Abstract
Echistatin is a viper venom disintegrin containing RGD loop maintained by disulfide bridges. It binds with a high affinity to αvβ3 and αIIbβ3 and it induces extensive conformational changes in these integrins resulting in expression of ligand-induced binding site (LIBS) epitopes. We investigated the activities of echistatin and its three analogues (R24A, D27W, echistatin 1-41). R24A echistatin did not react with αIIbβ3 and αvβ3 integrins and did not cause LIBS effect. D27W echistatin showed increased binding to αIIbβ3 and decreased binding to αvβ3. This substitution impaired the ability of echistatin to induce LIBS in αvβ3 integrin. Deletion of nine C-terminal amino acids of echistatin decreased its ability to bind αIIbβ3 and inhibit platelet aggregation. Truncated echistatin failed to induce LIBS epitopes on cells transfected with αIIbβ3 and αvβ3 genes. The ability of echistatin 1-41 to compete with binding of vitronectin to immobilized αvβ3 and monoclonal antibody 7E3 to platelets and to VNRC3 cells was decreased, although this analogue, after immobilization, retained its ability to bind purified αvβ3. We propose a hypothesis in which echistatin's RGD loop determines selective recognition of αIIbβ3 and αvβ3 integrin, whereas the C-terminal domain supports its binding to resting integrin and significantly contributes to the expression of LIBS epitope and to conformational changes of the receptor, leading to a further increase of the binding affinity of echistatin and of the inhibitory effect.
DISINTEGRINS represent a family of cysteine-rich, low molecular weight proteins occurring in venoms of various vipers. Most disintegrins contain RGD/KGD sequence. They bind with a high affinity to numerous integrins and are potent inhibitors of platelet aggregation and cell adhesion.1-4 Disintegrins demonstrate selectivity in interaction with various integrins that depends on several factors, including the amino acids adjacent to RGD, the pattern of intramolecular S-S bridges, and the regions of molecule beyond the RGD loop.
Echistatin, a 49 amino acid disintegrin containing four disulfide bridges, occurs in the venom of Echis carinatus, from which it was first purified into homogeneity by Gan et al.5 Chemical synthesis of echistatin by Garsky et al6 made it available for extensive biological and structural studies. The 1H-NMR study of echistatin showed that the RGD sequence is located in a mobile loop joining two strands of β-sheet protruding 14 to 17 Å from the protein core. Moreover, nuclear magnetic resonance (NMR) spectroscopy studies established echistatin coordinates essential for constructing the molecular models of various mutants of this disintegrin using homology methods.7-10 The pattern of S-S bridges established by NMR spectroscopy was confirmed by chemical analysis.11 12
Echistatin is a potent inhibitor of ligand binding to αIIbβ3, αvβ3, and α5β1 integrin.13,14 Its competition with fibrinogen for the RGD recognition sites on the αIIbβ3 integrin (glycoprotein IIb/IIIa complex) results in the inhibition of platelet aggregation5,15 and other antithrombotic properties such as an ability to prevent coronary thrombosis in animal models.16 Echistatin is also a potent inhibitor of bone resorption.17 This results from the blocking of the interaction of αvβ3 integrin on the surface of osteoclasts with bone extracellular matrix. In addition, echistatin's interaction with αvβ3 also inhibits adhesion of human umbilical vein endothelial cells (HUVEC) to immobilized vitronectin and fibronectin.18
In previous studies, we compared biologic activities of echistatin and eristostatin. Eristostatin is a disintegrin isolated from the venom of Eristocophis macmahoni. The polypeptide chains of echistatin and eristostatin both have 49 amino acids and show 60% amino acid sequence identity. Both disintegrins have cysteines at the same position and have an identical pattern of S-S bridges.11,19 However, they show a number of functional differences. Eristostatin is a more potent inhibitor of platelet aggregation than echistatin. Although both disintegrins bind to the same number of receptors on resting and on activated platelets, eristostatin binds with the same high affinity to resting and to activated platelets, whereas the binding affinity of echistatin to resting platelets is significantly lower than its binding affinity to adenosine diphosphate (ADP)-activated platelets.20
Echistatin blocks with similar potency the binding of specific ligands to purified αIIbβ3, α5β1, and αvβ3, whereas eristostatin is a much more selective inhibitor of αIIbβ3 binding to fibrinogen. It shows a low inhibitory effect on the binding of purified αvβ3 to vitronectin and α5β1 to fibronectin.14 Accordingly, in contrast to echistatin, eristostatin has little inhibitory effect on HUVEC adhesion to immobilized vitronectin, and HUVEC do not adhere to immobilized eristostatin.18 Further studies show that echistatin and eristostatin interaction with αIIbβ3 on platelets or on Chinese hamster ovary (CHO) cells transfected with the genes of this integrin results in an expression of ligand-induced binding site (LIBS) epitope. This was evidenced by increasing binding of monoclonal antibody 62 (MoAb 62), recognizing expression of LIBS epitope on the β3 subunit and extensive conformational changes of the receptor.21 This effect of eristostatin is much stronger than the effect of echistatin.22 Whereas echistatin induces significant conformational changes in the β3 subunit of VNRC3 cells, this is not true for eristostatin. Also, VNRC3 cells adhere to immobilized echistatin but not to immobilized eristostatin.22
Scarborough et al23 reported that eristocophin is equally potent antagonist of αIIbβ3 and αvβ3. Because eristocophin differs from eristostatin by the presence of two additional amino acids at its N-terminus and by substitution of D32 with N, it is possible that the latter substitution increases affinity of this disintegrin to αvβ3. We hypothesize that the difference in the ability of echistatin and eristostatin to recognize integrin ligand binding sites reflects the differences in the amino acid sequences of their RGD loops, C20KRARGDDMDDYC32 and C23RVARGDWNDDYC35, respectively. Homology modeling studies of echistatin and eristostatin suggested that the shape of the RGD loop is influenced both by the amino acid residues adjacent to the N-terminus (R22 in echistatin and V25 in eristostatin) and to the C-terminus (D27 in echistatin and W30 in eristostatin). We proposed a hypothesis in which the width and shape of the RGD loop are important ligand structural features that affect the fitting of ligand to the binding pocket of αIIbβ3 and αvβ3.19
The purpose of this study was to evaluate further structure-function relationship of disintegrins by comparing interaction of synthetic echistatin and its three analogues (R24A echistatin, D27W echistatin, and echistatin 1-41) with αIIbβ3 and αvβ3 integrins.
MATERIALS AND METHODS
Proteins, peptides, and antibodies.Echistatin was prepared from the Echis carinatus venom by high-performance liquid chromatography (HPLC) as described.20 Synthetic echistatin and its three analogues (R24A echistatin, D27W echistatin, and echistatin 1-41) were synthesized according to the method of Garsky et al6 and kindly provided by Dr V. Garsky (Merck Research Laboratories, West Point, PA). The purity of preparations was evaluated by means of reverse-phase HPLC using Vydac C-18 column and by mass spectrometry. We have previously shown that synthetic and natural echistatin show an identical pattern of S-S bridges.11 Circular dichroism has shown that spectra of echistatin and echistatin 1-41 are compatible with the contention that both disintegrins have identical pattern of folding. Polyclonal antibody against natural echistatin was raised in rabbits.
The following MoAbs were used as purified IgG. 7E3, obtained from Dr B. Coller (Mt Sinai Hospital, New York, NY), recognizes αIIbβ3 and αvβ3 complexes.24 MoAb 62, provided by Dr M. Ginsberg (Scripps Research Institute, La Jolla, CA), is specific for LIBS225 and recognizes an epitope in the C-terminal region of the extracellular domain of β3 subunit.21 AP5, recognizing LIBS epitope on the N-terminal region of β3 integrin,26 was provided by Dr T.J. Kunicki (Scripps Research Institute, La Jolla, CA). LM 609, obtained from Dr D. Cheresh (Scripps Research Institute), recognizes the αvβ3 complex.27 AP3 MoAb, recognizing β3 subunit,28 was obtained from hybridoma cells producing this antibody (line 240; ATCC, Rockville, MD).
Highly purified human fibrinogen and polyclonal antibody against fibrinogen were gifts from Dr A. Budzynski (Temple University, Philadelphia, PA). Antihuman vitronectin polyclonal antibody, developed in rabbit, was from GIBCO BRL (Gaithersburg, MD).
Fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG was purchased from Jackson Immune Research (West Grove, PA). GRGDSPK peptide was synthesized by Dr B. Jameson (Thomas Jefferson University, Philadelphia, PA). CNBr-Sepharose 4B and CMFDA (5-chloromethylfluorescein diacetate) were purchased from Pharmacia (Piscataway, NJ) and from Molecular Probes (Eugene, OR), respectively. Na125I and Bolton-Hunter reagent [125I] kit were acquired from ICN Radiochemicals (Irvine, CA). n-Octylglucoside and Hanks' Balanced Salt Solution (HBSS) were obtained from Boehringer Mannheim (Indianapolis, IN) and GIBCO BRL, respectively. All other reagents were purchased from Sigma (St Louis, MO).
Cell culture.A5 and VNRC3 cells, CHO cells expressing human αIIbβ3 and αvβ3, respectively,29 30 were kindly provided by Dr M. Ginsberg (Scripps Research Institute). Nontransfected CHO-K1 cells were purchased from ATCC. A5 and VNRC3 cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, nonessential amino acids, glutamine, penicillin, and streptomycin. CHO-K1 cells were maintained in Ham's F12 medium containing 10% fetal calf serum, glutamine, penicillin, and streptomycin. Cells were detached from plates with cation-free HBSS and 5 mmol/L EDTA and washed three times with HBSS containing Ca2+ and Mg2+.
Purification of αvβ3 and αIIbβ3 integrins.Vitronectin receptor αvβ3 was purified to homogeneity from VNRC3 cells by one-step affinity chromatography, as described.31 In brief, the method consists of two steps: lysis of the cells and affinity chromatography of the lysate on GRGDSPK-Sepharose column. αvβ3 obtained by this method showed higher degree of purity than αvβ3 purified from placenta and was not contaminated by hamster integrins. Fibrinogen receptor αIIbβ3 was purified from A5 cells by the same procedure, except that elution from affinity column was accomplished using RGDS (5 mmol/L) rather than EDTA.
Preparation of gel-filtered platelets (GFP).Platelets were isolated from acid citrate dextrose anticoagulated fresh human blood. In some experiments, 1 μg/mL prostaglandin E1 was added with anticoagulant to prevent platelet activation during preparation. GFP were obtained as described.32
Binding of 125I-labeled disintegrins and 125I-MoAb 62 to platelets and CHO cells.Echistatin and its analogues were labeled with 125I using Bolton-Hunter reagent according to the original method33 and the manufacturer's recommendations. This method of radiolabeling did not alter disintegrins' biologic activity as tested in the platelet aggregation inhibition assay. In contrast, we observed that 125I radiolabeling with the Pierce (Rockford, IL) iodobeads resulted in a decrease of biologic activity of echistatin. MoAb 62 (IgG) was radiolabeled using chloramine T according to the procedure described previously.34
Binding of radiolabeled echistatin and its analogues and 125I-MoAb 62 to platelets was performed as described,20 and the results were analyzed by the method of Scatchard. Nonspecific binding under each condition was measured in the presence of 50 molar excess cold disintegrin.
Binding of 125I-MoAb 62 IgG to the cell suspension was performed as described.22
Flow cytometry analysis.Samples for flow cytometry analysis were prepared as described earlier22 and analyzed in a Coulter Epics Elite flow cytometer (Miami, FL).
Adhesion studies.Adhesion of cultured cells labeled with 5-chloromethylfluorescein diacetate (CMFDA) was performed as described.22
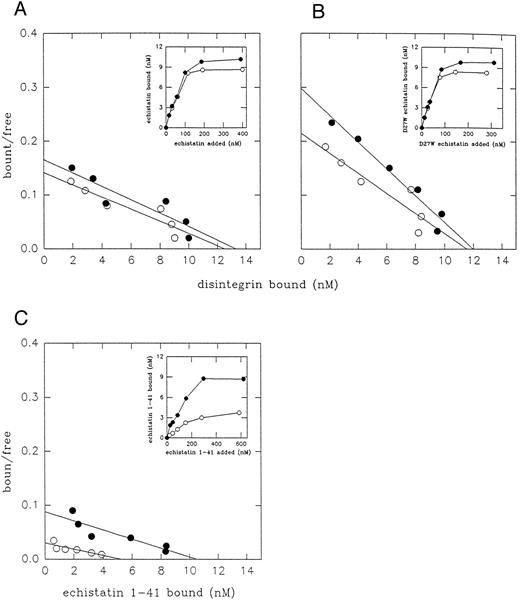
Scatchard analysis of 125I-labeled disintegrins binding to resting platelets (○) and to ADP-activated platelets (•). Platelets (2 × 108/mL) were incubated with increasing concentration of 125I-labeled echistatin (A), D27W echistatin (B), and echistatin 1-41 (C) for 15 minutes at room temperature and then 400 μL of platelet suspension was placed over silicone oil and centrifugated. The radioactivities of supernatant and pellet were measured with a γ-counter. Nonspecific binding was measured after preincubation of platelets (5 minutes at room temperature) with 5 mmol/L EDTA. The results are means from three independent experiments.
Scatchard analysis of 125I-labeled disintegrins binding to resting platelets (○) and to ADP-activated platelets (•). Platelets (2 × 108/mL) were incubated with increasing concentration of 125I-labeled echistatin (A), D27W echistatin (B), and echistatin 1-41 (C) for 15 minutes at room temperature and then 400 μL of platelet suspension was placed over silicone oil and centrifugated. The radioactivities of supernatant and pellet were measured with a γ-counter. Nonspecific binding was measured after preincubation of platelets (5 minutes at room temperature) with 5 mmol/L EDTA. The results are means from three independent experiments.
Solid-phase assay.The microplates (Costar, Cambridge, MA) were coated with 300 ng/well of purified integrin in 0.05 mol/L carbonate/bicarbonate buffer, pH 9.2, and incubated at 4°C overnight. To block nonreacted surfaces, the plates were incubated for 60 minutes at 37°C with phosphate-buffered saline (PBS)/0.5% Tween 20 containing 5% nonfat milk (PBST). After washing three times with PBST, varying concentrations of disintegrins were mixed with ligand (fibrinogen or vitronectin, 1 μg per sample) in a buffer containing 20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L CaCl2 , 1 mmol/L MgCl2 , and 1% bovine serum albumin and incubated at 37°C for 30 minutes. After washing with PBST, polyclonal antifibrinogen or antivitronectin antibody was added and the plates were incubated for 60 minutes at 37°C. After washing, the binding of polyclonal antibodies to integrin ligands was detected using alkaline phosphatase-conjugated goat antimouse IgG (Sigma), as described previously.35 To study binding of purified integrins to immobilized echistatin and its analogues, the microplates were coated with 300 ng/well (245 ng for echistatin 1-41) of purified disintegrins in 50 mmol/L carbonate/bicarbonate buffer (pH 9.2) and incubated overnight at 4°C. The wells were blocked with 5% nonfat milk in PBST buffer and then purified integrin containing 50 mmol/L n-octylglucoside, 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 2 mmol/L MgCl2 , 0.1 mmol/L CaCl2. The plates were incubated for 30 minutes at 37°C. The level of bound αvβ3 was tested by adding LM 609 MoAb, then alkaline phosphatase-conjugated antimouse IgG (Sigma), and finally substrate for alkaline phosphatase (p-nitrophenyl phosphate; Sigma). The level of αIIbβ3 integrin was tested using AP3 MoAb. The absorbance was measured using a microplate autoreader (Bio-Tek Instruments, Wimooski, VT) at 405 nm. Efficient immobilization of echistatin and its analogues on the plates was confirmed by studying binding of the polyclonal antiechistatin antibody.
Homology modeling of echistatin and its analogues.The three-dimensional models of echistatin and its two analogues were built on the basis of echistatin coordinates determined by NMR studies9 using the computer program FRODO36 on an ESV graphic system (Evans and Sutherland Computer Cooperation). The amino acid mutations were incorporated in the three-dimensional structure of echistatin on the graphics, optimized for stereochemistry, and then refined using the conjugate gradient energy minimization method of Powell.37 The energy minimized structures were further refined with a slow cooling simulated annealing molecular dynamics protocol using X-PLOR.38 Although the orientations of the side chain atoms are optimized using rotomer dictionary, the conformational angles of side chains located on the surface of the molecule depend on their interactions with its receptor, ligand, or solvent molecules.
RESULTS
Interaction of echistatin and its analogues with human platelets.Natural echistatin, synthetic echistatin, and its three analogues inhibited ADP-induced platelet aggregation in platelet-rich plasma. The IC50 values for natural (136 ± 29 nmol/L) and synthetic echistatin (139 ± 17 nmol/L) were almost identical to IC50 values reported previously.20 R24A echistatin had very low inhibitory activity (IC50 = 2,725 ± 225 nmol/L), in agreement with the previous report by Garsky et al.6 D27W echistatin was significantly more active (IC50 = 83 ± 16 nmol/L), whereas truncation of the C-terminal end of echistatin resulted in the decrease of platelet aggregation inhibitory activity from IC50 139 nmol/L to 355 ± 25 nmol/L.
Figure 1 and Table 1 compare the number of binding sites and binding affinity of synthetic echistatin and its analogues to human resting and ADP-activated gel-filtered platelets. D27W echistatin bound with increased affinity to resting and ADP-stimulated platelets as compared with wild-type echistatin. Truncated echistatin bound with much lower affinity and to a lower number of sites on resting platelets. However, activation of platelets with ADP significantly increased binding of truncated echistatin almost to the level observed with wild-type echistatin. It should be noted that in a previous study from our laboratory, the binding affinity of echistatin to resting platelets was lower than in a current study (153 v 106 nmol/L).20 This difference could result from the fact that, in the previous studies, Pierce iodobeads rather than Bolton Hunter reagent were used for labeling echistatin with 125I.
Binding Site Number and Affinity of Echistatin and Its Analogs to Resting and ADP-Activated Platelets
| . | Platelets (resting) . | Platelets (ADP) . |
|---|---|---|
| Echistatin | 41,890 ± 3,430 | 46,670 ± 5,430 |
| 106.5 ± 18.2 nmol/L | 90.0 ± 18.6 nmol/L | |
| Echistatin D27W | 45,430 ± 6,330 | 47,280 ± 4,400 |
| 64.1 ± 13.8 nmol/L | 55.6 ± 18.0 nmol/L | |
| Echistatin 1-41 | 17,690 ± 1,600 | 38,970 ± 930 |
| 210.3 ± 31.4 nmol/L | 108.2 ± 27.3 nmol/L |
| . | Platelets (resting) . | Platelets (ADP) . |
|---|---|---|
| Echistatin | 41,890 ± 3,430 | 46,670 ± 5,430 |
| 106.5 ± 18.2 nmol/L | 90.0 ± 18.6 nmol/L | |
| Echistatin D27W | 45,430 ± 6,330 | 47,280 ± 4,400 |
| 64.1 ± 13.8 nmol/L | 55.6 ± 18.0 nmol/L | |
| Echistatin 1-41 | 17,690 ± 1,600 | 38,970 ± 930 |
| 210.3 ± 31.4 nmol/L | 108.2 ± 27.3 nmol/L |
The number of binding sites and binding affinity were estimated by Scatchard analysis of 125I binding data from three experiments. The results correspond to mean and standard deviation. Binding affinity from these experiments are expressed in nanomoles per liter.
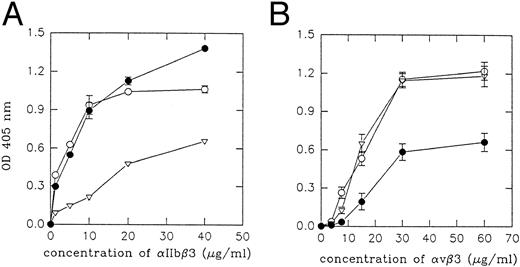
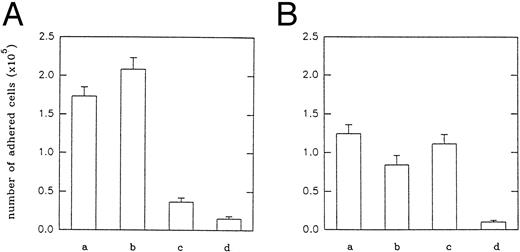
Interaction of echistatin and its analogues with purified integrins and with transfected cells.We examined the inhibitory effect of echistatin and its analogues on the binding of fibrinogen to immobilized αIIbβ3 (Fig 2A) and on the binding of vitronectin to immobilized αvβ3 (Fig 2B) in solid-phase assay. D27W echistatin was the strongest inhibitor of fibrinogen binding to αIIbβ3, followed by wild-type echistatin and truncated echistatin. The latter analogue showed a low inhibitory effect. In agreement with previous experiments, substitution of aspartic acid 27 in echistatin with tryptophane resulted in a decrease of ability of this disintegrin to inhibit vitronectin binding to immobilized αvβ3.19 Truncated echistatin was also much less active in this system. In another series of experiments, we examined binding of purified integrins and transfected cells to immobilized echistatin and its analogues. Purified integrins αIIbβ3 and αvβ3 did not bind to immobilized R24A echistatin. Purified αIIbβ3 bound most efficiently to immobilized D27W echistatin and slightly less to immobilized wild-type echistatin. The binding of this integrin to immobilized truncated echistatin was significantly impaired (Fig 3A). Purified αvβ3 bound with the same potency to immobilized truncated echistatin and with a much lower potency to immobilized D27W echistatin (Fig 3B). Similar results were obtained by studying adhesion of CMFDA-labeled CHO cells transfected with the genes of αIIbβ3 integrin (A5 cells) or with the genes of αvβ3 integrin (VNRC3 cells). Adherence of both types of cells to immobilized R24A echistatin was negligible (Fig 4). A5 cells adhered most efficiently to immobilized D27W echistatin and slightly less to wild echistatin. The number of A5 cells adhering to immobilized truncated echistatin was about fivefold less than the number of cells adhering to wild immobilized echistatin (Fig 4A). By contrast, VNRC3 cells adhered as well to immobilized wild echistatin as to immobilized truncated echistatin. However, adhesion of these cells to immobilized D27W echistatin was significantly decreased (Fig 4B).
Inhibition of fibrinogen binding to immobilized αIIbβ3 (A) and vitronectin binding to immobilized αvβ3 (B) by echistatin and its analogues. Three hundred nanograms of integrin was immobilized on a 96-well enzyme-linked immunosorbent assay (ELISA) plate under conditions described in the Materials and Methods. After blocking the plate, the ligand (fibrinogen or vitronectin, 1 μg per sample) was added to the wells together with different concentrations of echistatin (○), D27W echistatin (•), echistatin 1-41 (▿), or R24A echistatin (▾) and incubated at 37°C for 30 minutes. The amount of bound ligand was detected using antifibrinogen or antivitronectin polyclonal antibodies as described in the Materials and Methods. Error bars represent the standard deviation for three independent experiments.
Inhibition of fibrinogen binding to immobilized αIIbβ3 (A) and vitronectin binding to immobilized αvβ3 (B) by echistatin and its analogues. Three hundred nanograms of integrin was immobilized on a 96-well enzyme-linked immunosorbent assay (ELISA) plate under conditions described in the Materials and Methods. After blocking the plate, the ligand (fibrinogen or vitronectin, 1 μg per sample) was added to the wells together with different concentrations of echistatin (○), D27W echistatin (•), echistatin 1-41 (▿), or R24A echistatin (▾) and incubated at 37°C for 30 minutes. The amount of bound ligand was detected using antifibrinogen or antivitronectin polyclonal antibodies as described in the Materials and Methods. Error bars represent the standard deviation for three independent experiments.
Binding of purified αIIbβ3 and αvβ3 to immobilized echistatin and its analogues. Three hundred nanograms per sample of echistatin (○), 300 ng per sample of D27W echistatin (•), and 245 ng per sample of echistatin 1-41 (▿) were immobilized overnight at 4°C on a 96-well ELISA plate in 50 mmol/L carbonate/bicarbonate buffer, pH 9.2. After blocking, the purified receptors were incubated on plate at 37°C for 30 minutes. The amount of bound receptor to disintegrin was detected using MoAb as described in the Materials and Methods. Error bars represent the standard deviations from three experiments.
Binding of purified αIIbβ3 and αvβ3 to immobilized echistatin and its analogues. Three hundred nanograms per sample of echistatin (○), 300 ng per sample of D27W echistatin (•), and 245 ng per sample of echistatin 1-41 (▿) were immobilized overnight at 4°C on a 96-well ELISA plate in 50 mmol/L carbonate/bicarbonate buffer, pH 9.2. After blocking, the purified receptors were incubated on plate at 37°C for 30 minutes. The amount of bound receptor to disintegrin was detected using MoAb as described in the Materials and Methods. Error bars represent the standard deviations from three experiments.
Adhesion of A5 (A) and VNRC3 (B) cells to immobilized echistatin and its analogues. Five micrograms per sample of echistatin (a), 5 μg per sample of D27W echistatin (b), 4 μg per sample of echistatin 1-41 (c), and 5 μg per sample of R24A echistatin (d) were immobilized overnight at 4°C on a 96-well plate (Costar) in carbonate/bicarbonate buffer, pH 9.2. After blocking, the CMFDA-labeled cells were added to each well and incubated as described in the Materials and Methods. Adhesion of CHO-K1 cells was negligible and at the level of nonspecific adhesion of transfected cells to bovine serum albumin. Error bars represent the standard deviations from three independent experiments.
Adhesion of A5 (A) and VNRC3 (B) cells to immobilized echistatin and its analogues. Five micrograms per sample of echistatin (a), 5 μg per sample of D27W echistatin (b), 4 μg per sample of echistatin 1-41 (c), and 5 μg per sample of R24A echistatin (d) were immobilized overnight at 4°C on a 96-well plate (Costar) in carbonate/bicarbonate buffer, pH 9.2. After blocking, the CMFDA-labeled cells were added to each well and incubated as described in the Materials and Methods. Adhesion of CHO-K1 cells was negligible and at the level of nonspecific adhesion of transfected cells to bovine serum albumin. Error bars represent the standard deviations from three independent experiments.
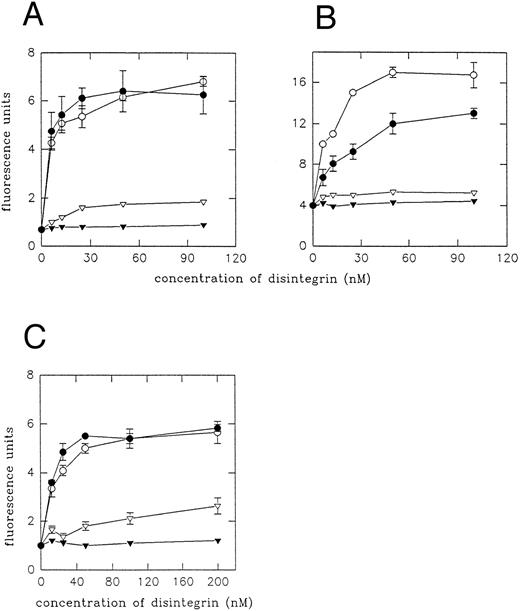
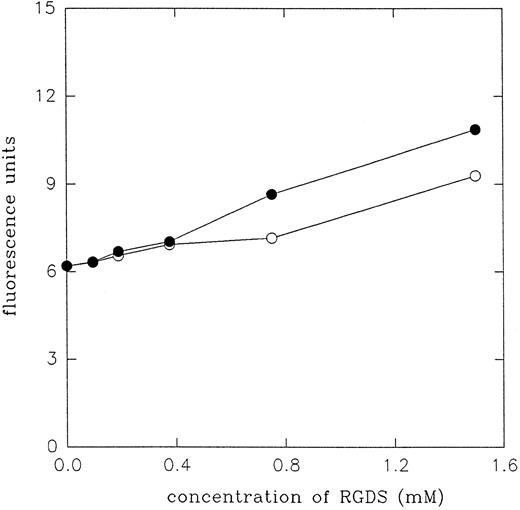
Effect of echistatin and its analogues on the expression of LIBS epitopes on β3 subunit of αIIbβ3 and αvβ3 integrin.In this series of experiments, we studied expression of LIBS epitopes on platelets and on transfected cells by means of flow cytometry and radioligand assay. Binding of two MoAbs, MoAb 62 and AP5, has been determined using FITC-conjugated secondary antibody. Figure 5 shows the effect of echistatin and its analogues on the expression of LIBS2 (MoAb 62) epitope on A5 cells (Fig 5A), on VNRC3 cells (Fig 5B), and on resting platelets (Fig 5C). It can be seen that wild-type echistatin induced significant increase of MoAb 62 binding to all three types of cells. Substitution of aspartic acid 27 with tryptophane did not significantly alter echistatin's effect on the induction of conformational changes in αIIbβ3 integrin either on platelets or on transfected cells. However, D27W echistatin showed less activity with αvβ3 integrin on VNRC3 cells. R24A echistatin and truncated echistatin were inactive in all three systems, whereas truncated echistatin expressed low levels of LIBS epitope on platelets. Figure 6 shows that RGDS is at least 104 times less potent than echistatin in inducing LIBS in platelets and A5 cells. Similar results to MoAb 62 were obtained studying the effect of echistatin and its analogues on the binding of AP5 MoAb to platelets and transfected cells (Fig 7).
Expression of MoAb 62 LIBS epitope on A5 cells (A), VNRC3 cells (B), and resting platelets (C) by echistatin and its analogues. A5 or VNRC3 cells (5 × 105 of each) were incubated with echistatin (○), D27W echistatin (•), echistatin 1-41 (▿), or R24A echistatin (▾) for 15 minutes at room temperature. After being washed, MoAb 62 (1 μg per sample) was added for 45 minutes at 4°C. Cells were then incubated with FITC-conjugated goat antimouse IgG, fixed, and analyzed by flow cytometry as described in the Materials and Methods. In the case of platelets (6 × 106 per sample), the washing steps were omitted and incubations with antibodies were performed at room temperature for 30 minutes. Error bars represent the standard deviations for three independent experiments.
Expression of MoAb 62 LIBS epitope on A5 cells (A), VNRC3 cells (B), and resting platelets (C) by echistatin and its analogues. A5 or VNRC3 cells (5 × 105 of each) were incubated with echistatin (○), D27W echistatin (•), echistatin 1-41 (▿), or R24A echistatin (▾) for 15 minutes at room temperature. After being washed, MoAb 62 (1 μg per sample) was added for 45 minutes at 4°C. Cells were then incubated with FITC-conjugated goat antimouse IgG, fixed, and analyzed by flow cytometry as described in the Materials and Methods. In the case of platelets (6 × 106 per sample), the washing steps were omitted and incubations with antibodies were performed at room temperature for 30 minutes. Error bars represent the standard deviations for three independent experiments.
Expression of MoAb 62 LIBS epitope on A5 cells and platelets by RGDS peptide. The applied procedure was the same as described in Fig 5. (○) LIBS expression on A5 cells. (•) LIBS expression on resting platelets.
Expression of MoAb 62 LIBS epitope on A5 cells and platelets by RGDS peptide. The applied procedure was the same as described in Fig 5. (○) LIBS expression on A5 cells. (•) LIBS expression on resting platelets.
Expression of AP5 LIBS epitope on A5 cells (A), VNRC3 cells (B), and resting platelets (C) by echistatin and its analogues. The applied procedure was the same as described in Fig 5, except for the use of the buffer for AP5 MoAb, which did not contain ions Ca2+ and Mg2+.
Expression of AP5 LIBS epitope on A5 cells (A), VNRC3 cells (B), and resting platelets (C) by echistatin and its analogues. The applied procedure was the same as described in Fig 5, except for the use of the buffer for AP5 MoAb, which did not contain ions Ca2+ and Mg2+.
Assay of the number of binding sites of MoAb 62 antibody on platelets and on transfected cells yielded results that were generally consistent with the flow cytometry study (Table 2). However, it should be noted that echistatin and D27W echistatin induced a similar number of MoAb 62 binding sites on A5 cells. Truncated echistatin did not express MoAb 62 sites on A5 cells, but it did cause expression of 8,000 sites per platelet with moderate affinity (kd, 45 nmol/L) on resting platelets (compared with 17,180 sites expressed by wild-type echistatin with a kd of 22 nmol/L). This difference may be explained assuming that the conformational change of fibrinogen receptor during platelet isolation is sufficient to make it reactive with truncated echistatin.
Binding Sites Number and Binding Affinity of MoAb 62 to CHO Cells and to Resting Platelets in the Absence or Presence of Echistatin and Its Analogs
| Disintegrin . | A5 Cells . | VNRC3 Cells . | Platelets . |
|---|---|---|---|
| None | 15,010 ± 2,600 | 14,430 ± 1,200 | 1,690 ± 200 |
| 2.7 ± 0.4 nmol/L | 3.5 ± 0.9 nmol/L | 22.0 ± 5.0 nmol/L | |
| Echistatin | 26,930 ± 1,080 | 28,650 ± 2,600 | 17,180 ± 2,420 |
| 3.0 ± 0.4 nmol/L | 4.0 ± 1.0 nmol/L | 22.0 ± 3.0 nmol/L | |
| Echistatin D27W | 23,770 ± 1,470 | 19,770 ± 930 | 17,510 ± 3,150 |
| 2.5 ± 0.4 nmol/L | 2.9 ± 0.4 nmol/L | 22.0 ± 7.0 nmol/L | |
| Echistatin 1-41 | 16,300 ± 3,100 | 16,280 ± 1,110 | 8,084 ± 1,500 |
| 2.6 ± 0.5 nmol/L | 3.4 ± 1.0 nmol/L | 45.0 ± 6.0 nmol/L | |
| Echistatin R24A | 14,750 ± 1,500 | 15,160 ± 1,010 | ND |
| 3.0 ± 0.4 nmol/L | 3.1 ± 0.2 nmol/L |
| Disintegrin . | A5 Cells . | VNRC3 Cells . | Platelets . |
|---|---|---|---|
| None | 15,010 ± 2,600 | 14,430 ± 1,200 | 1,690 ± 200 |
| 2.7 ± 0.4 nmol/L | 3.5 ± 0.9 nmol/L | 22.0 ± 5.0 nmol/L | |
| Echistatin | 26,930 ± 1,080 | 28,650 ± 2,600 | 17,180 ± 2,420 |
| 3.0 ± 0.4 nmol/L | 4.0 ± 1.0 nmol/L | 22.0 ± 3.0 nmol/L | |
| Echistatin D27W | 23,770 ± 1,470 | 19,770 ± 930 | 17,510 ± 3,150 |
| 2.5 ± 0.4 nmol/L | 2.9 ± 0.4 nmol/L | 22.0 ± 7.0 nmol/L | |
| Echistatin 1-41 | 16,300 ± 3,100 | 16,280 ± 1,110 | 8,084 ± 1,500 |
| 2.6 ± 0.5 nmol/L | 3.4 ± 1.0 nmol/L | 45.0 ± 6.0 nmol/L | |
| Echistatin R24A | 14,750 ± 1,500 | 15,160 ± 1,010 | ND |
| 3.0 ± 0.4 nmol/L | 3.1 ± 0.2 nmol/L |
The number of binding sites and binding affinity for MoAb 62 were estimated in absence or presence of disintegrin at a concentration of 100 nmol/L by Scatchard analysis of the 125I binding data from three experiments. The values correspond to the mean and standard deviation from three independent experiments. Binding affinity are expressed in nanomoles per liter. P = .002, .0076, .616, and .894 when comparing A5 cells without distintegrins and A5 cells with wild-type echistatin, D27W echistatin, echistatin 1-41, and R24A echistatin, respectively; P = .040 when comparing A5 cells with wild-type echistatin and A5 cells with D27W echistatin; P = .0011, .0039, .124, and .468 when comparing VNRC3 cells without disintegrins and VNRC3 cells with wild-type echistatin, D27W echistatin, echistatin 1-41, and R24A echistatin, respectively; P = .0056 when comparing VNRC3 cells with wild-type echistatin and VNRC3 cells with D27W echistatin; P = .0004, .001, and .002 when comparing resting platelets without disintegrins and resting platelets with wild-type echistatin, D27W echistatin, and echistatin 1-41, respectively.
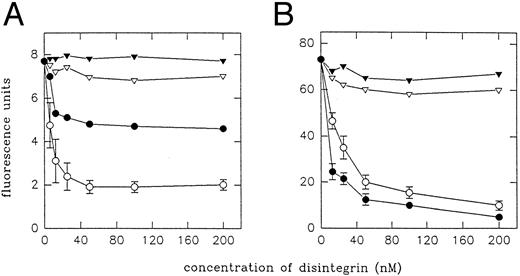
Effect of echistatin and its analogues on the binding of MoAb 7E3 to resting platelets and to VNRC3 cells.MoAb 7E3 binds to αIIbβ3 and αvβ3 integrin in a complex dependent manner with a higher affinity than natural ligands. For this reason we decided to study the effect of echistatin and its analogues on 7E3 binding to platelets and to CHO cells transfected with αvβ3 (VNRC3 cells). Figure 8A shows that wild echistatin and D27W echistatin blocked binding of 7E3 to platelets, with D27W being more effective. R24A echistatin and truncated echistatin did not show any significant inhibition. Echistatin, in agreement with previous studies,18 22 potently blocked binding of 7E3 to VNRC3 cells, whereas D27W echistatin showed only a partial inhibitory effect. Previous studies22 also have shown little activity of eristostatin in this system. Truncated echistatin showed as little effect as R24A echistatin (Fig 8B).
Inhibition of binding of MoAb 7E3 to VNRC3 cells (A) and resting platelets (B) by echistatin and its analogues. The VNRC3 cells (5 × 105) were preincubated with echistatin (○), D27W echistatin (•), echistatin 1-41 (▿), or R24A echistatin (▾) at room temperature for 15 minutes and then 7E3 (1 μg per sample) was added. Samples with platelets (6 × 106 per sample) were prepared in similar manner except for the washing steps. The flow cytometry analysis was performed with FITC-conjugated goat antimouse IgG as described in the Materials and Methods. Error bars represent the standard deviation for three independent experiments.
Inhibition of binding of MoAb 7E3 to VNRC3 cells (A) and resting platelets (B) by echistatin and its analogues. The VNRC3 cells (5 × 105) were preincubated with echistatin (○), D27W echistatin (•), echistatin 1-41 (▿), or R24A echistatin (▾) at room temperature for 15 minutes and then 7E3 (1 μg per sample) was added. Samples with platelets (6 × 106 per sample) were prepared in similar manner except for the washing steps. The flow cytometry analysis was performed with FITC-conjugated goat antimouse IgG as described in the Materials and Methods. Error bars represent the standard deviation for three independent experiments.
Molecular models of echistatin and its analogues.We developed previously molecular models for D27W echistatin and eristostatin using homology and molecular dynamics simulation methods.19 These structures are based on echistatin coordinates experimentally determined by NMR methods. Echistatin is predominantly a loop structure with four nonstandards turns and has no secondary structure elements. The folding forces are largely due to the four disulfide bridges between eight cysteine residues. The structure does not have a hydrophobic core. The three loops and the N-terminal part of the molecule are held together through four cysteine bridges. We previously reported that the substitution of aspartic acid 27 in echistatin with tryptophan, which is a large hydrophobic residue, makes the RGD loop significantly wider.19 The C-terminal tail, which consists of eight residues following the cysteine 39, has no significant role on the protein molecule. The truncation of the C-terminal end of echistatin (residues 40 through 47) does not alter either the shape or width of the RGD loop (Fig 9).
Structures of echistatin (A) and echistatin 1-41 (B). Residues backbones are color-coded as follows: green, hydrophobic; blue, positively charged; red, negatively charged; yellow, containing sulfur; pink/pale blue, hydrophilic; white, tryptophane; magenta, asparagine/glutamine; cyan, glycine/proline. The side chains of the amino acids at the RAR6DD are shown as ball-and-stick models.
Structures of echistatin (A) and echistatin 1-41 (B). Residues backbones are color-coded as follows: green, hydrophobic; blue, positively charged; red, negatively charged; yellow, containing sulfur; pink/pale blue, hydrophilic; white, tryptophane; magenta, asparagine/glutamine; cyan, glycine/proline. The side chains of the amino acids at the RAR6DD are shown as ball-and-stick models.
DISCUSSION
The results of our experiments suggest that the structure of the RGD loop of echistatin is critical for receptor recognition and that the C-terminal domain of echistatin beyond the RGD loop is critical for the induction of conformational changes of the receptors and expression of LIBS epitopes. Moreover, the C-terminal domain of echistatin appears to increase binding affinity of this disintegrin to receptor in conformationally inactive state.
Scarborough et al13 observed that disintegrins with RGDW sequences show higher avidity to αIIbβ3 integrins and that disintegrins with RGDN sequences interact better with αvβ3 and α5β1 integrins. Other investigators39 40 observed that other amino acids adjacent to RGD sequence also affect selectivity of disintegrins.
We have previously reported19 that substitution of D27 with W made echistatin a more potent inhibitor of platelet aggregation, increased its potency to block binding fibrinogen to immobilized αIIbβ3, and decreased its ability to inhibit binding of vitronectin to immobilized αvβ3. In fact, this substitution made some properties of echistatin similar to eristostatin. In this study, we confirm our previous observation.19 In addition, we report that D27W substitution also enhanced binding affinity of echistatin to resting and to activated platelets and enhanced its ability to inhibit binding of 7E3 antibody to platelets (Fig 8). CHO cells transfected with αIIbβ3 adhered more extensively to immobilized D27W echistatin than to immobilized wild-type echistatin. However, D27W echistatin and wild-type echistatin induced LIBS epitope in αIIbβ3 integrin to the same extent (Figs 4 and 5). In all assays, D27W echistatin was much less reactive with αvβ3 than wild-type echistatin.
Disintegrins are the most potent inducers of LIBS epitopes on αIIbβ3 and αvβ3 integrins. Their LIBS inducing activity is 4 to 5 orders of magnitude higher than the LIBS inducing activity of short linear RGDX peptides (Figs 5 and 6) and at least three orders of magnitude higher than the activity of RGD peptidomimetic Ro-435054 (Marcinkiewicz et al, unpublished observation). Figures 5-7 show that the effect of echistatin on expression of LIBS epitope in αIIbβ3 integrin saturated at 30 nmol/L, whereas the effect of RGDS was not observed at concentrations lower than 0.4 mmol/L. Echistatin and its substituted analogue D27W induced LIBS epitopes in both integrins, as detected by two MoAbs, in all assays at concentrations between 5 and 100 nmol/L. By contrast, truncation of the C-terminal domain of echistatin beyond the RGD loop resulted in a complete loss of the ability to induce LIBS in transfected cells. In platelet assays, the ability of echistatin 1-41 to induce LIBS at 100 nmol/L was diminished, probably reflecting partial activation of the receptor.
Du et al21 previously reported that MoAb 62 antibody binds to low-affinity sites on resting platelets and that the high concentration of RGDS causes expression of about 26,000 high-affinity sites (kd, 16 nmol/L) per platelet. In our experimental system, we only measured the expression of high-affinity sites on platelets and on transfected cells. We reported previously that eristostatin expresses 10,500 (kd, 15 nmol/L) MoAb 62 binding sites per platelet and 47,850 (kd, 4.5 nmol/L) MoAb 62 sites per CHO cell transfected with αIIbβ3.22
Truncated echistatin, after immobilization, retained its ability to bind αvβ3 in adhesion assays (Figs 3B and 4B). However, this deletion resulted in a decreased ability of echistatin to inhibit ligand binding to immobilized αIIbβ3 and αvβ3 (Fig 2), to inhibit platelet aggregation, and to bind to αIIbβ3 on resting platelets. However, binding of truncated echistatin to resting platelets was significantly enhanced after platelet activation with ADP (Fig 1 and Table 1). It should be noted that the pattern of interaction of truncated echistatin with platelets resembled that of decorsin. Decorsin is a low molecular weight peptide isolated from leech extract of Macrobdella decora that acts as a potent fibrinogen receptor antagonist.41 RGD loop of decorsin maintained by two disulfide bridges is very well defined by NMR spectroscopy.42 Decorsin, which does not contain C-terminal amino acids beyond the RGD loop, binds poorly to resting platelets and does not induce LIBS effect in platelet αIIbβ3. After platelet activation with ADP and thrombin, binding affinity of decorsin to platelet αIIbβ3 is restored.43
A number of investigators suggested that C-terminal domains of disintegrins beyond the RGD loop may play a role in their interaction with integrins. Gould et al3 reported that deletion of the sequence PRNP from the C-terminal domain of echistatin reduces its ability to inhibit platelet aggregation. Wright et al44 reported that the synthetic C-terminal peptide of echistatin (PRNPHKGPAT) inhibited binding of αIIbβ3 to immobilized echistatin, stimulated binding of 125I-fibrinogen to human platelets, and did not inhibit echistatin binding to immobilized fibrinogen. It has been suggested the alteration of the amino acid sequences in C-terminal region of gamma echistatin can be also responsible for the decrease of its antiplatelet aggregation ability.45 On the basis of NMR studies, Senn and Klaus46 suggested that the C-terminus of flavoridin could act as a secondary binding determinant for specific interaction with integrin-type receptors. Molecular structure of echistatin (Fig 9) is compatible with this suggestion. Moreover, deletion of the C-terminus does not affect structure of RGD loop as determined by homology modeling with energy minimization and molecular dynamics simulation methods (Fig 9), in contrast to D27W substitution in echistatin that made the RGD loop wider.19 We hypothesize that the putative secondary binding site of echistatin plays a major role in induction of LIBS epitope and in disintegrin interaction with receptor expressed on the cell surface in the nonactive state.
It is difficult to understand why immobilized truncated echistatin retained its ability to interact with αvβ3, although it interacted poorly with αIIbβ3 (Figs 3B and 4B). The results of these experiments should be interpreted with caution. It is conceivable that the ability of truncated echistatin to interact with αvβ3 may reflect conformational changes occurring with immobilization. It is well known that the immobilization of natural ligands such as fibrinogen may increase their binding avidity to integrins. Savage and Ruggeri47 reported that αIIbβ3 expressed on nonstimulated platelets is fully competent to bind immobilized fibrinogen, although it does not significantly interact with soluble fibrinogen. It should be noted that the isoelectric point of truncated echistatin (pH 6.18) is significantly lower than the isoelectric point of wild-type echistatin (pH 7.45). This shift may affect orientation of the residues of truncated echistatin bound to plastic even if the amount of protein, as determined by interaction with antiechistatin antibody, was the same.
In conclusion, we propose a hypothesis that the structure of echistatin's RGD loop determines its selective recognition of αvβ3 and αIIbβ3, whereas the C-terminal domain of this disintegrin significantly contributes to the expression of LIBS and it facilitates binding to nonactivated αIIbβ3. We propose that initially echistatin's RGD loop binds to a recognition site on resting αIIbβ3 integrin with low affinity and that this interaction results in a low level of conformational changes. The second step of interaction involves conformational change of the receptor by the C-terminal domain that leads to a further increase of binding affinity of the molecule to the recognition site. The C-terminal domain of echistatin appears to mimic anti-LIBS antibodies that increase ligand binding to integrins.21 26 Obviously, this second step of interaction cannot occur without initial binding of the RGD loop to the receptor because R24A echistatin did not induce any conformational changes in both integrins. These two steps of interaction are essential to produce maximal inhibitory effect of echistatin on platelet aggregation. Separation of the two steps interaction of echistatin with αIIbβ3 confirms and extends observation by Kouns et al,48,49 who reported that peptidomimetic inhibitor of platelet fibrinogen receptor RO 43-5054 binds to the receptor and induces conformational changes, whereas peptidomimetic RO 44-9883 does not induce significant conformational changes, although it does bind to the receptor.
Induction of LIBS epitopes on αvβ3 was previously observed in our laboratory18,22 and by Pelletier et al.50 We report here that truncated echistatin, in contrast to wild-type echistatin, did not induce LIBS effect in αvβ3 integrin and did not compete as well as wild-type echistatin with the binding of vitronectin to immobilized αvβ3 (Fig 2B) and with the binding of 7E3 MoAb to CHO cells transfected with αvβ3 (Fig 8A). Pelletier et al50 described activation of αvβ3 on the membrane of melanoma cells by anti-LIBS antibody AP5 and suggested that αvβ3 receptors are expressed on cell membrane in two forms: basal and active. Both forms are active but functionally distinct; transition of basal to active form by AP5 appears to stimulate adhesion that is more efficient to stimulate cell migration. In our own study, we observed that anti-LIBS MoAb 62 antibody enhances binding of FITC-echistatin to HUVEC.18 Therefore, it can be proposed that the two-step interaction of echistatin with αvβ3 is also required to produce maximal inhibitory effect of this disintegrin.
ACKNOWLEDGMENT
The authors thank Dr Victor Garsky for the generous gift of synthetic echistatin and its analogues; Dr Mark H. Ginsberg for providing transfected cells and MoAb 62; Dr Barry Coller, Dr Tom Kunicki, and Dr David Cheresh for MoAbs 7E3, AP5, and LM 609, respectively. The invaluable help Dr Yuqin Wang and Mariola Marcinkiewicz in a number of experiments is gratefully acknowledged. The authors also thank Diana Juliano for reading this manuscript and helpful discussion.
Supported by National Institutes of Health Grant No. HL45486 (S.N.); by postdoctoral fellowship 1T32HL07777 (M.A.M.); by a postdoctoral fellowship from the American Heart Association, Southeastern Pennsylvania Affiliate (M.A.M.); and by the Core Grant No. P30-CA-1227 awarded to the Fels Research Institute (S.V.-K.).
Address reprint requests to Stefan Niewiarowski, MD, PhD, Department of Physiology, Temple University School of Medicine, 3400 N Broad St, Philadelphia, PA 19140.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal