Abstract
CD4 molecules are the primary receptors for human immunodeficiency virus (HIV) and bind the envelope glycoprotein gp120 of HIV with high-affinity. We have previously shown that cross-linking of CD4 molecules (CD4XL) in normal peripheral blood mononuclear cells (PBMC) results in secretion of cytokines tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), but not of interleukin-2 (IL-2) or IL-4. To investigate the intracellular signaling events associated with CD4-gp120 interaction, we incubated CD4+ T cells from peripheral blood of HIV-negative healthy donors with HIV envelope protein gp160 alone or performed CD4XL with gp160 and anti-gp160 antibody. This procedure resulted in tyrosine phosphorylation of intracellular substrates p59fyn, zap 70, and p95vav and also led to ras activation, as assessed by conversion of rasGDP to rasGTP. The role of ras in CD4 signaling was further investigated using CD4+ Jurkat cells transfected with a dominant negative ras mutant. CD4+ T cells expressing dn-ras secreted significantly reduced levels of TNF-α in response to CD4XL. These studies indicate that interaction of HIV gp160 with CD4 molecules activates the ras pathway in T cells, which may result in the cells becoming unresponsive to subsequent stimulation.
TRIGGERING OF THE T-cell receptor (TCR) complex initiates a cascade of biochemical events resulting in the transcription of numerous genes leading to functional T-cell responses.1-3 CD4 molecules serve as coreceptors for TCR and act synergistically with TCR in T-cell activation.4 Ligation of antigen/major histocompatibility complex (MHC) with the TCR complex results in activation of protein tyrosine kinases (PTKs) including p56lck,5 which mediate tyrosine phosphorylation of several substrates including phospholipase C-γ1 (PLC-γ1), phosphatidylinositol 3-kinase (PI-3 kinase), p95vav, and p21ras.6-9 The rate limiting step in ras activation is the exchange of bound GDP to GTP, which leads to the formation of an active ras-GTP complex.10,11 Several candidate molecules including Grb2/sos,12,13 Crk/Cbl/C3G,14 and vav9 have been implicated in ras activation in T cells. Further downstream events of T-cell signaling include intracellular calcium mobilization,15 activation of protein kinase C (PKC), and transcriptional activation of several cytokines.
Intracellular signaling via CD4 molecules has been a subject of intense investgation in human immunodeficiency virus (HIV) disease pathogenesis. CD4, which is the primary receptor for HIV envelope glycoprotein, binds HIV envelope glycoprotein with strong affinity. Many laboratories including our own have shown that interaction with HIV-gp120 in vitro significantly influences CD4+ T cells rendering them unresponsive to subsequent TCR activation. In the absence of secondary stimulation, the gp120-CD4 interaction can result in induction of cytokines interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF ), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-6 (IL-6).16-18 Other reported effects of CD4 signaling include activation of p56lck,19-21 tyrosine phosphorylation of PI-3 and PI-4 kinases,22 and activation of raf-1.3 After CD4 ligation, the cells also upregulate expression of IL-2R and Fas (CD95) antigen, and if the CD4 receptor is cross-linked, they are primed to undergo apoptosis.18,23 24
The current study was aimed at elucidating further the intracellular signaling events resulting from CD4 ligation by HIV gp120. In particular, we investigated the role of ras, which is pivotal to several intracellular signaling pathways. We show that CD4 ligation with gp160 or with anti-CD4 antibody (Ab) with and without a second antibody as a means of CD4 cross-linking (CD4XL) results in similar patterns of tyrosine phosphorylation of various substrates, and that ras activation plays an essential role in the induction of the cytokine TNF-α.
MATERIALS AND METHODS
Envelope glycoproteins.HIV-1–derived gp160 and gp120 were purified from culture supernatants of a clone of HIV-infected Hut-78 cells, 6D4451 , as described earlier.25 Briefly, supernatant of cells grown in serum-free HB104 medium was concentrated and passed through a lentil-lectin Sepharose column (Pharmacia Biotech, Piscataway, NJ). Glycoproteins were eluted with 400 mmol/L methyl mannoside. gp160 was further purified by affinity chromatography over anti-HIV451 monoclonal antibody (MoAb)-Speharose 4B column. The envelope glycoprotein preparations were > 95% pure and were not contaminated with endotoxins, as tested by the Limulus amoebocyte lysate assay (E-TOXATE, Sigma Chemical Co, St Louis, MO).
Antibodies and reagents.The following reagents were used: MoAb to CD4 (Leu3a, IgG1; Becton Dickinson, Mountainview, CA); MoAb to CD3 (MoAb 454, IgG2a, gift from Dr N. Chiorazzi, North Shore University Hospital, Manhasset, NY); nonimmune mouse Ig (mIg; Chrompure IgG, Jackson ImmunoResearch, West Grove, PA); Herbimycin A (GIBCO-BRL, Grand Island, NY); antihuman phosphotyrosine (4G10), anti-p56lck, anti-PLC-γ1, anti-p59fyn, anti-ZAP-70, anti-p95vav antibodies (Upstate Biotechnology, Lake Placid, NY); anti-ras antibody Y13-259, rabbit antirat Ig antibody and Protein A-agarose (Oncogene Science Inc, Manhasset, NY); PEI-cellulose plates (Sigma); 32P-orthophosphate (NEN, DuPont, Boston, MA). The ras N17 dominant negative plasmid in the p1017 vector was a generous gift from Dr Roger Perlmutter (University of Washington, Seattle, WA).26
Cells.Peripheral blood mononuclear cells (PBMC) were isolated from healthy volunteers by Ficoll Hypaque (Lymphoprep, Nycomed, Oslo, Norway) density gradient centrifugation. T cells were enriched by double rosetting with neuraminidase-treated sheep red blood cells (SRBC). Adherent cells were removed by incubating cells in the dishes at 37°C for 2 hours in complete RPMI. CD4+ T cells were purified by negative selection with anti-CD8 MoAb coated magnetic beads (Dynal, Great Neck, NY) and were used in the cross-linking studies. A CD4 positive clone of Jurkat T cells, E6-1, obtained from the NIH AIDS Reference Reagent Program, Bethesda, MD, (donated by Dr A. Weiss27 ) was maintained in RPMI 1640 media supplemented with penicillin, streptomycin, and 10% fetal calf serum (FCS).
Cell activation and lysis.Purified CD4+ T cells were incubated with gp160 (5 μg/L × 106 cells) overnight at 4°C on a rotator. Cells were washed and incubated at 37°C for various time intervals. Reactions were terminated by washing cells in ice cold phosphate-buffered saline (PBS) + EDTA + sodium orthovanadate and lysed in lysis buffer (0.04 mol/L Tris-Hcl, 0.276 mol/L NaCl, 20% glycerol, 2% NP40, 0.002 mol/L sodium orthovanadate, 0.02 mol/L NaF, 10 μg/mL Aprotinin, 10 μg/mL Leupeptin, 0.004 mol/L EDTA, and 1 mmol/L phenylmethylsulfonyl fluoride [PMSF]). For CD4XL, cells were pretreated with gp160 overnight, washed, and incubated for an additional 30 minutes with goat anti-gp160 antibodies25 at 4°C, then exposed to 37°C for various time intervals. Reactions were terminated as described above. Cells were cross-linked with anti-CD3 MoAb and goat antimouse IgG (GAM), as a positive control for T-cell activation and tyrosine phosphorylation of various kinases.
Immunoprecipitation and immunoblotting.Cell lysates were precleared with Protein A-agarose and were incubated overnight at 4°C with appropriate antibodies as indicated on a rotator, mixed with second antibody-coated protein A-agarose beads, and allowed to precipitate at 4°C on a rotator overnight. The immunoprecipitates were washed extensively with lysis buffer, and pellets were boiled with sample buffer. Samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 4% to 20% gradient gels, followed by immunoblotting with either specific antibody or with antiphosphotyrosine antibody (4G10).
Biosynthetic labelling of cells with 32P orthophosphate to examine ras activation.Purified CD4+ T cells were labelled with 32P orthophosphate 400 mCi/mL (1 × 108 cells) in phosphate-free medium containing 10% dialyzed FCS for 4 hours at 37°C. Following labeling, cells were stimulated by cross-linking CD4 or CD3 molecules as described earlier. Ras proteins from the cell lysates of 32P-labeled, activated, or unstimulated CD4+ T cells were immunoprecipitated with anti-ras antibody Y13-259 precoupled to Protein A-agarose via rabbit antirat Ig. Immunoprecipitates were washed eight times using 1 mL of lysis buffer for each wash, and GDP and GTP nucleotides were eluted with elution buffer (2 mm EDTA, 2 mmol/L dithiothreitol and 0.2% SDS) at 68°C for 20 minutes. Separation of eluted nucleotides was performed on PEI-cellulose TLC plates, using the solvent (1.2 mol/L ammonium formate and 0.8 mol/L HCI). Labeled nucleotides separated by thin layer chromatography (TLC), were visualized by autoradiography and quantitated by densitometry.
Transient transfection of dominant negative rasN17.Jurkat T cells in 0.4 mL complete RPMI were equilibriated on ice and transfected by electroporation with 2 to 40 μg rasN17 or control vector, using a BRL electroporator (GIBCO-BRL). Cells were kept on ice for an additional 10 minutes, diluted to 5 mL complete culture medium and cultured for 24 hours. Transfection efficiency was analyzed by using the Green Fluorescent Plasmid (GFP, Clontech, Palo Alto, CA), and determined to be approximately 40% to 60%. Cells were stimulated with various stimuli for 24 hours and cytokines measured in the supernatants and IL-2 receptor expression on cell surface.
Enzyme-linked immunosorbent assay (ELISA).Cell supernatants from various culture conditions were harvested and analyzed for cytokines, IL-2, and TNF-α using commecial kits from Biosource (Camarillo, CA) according to the manufacturer's instructions.
RESULTS
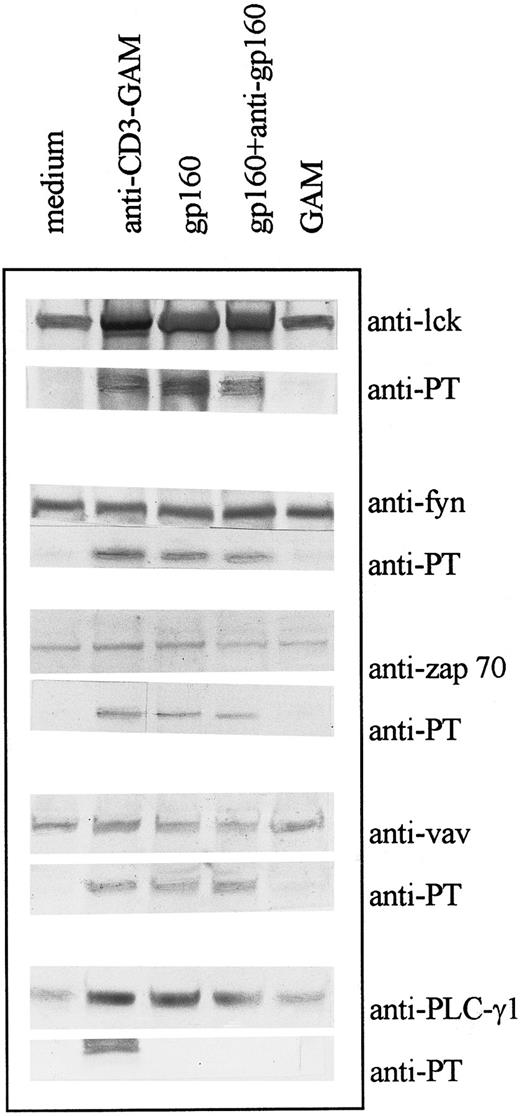
Ligation of CD4 molecule with HIV-gp160 or CD4XL with HIV-gp160 and anti-gp160 Ab induces tyrosine phosphorylation of cellular substrates.CD4+ T cells were isolated from PBMC as described and treated with gp160 with and without anti-gp160 Ab treatment. Tyrosine phosphorylation of individual substrates was evaluated by immunoprecipitation with specific antibodies followed by immunoblotting with specific antibody or with antiphosphotyrosine antibody. Several substrates, including p56lck, p59fyn, ZAP-70, and p95vav, but not PLC-γ1 were tyrosine phosphorylated following CD4 ligation (Fig 1). Treatment of CD4+ T cells with soluble gp160 with or without subsequent treatment with anti-gp160 Ab resulted in similar patterns of tyrosine phosphorylation. Preincubation of the gp160 with soluble CD4 abrogated the effect of the envelope proteins (data not shown). Because glycan moities of envelope proteins have high mannose content,28 possible nonspecific effects mediated by lymphocyte-mannose binding proteins were ruled out by using glycosylated-human serum albumin (HSA); which did not induce detectable tyrosine phosphorylation in CD4+ T cells (data not shown). Cross-linking CD3 molecules with anti-CD3 and GAM resulted in tyrosine phosphorylation of all the above substrates as well as that of PLC-γ1; this effect was inhibited in CD3XL and CD4XL systems by the PTK inhibitor, herbimycin (data not shown).
CD4 ligation with gp160 or CD4XL with gp160 and anti-gp160 induces tyrosine phosphorylation of various cellular substrates: CD4+ T cells were treated with gp160 alone or with gp160 plus anti-gp160. Cell lysates were precleared and immunoprecipitated with antibodies to substrates p56lck, p59fyn, ZAP-70, PLC-γ1 (145 kD), and p95vav as indicated and immunoblotted with either the specific antibody or with antiphosphotyrosine antibody (4G10).
CD4 ligation with gp160 or CD4XL with gp160 and anti-gp160 induces tyrosine phosphorylation of various cellular substrates: CD4+ T cells were treated with gp160 alone or with gp160 plus anti-gp160. Cell lysates were precleared and immunoprecipitated with antibodies to substrates p56lck, p59fyn, ZAP-70, PLC-γ1 (145 kD), and p95vav as indicated and immunoblotted with either the specific antibody or with antiphosphotyrosine antibody (4G10).
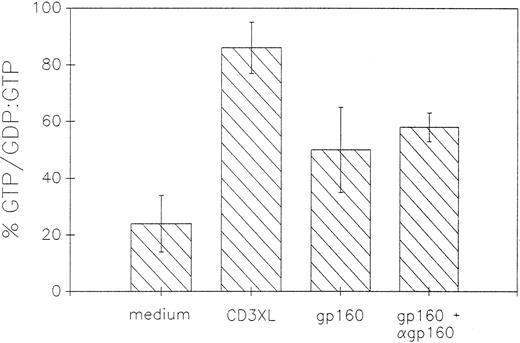
Ligation of CD4 molecule with HIV-gp160 or CD4XL with HIV-gp160 plus anti-gp160 Ab induces p21ras activation in CD4+ T cells as determined by conversion of rasGDP to rasGTP.To elucidate the involvement of ras in CD4 signaling, we examined the GDP/GTP exchange of ras protein following CD4 ligation with either gp160 or after CD4XL with gp160 and anti-gp160 Ab. As shown in Fig 2, CD4 ligation resulted in conversion of rasGDP to rasGTP. The percent conversion of rasGDP to rasGTP was 30% in resting cells, 47% in gp160-treated cells, and 55% after CD4XL with anti-gp160 Ab. Ligation of the TCR/CD3 complex with anti-CD3 MoAb, which is known to cause ras activation,10,11 was used as a positive control, and resulted in 89% conversion of rasGDP to rasGTP.
Activation of p21ras as measured by conversion of rasGDP to rasGTP following CD4XL. Labeling with 32 P-orthophosphate, cross-linking with gp160 and anti-gp160, cell lysing, and immunoprecipitation was performed as described in Materials and Methods. Ras bound GDP and GTP were eluted and analyzed by thin layer chromatography, visualized by autoradiography, and quantitated by densitometry. Conversion of rasGDP to rasGTP was calculated as a ratio of percent rasGTP/GDP:GTP. Data represents mean of two experiments.
Activation of p21ras as measured by conversion of rasGDP to rasGTP following CD4XL. Labeling with 32 P-orthophosphate, cross-linking with gp160 and anti-gp160, cell lysing, and immunoprecipitation was performed as described in Materials and Methods. Ras bound GDP and GTP were eluted and analyzed by thin layer chromatography, visualized by autoradiography, and quantitated by densitometry. Conversion of rasGDP to rasGTP was calculated as a ratio of percent rasGTP/GDP:GTP. Data represents mean of two experiments.
Ligation of CD4 molecule with HIV-gp160 or CD4XL with HIV-gp160 and anti-gp160 Ab induces secretion of TNF-α.Cytokines TNF-α and IL-2 were quantitated by ELISA in 48-hour culture supernatants of CD4+ T cells following CD4 ligation (Table 1). Induction of TNF-α secretion (but not of IL-2) was noted to occur on treatment with gp160 and after CD4XL with gp160 plus anti-gp160 Ab (600 pg/mL and 811 pg/mL, respectively). As expected, CD3 receptor cross-linking in CD4+ T cells resulted in maximum induction of both, TNF-α and IL-2.
Induction of TNF-α Secretion After CD4XL With gp160 and Anti-gp160
| Treatment . | IL-2 Secretion . | TNF-α Secretion . |
|---|---|---|
| Medium | < | 65 ± 10 |
| Anti-CD3-GAM | 3,310 ± 210 | 2,548 ± 180 |
| gp160 | < | 600 ± 50 |
| gp160 + anti-gp160 | < | 811 ± 65 |
| Treatment . | IL-2 Secretion . | TNF-α Secretion . |
|---|---|---|
| Medium | < | 65 ± 10 |
| Anti-CD3-GAM | 3,310 ± 210 | 2,548 ± 180 |
| gp160 | < | 600 ± 50 |
| gp160 + anti-gp160 | < | 811 ± 65 |
CD4XL with gp160 and anti-gp160 was performed as described in Materials and Methods. Cell supernatants were harvested after 48 hours, and cytokine concentrations (in picograms per milliliter) were measured by ELISA using commercial kits according to manufacturer's instructions. < denotes undetectable levels.
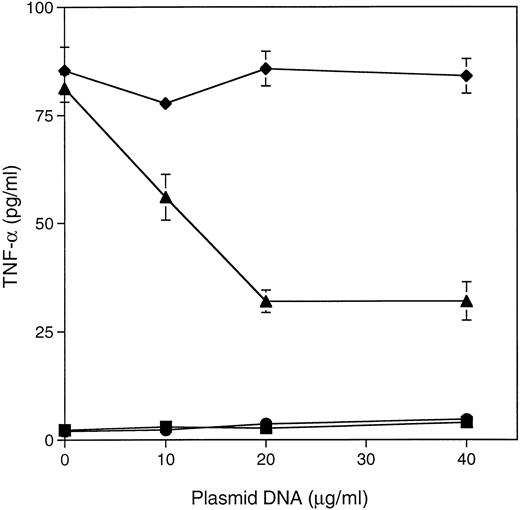
Inhibition of TNF-α secretion in rasN17 transfected Jurkat cells following CD4XL.To determine whether CD4 ligation-induced ras activation is an obligatory event in the signaling pathway, we used jurkat cells transfected with rasN17, a dominant negative ras mutant. As shown in Fig 3, in contrast to peripheral CD4+ T cells, rasN17 transfected cells secreted less than 50% TNF-α after CD4XL (85 pg/mL and 35 pg/mL, respectively).
CD4 cross-linking with gp160 and anti-gp160 in dominant negative ras mutant-transfected cells results in decreased TNF-α secretion. CD4+ Jurkat T cells were transiently transfected with the dominant negative ras mutant (rasN17) or control vector by electroporation as described in Materials and Methods. Concentration of plasmid DNA used for transfection (μg/mL) is indicated. CD4XL was performed with gp160 and anti-gp160 antibody. TNF-α in the culture supernatants was measured by ELISA. Mock-medium (▪), Mock-CD4XL (♦), RasN17-medium (•), RasN17-CD4XL (▴).
CD4 cross-linking with gp160 and anti-gp160 in dominant negative ras mutant-transfected cells results in decreased TNF-α secretion. CD4+ Jurkat T cells were transiently transfected with the dominant negative ras mutant (rasN17) or control vector by electroporation as described in Materials and Methods. Concentration of plasmid DNA used for transfection (μg/mL) is indicated. CD4XL was performed with gp160 and anti-gp160 antibody. TNF-α in the culture supernatants was measured by ELISA. Mock-medium (▪), Mock-CD4XL (♦), RasN17-medium (•), RasN17-CD4XL (▴).
DISCUSSION
It is now well documented that HIV envelope proteins can exert powerful biological effects on the immune system, resulting largely from ligation of CD4 molecules and subsequent intracellular signaling in CD4+ cells. The signaling cascade resulting from gp120 binding to CD4 molecules on T cells has not yet been fully elucidated; in this study, we have shown that ligation of CD4 molecules in CD4+ T cells with HIV-1 gp160 results in tyrosine phosphorylation of several intracellular kinases, notably p95vav, activation of ras, as well as induction of cytokine TNF-α. Furthermore, we have shown that the TNF-α induction resulting from CD4 signaling is dependent on ras activation.
CD4 molecules serve as coreceptors for TCR, and act synergistically with TCR in T cell activation.4 In the absence of a TCR signal, signals elicited from CD4 molecules are often referred to as negative signaling, as they render the cells unresponsive to subsequent stimuli, which normally elicit full TCR activation.29,30 It should be noted that the nature of CD4 signaling depends on the site of CD4 molecule being activated.31-33 Because CD4 molecules serve as the primary receptors for HIV-gp120 through a CDR2-like domain in D1 region, CD4-mediated intracellular signaling resulting from this interaction has special relevance in understanding HIV disease pathogenesis.34,35 To delineate the biochemical events that ensue following CD4 ligation, we examined early and late signaling events and cellular responses in purified CD4+ T cells in which CD4 molecules were ligated with gp160 alone or with subsequent treatment with gp160 antibodies. Previous studies have shown that CD4 ligation results in tyrosine phospohrylation of p56lck.36-38 In this study, we show that CD4XL with gp160 and anti-gp160 leads to tyrosine phosphorylation of p59fyn and in the recruitment and tyrosine phosphorylation of ZAP-70, but fails to induce tyrosine phosphorylation of PLC-γ1 or in the mobilization of calcium in T cells.39 The failure of CD4 signaling to elicit PLC-γ1 phosphorylation marks a clear distinction from TCR signaling wherein PLC-γ1 is phosphorylated and plays a key role in PTK- and PKC-mediated signaling, calcium mobilization and IL-2 secretion. Although data regarding calcium mobilization on engagement of CD4 molecules with gp120 is controversial,36 39 the failure of CD4-ligation to induce IL-2 secretion despite phosphorylation of proximal kinases could be attributable to the absence of PLC-γ1 phosphorylation following CD4-gp120 interaction.
Engagement of CD4 molecules in CD4+ T cells resulted in tyrosine phosphorylation of p95,vav which has been implicated to be a substrate for tyrosine kinase p56lck 9 and in activation of ras as measured by conversion of rasGDP to rasGTP. It is possible that p95vav may be involved in the activation of ras in CD4+ T cells; however, the role of vav as a guanine nucleotide exchange protein is controversial and structurally vav is a more likely candidate as an exchange protein for the ras related rho/rac proteins.40 The activation of ras by CD4 ligation is a major finding of this study and is of significance because ras appears to be critically important for many functional responses of T cells.26 41 The use of ras by preligation of CD4 molecules may be responsible for rendering the cells unresponsive to subsequent TCR activation, which also requires ras activation.
Signaling events downstream of ras activation have recently been shown to involve multiple pathways including raf-1 and MEKK, which lead to activation of MAPK and JNK/SAPK, respectively.3,42,43 We have recently observed that CD4XL induces ERK2 activation but not JNK in CD4+ T cells (Chirmule et al, manuscript in preparation). Heat inactivated HIV-1 has been reported to elicit signals in CD4+ T cells such as protein tyrosine phosphorylation, PI-4 kinase, and MAP kinase activation.44 These serine threonine kinases have been implicated in regulation of several transcription factors including AP-1,45 which is a collection of homodimeric and heterodimeric complexes composed of c-jun and c-fos products and is also activated following CD4 ligation.46 These findings, taken together with the studies reported here indicate that activation of ras pathway may be involved in the signals transduced through the CD4 molecule, which culminate in activation of AP-1. We have observed in preliminary studies that CD4XL induces ERK2, but not JNK (Chirmule et al, manuscript in preparation). Considered in the context of AP-1, which is more of a target for JNK than ERK, these downstream effectors need to be investigated further. The role of ZAP-70 in AP-1 activation in our culture system is uncertain. Although CD4 signaling resulted in tyrosine phosphorylation of ZAP-70, preliminary studies suggest that CD4+ Hela cells, which lack CD3 and ZAP-70, can still activate AP-1 (data not shown).
It has previously been shown by our laboratory,16,17,18,23,24 and by others23,24 that HIV-1 gp120/CD4 interaction results in the induction of cytokines IFN-γ, TNF-α, IL-3, GM-CSF, and IL-6. Results reported here suggest that ras activation may be critical for CD4 ligation-induced TNF-α secretion; Jurkat cells transfected with the negative dominant ras mutant (rasN17) secreted significantly less TNF-α following CD4XL than did mock transfected cells. Although the constitutive TNF-α secretion in CD4+ Jurkat cells was considerably less than that in peripheral CD4+ T cells (65 pg/mL), it was augmented by CD4XL (85 pg/mL). Cells transfected with the negative dominant ras mutant, however, secreted ≤ 50% TNF-α (35 pg/mL) on CD4XL than the mock transfected cells, suggesting that ras may be playing an important role in this process. We have previously shown that CD4XL in PBMC induces cytokines TNF-α and IFN-γ which contribute to Fas Ag upregulation and prime CD4+ T cells for apoptosis.16 Serum levels of TNF-α and IFN-γ have both been reported to be increased in patients with HIV infection,47,48 and these cytokines, especially TNF-α, have been shown to upregulate HIV in latently infected cells.49 TNF-α also activates NF-kB and thereby promotes viral replication.50 Recent in vitro studies from our laboratory51 provide evidence that CD4XL in vitro of PBMC from HIV-infected patients leads to TNF-α upregulation and also increases p24 antigen production in a subset of patients. The elucidation of CD4 signaling events downstream to ras, eq, ERK2 in the induction of TNF-α would clearly be important in understanding the mechanisms involved in dysregulation of TNF-α in HIV disease pathogenesis.
We have shown that the envelope glycoproteins of HIV-1 have the ability to signal via CD4 molecules and thereby lead to activation of tyrosine kinases, p59fyn, ZAP-70, tyrosine phosphorylation of p95,vav ras, and induction of TNF-α. In HIV infection, a strong likelihood exists that CD4 ligation occurs in vivo by both free and cell-associated gp12052,53 or by the HIV virions themselves, the latter in the lymph nodes where abundant virions are trapped in the processes of follicular dendritic cells (FDC) and come in contact with trafficking T cells. Such a mechanism of CD4+ T cell activation could contribute in driving the disease process such that it is deleterious to the host resulting in lymphocyte anergy or apoptosis, while being favorable to the virus by promoting HIV replication.
Supported by National Institutes of Health Grants No. DA 05161 and AI 128281.
Address reprint requests to Savita Pahwa, MD, North Shore University Hospital, Biomedical Research Building, Rm 303, 350 Community Dr, Manhasset, NY 11030.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal