 6 | |
| Chloroquine | — | 5 | 0 | 2 |
| OVA-peptide | 69 | 49 | 26 | |
| OVA-protein | 47 | 30 | 10 | |
| Treatment* . | Antigen . | % Specific Lysis at E:T . | ||
|---|---|---|---|---|
| . | . | 10 . | 3 . | 1 . |
| — | — | 5 | 2 | 1 |
| OVA-peptide | 76 | 56 | 32 | |
| OVA-protein | 48 | 28 | 14 | |
| Amiloride | — | 7 | 3 | 0 |
| OVA-peptide | 70 | 52 | 28 | |
| OVA-protein | 12 | 4 | 0 | |
| Brefeldin A | — | 4 | 5 | 0 |
| OVA-peptide | 73 | 54 | 26 | |
| OVA-protein | 10 | 4 | 2 | |
| LLnL | — | 7 | 1 | 4 |
| OVA-peptide | 72 | 52 | 31 | |
| OVA-protein | 6 | 5 | 3 | |
| LLM | — | 6 | 1 | 0 |
| OVA-peptide | 78 | 54 | 29 | |
| OVA-protein | 31 | 15 | 6 | |
| Chloroquine | — | 5 | 0 | 2 |
| OVA-peptide | 69 | 49 | 26 | |
| OVA-protein | 47 | 30 | 10 | |
bmDC were incubated with 20 ng/mL GM-CSF and 2 mg/mL ovalbumin in the presence or absence of 50 μmol/L amiloride or 5 μg/mL brefeldin A or 50 μmol/L LLnL or LLM or chlorquine. Inhibitors were added to cell medium 30 minutes before incubation with ovalbumin and were present during the chase period of 3 hours. Lysis of bmDC pulsed with OVA peptide (1 μmol/L) or OVA protein (2 mg/mL) by OVA-specific CTL clone B3 was assessed in a standard 51Cr-release assay.
In the ER, peptides from cytosolic antigens bind to newly synthesized MHC class I molecules. These processed complexes are then transported to the cell surface for recognition by CD8+ CTL. Brefeldin A inhibits vesicular egress from the ER and Golgi complex and thus prevents the presentation of the peptide-MHC class I complexes. Treatment of DC with brefeldin A completely inhibited the presentation of exogenously added ovalbumin but had no effect on presentation of exogenously added synthetic peptide (Table 1). This provides further evidence that peptides processed from soluble ovalbumin are loaded on MHC class I molecules in the ER.
To analyze the involvement of the proteasome in the pathway of presentation of exogenous antigens, we tested LLnL (N-acetyl-L-leucinyl-L-leucinyl-L-norleucinal) and ist methional analogue LLM for their ability to block soluble ovalbumin presentation to the B3 CTL clone. Preincubation of cells with the proteasome inhibitor LLnL blocked the presentation of ovalbumin. However, the presence of LLM, an analogue of LLnL with lower potency against the proteasome, did not affect ovalbumin processing and presentation in this assay.
The addition of chloriquine, an agent that increases the pH of distal acidic vesicles and inhibits proteolysis in these compartments, had no effect on the MHC class I presentation of soluble ovalbumin, indicating that the peptides were produced in the nonlysosomal (probably cytosolic) compartment.
Influence of cytokines on MHC class I presentation of soluble antigens.It has been recently shown that activation of DC by inflammatory mediators such as IL-1, TNF-α, or LPS induces upregulation of costimulatory and MHC molecules and reduction of endocytosis.28-30 This results in an increased capacity of T-lymphocyte priming and lower ability of DC to capture and present soluble antigens on class II molecules.
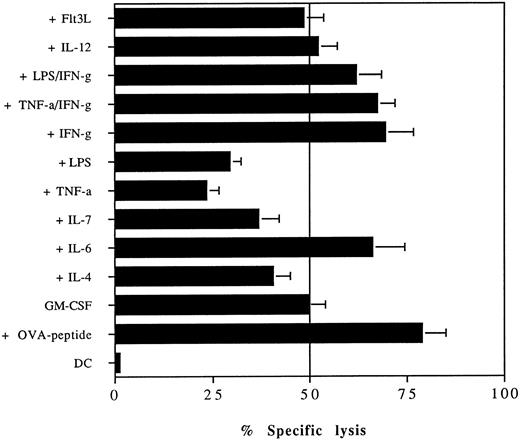
We therefore analyzed the influence of several cytokines on MHC class I presentation of soluble proteins. bmDC grown in 20 ng/mL GM-CSF were preincubated for 24 hours with the indicated cytokines before the addition of 2 mg/mL ovalbumin for 16 hours. After extensive washing, these cells were used as targets in a CTL assay (Fig 3). The presence of TNF-α or LPS in the medium reduced the ability of DC to capture and present soluble ovalbumin, consistent with previous studies on MHC class II presentation of soluble antigens showing that these cytokines inhibit the uptake and presentation of soluble MHC class II-restricted antigens. There was some inhibition of ovalbumin presentation by IL-7 and IL-4. IL-12 and Flt3 ligand (Flt3L) had no effect on presentation. The addition of IFN-γ or IL-6 increased the level of ovalbumin presentation. The presence of IFN-γ could also overcome the inhibitory effect mediated by LPS or TNF-α. The inhibition of ovalbumin presentation by LPS or TNF-α was not due to downregulation of MHC or costimulatory molecules because both cytokines increased the expression of B7.1, B7.2, and MHC class I molecules comparable to the levels induced by IFN-γ (data not shown).
The presentation of soluble antigens on MHC class I molecules by DC is altered by proinflammatory mediators. bmDC grown in media containing 20 ng/mL GM-CSF were cultured in the presence or absence of TNF-α (50 ng/mL), IL-12 (50 ng/mL), IL-7 (50 ng/mL), IL-6 (100 ng/mL), IL-4 (20 ng/mL), IFN-γ (100 U/mL), LPS (10 μg/mL), or Flt3L (100 ng/mL). After 24 hours of incubation, 2 mg/mL of soluble ovalbumin was added and 16 hours later the presentation of ovalbumin by bmDC to the OVA-specific B3 clone was analyzed in a standard 51Cr-release assay. DC pulsed with 1 μmol/L OVA peptide (+OVA peptide) or untreated DC (DC) were included as a control. CTL were added at an E:T ratio of 10:1. The assay was conducted in quadriplicates and error bars show the means and standard deviation.
The presentation of soluble antigens on MHC class I molecules by DC is altered by proinflammatory mediators. bmDC grown in media containing 20 ng/mL GM-CSF were cultured in the presence or absence of TNF-α (50 ng/mL), IL-12 (50 ng/mL), IL-7 (50 ng/mL), IL-6 (100 ng/mL), IL-4 (20 ng/mL), IFN-γ (100 U/mL), LPS (10 μg/mL), or Flt3L (100 ng/mL). After 24 hours of incubation, 2 mg/mL of soluble ovalbumin was added and 16 hours later the presentation of ovalbumin by bmDC to the OVA-specific B3 clone was analyzed in a standard 51Cr-release assay. DC pulsed with 1 μmol/L OVA peptide (+OVA peptide) or untreated DC (DC) were included as a control. CTL were added at an E:T ratio of 10:1. The assay was conducted in quadriplicates and error bars show the means and standard deviation.
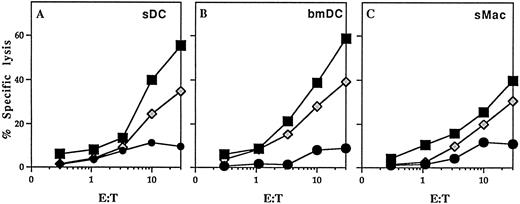
In vivo CTL induction using DC pulsed with soluble ovalbumin.To analyze the ability of DC pulsed with soluble ovalbumin to induce antigen-specific CTL, 4 × 105 bmDC in 200 μL saline were injected intraperitoneally on days 0 and 7 into C57BL/6 mice. Splenocytes from immunized mice were harvested 1 week after the second injection and stimulated with irradiated syngenic splenocytes pulsed with 10 μmol/L OVA peptide. After 5 days of culture, primed CTL were assayed for their ability to lyse syngenic EL-4 tumor cells pulsed with 1 μmol/L OVA-peptide (Fig 4). DC pulsed with soluble ovalbumin were able to prime an ovalbumin-specific CTL response more efficiently than bone marrow-derived macrophages. Interestingly, no differences in induction of antigen-specific CTL was observed when sDC or bmDC were used. However, it is unclear whether the induction of ovalbumin-specific CTL resulted from a direct priming by injected DC or from an indirect priming through the cross-priming phenomenon. Mice previously injected with phosphate-buffered saline or untreated DC did not elicit a measurable CTL response to OVA under the same conditions (data not shown).
In vivo induction of OVA-specific CTL by DC. DC isolated from bone marrow (bmDC) or spleens (sDC) and splenic macrophages (sMac) from C57BL/6 mice were pulsed with soluble ovalbumin and 4 × 105 DC were injected intraperitoneally on day 0 and 7 into C57BL/6 mice. Splenocytes from immunized mice were harvested on day 14 and stimulated with syngeneic splenocytes pulsed with 10 μmol/L OVA peptide. The primed CTL were then assayed for their ability to lyse E.G7 cells, transfectants expressing the OVA peptide, or EL-4 tumor cells either pulsed with 1 μmol/L OVA peptide or left unpulsed. Mice injected with saline or unpulsed DC gave no response. (▪) EL-4 + OVA peptide; (•) EL-4; (⋄) EG.7.
In vivo induction of OVA-specific CTL by DC. DC isolated from bone marrow (bmDC) or spleens (sDC) and splenic macrophages (sMac) from C57BL/6 mice were pulsed with soluble ovalbumin and 4 × 105 DC were injected intraperitoneally on day 0 and 7 into C57BL/6 mice. Splenocytes from immunized mice were harvested on day 14 and stimulated with syngeneic splenocytes pulsed with 10 μmol/L OVA peptide. The primed CTL were then assayed for their ability to lyse E.G7 cells, transfectants expressing the OVA peptide, or EL-4 tumor cells either pulsed with 1 μmol/L OVA peptide or left unpulsed. Mice injected with saline or unpulsed DC gave no response. (▪) EL-4 + OVA peptide; (•) EL-4; (⋄) EG.7.
DISCUSSION
DC are recognized as the most efficient professional APC for induction of primary immune responses. DC migrate from the bone marrow to peripheral tissues, where they can be found in an immature or inactivated form characterized by a high rate of uptake and processing of environmental antigens for presentation in the context of MHC molecules. Upon activation by proinflammatory mediators, DC loose their endocytotic capacity and are thought to migrate to the secondary lymphoid organs, where they present previously captured antigens to T cells and initiate immune responses.28
Recently, several studies showed that DC can present antigenic epitopes derived from exogenous antigens onto MHC class I molecules and induce an antigen-specific CTL response when pulsed with soluble proteins.26 27 We provide here evidence that DC use macropinocytosis to capture and load soluble antigens on MHC class I molecules. The presentation of exogenous ovalbumin was more efficient when bmDC were used as compared with sDC or macrophages. However, no differences in induction of antigen-specific CTL were observed when sDC or bmDC were used.
The pathway through which exogenous antigens are presented on MHC class I molecules is controversial. In addition to a cytosolic pathway in which the presentation of peptides derived from exogenous antigens can be inhibited by brefeldin A and a mutation that disrupts TAP,5,8 9 there is some evidence for a noncytosolic pathway, suggesting that the peptides are generated by proteolysis in the endocytic compartment.39-41The presentation of these antigens as demonstrated for bacteria-associated or Latex-bound ovalbumin and viral antigens was not blocked by brefeldin A or by a mutation in the TAP transporter.
We show that the presentation of soluble ovalbumin by DC was TAP-dependent, brefeldin A-sensitive, blocked by inhibitors of proteosomes, and resistant to chloroquine. These data suggest that macropinocytosed exogenous antigens access the cytosol of DC and then are processed and presented via the conventional pathway described for presentation of cytosolic proteins by MHC class I. This pathway resembles in vivo cross-priming and thus DC might be involved in this phenomenon.
It is believed that proinflammatory cytokines promote the maturation of DC. They reduce the ability of DC to capture antigens and increase the expression of MHC and costimulatory molecules. Sallusto et al30 demonstrated that cytokines such as TNF-α, IL-1, or LPS can regulate the function of DC by decreasing the formation of macropinosomes and intracellular MII vesicles. Recent studies performed in macrophages analyzing the regulation of presentation of exogenous antigens showed that LPS and IFN-γ can also modulate the capacity of bone marrow and peritoneal macrophages to present exogenous ovalbumin on MHC class I molecules.42
We show here that proinflammatory cytokines can also affect the class I presentation of soluble proteins by DC. Incubation of DC with TNF-α or LPS resulted in reduction of ovalbumin presentation. This was apparently not due to decreased expression of MHC class I molecules on cell surface and may reflect the effect of these stimuli on antigen uptake and processing because these cytokines upregulated the expression of Kb-molecules comparable to the effect mediated by IFN-γ. Interestingly, even in the prsence of these stimuli, the addition of IFN-γ to the cell cultures increased the ovalbumin presentation. This indicates that IFN-γ has a dominant effect on presentation of exogenous antigens by DC.
The involvement of DC in the cross-priming phenomenon could offer the immune system an additional pathway for an effective priming of cytotoxic T cells and provide the possibility to activate both CD4- and CD8-directed immune responses. Extensive studies performed in the past several years led to the identification of a number of genes encoding antigens recognized by tumor-reactive T cells.43 This has opened an opportunity to develop new anticancer therapies that have now begun to be evaluated in clinical trials. The use of DC pulsed with antigenic protein could provide an alternative approach to generate an effective T-cell response against tumors, especially when the immunodominant T-cell epitopes are not known.
ACKNOWLEDGMENT
The authors thank L.L. Lenz and W. Brugger for critical reading of the manuscript and helpful discussions.
Supported by the Howard Hughes Medical Institute. P.B. was supported by a fellowship from Deutsche Krebshilfe, Dr. Mildred-Scheel-Stiftung für Krebsforschung.
Address reprint requests to Michael J. Bevan, PhD, Howard Hughes Medical Institute, Department of Immunology, University of Washington, Box 357370, Seattle, WA 98195.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal