Abstract
Fluid shear stress generated by blood flow on arterial wall may play a role in the process of atherosclerosis, not only affecting the mass transport phenomena that take place in blood, but also by modulation of synthesis and secretion of humoral factors released by vascular endothelium that mediate platelet-vessel wall interactions. The present study was designed to investigate whether shear stress, induced by laminar flow, modulates von Willebrand factor (vWF ) release from cultured human umbilical vein endothelial cells (HUVEC) and whether this physical stimulation can affect vWF synthesis. Monolayers of HUVEC were exposed to laminar flow of varying magnitude (from 2 to 12 dynes/cm2) using a cone-and-plate device. The release of vWF in cell supernatant and in extracellular matrix by cells exposed to flow or maintained in static conditions was evaluated by enzyme-linked immunosorbent assay. HUVEC exposed to laminar flow released higher amounts of vWF into the cell supernatant within few hours of exposure and vWF secretion was dependent on shear stress magnitude. vWF released in extracellular matrix was also higher in cell monolayers exposed to shear than in static controls. vWF mRNA expression in HUVEC was not affected by exposure of cells to laminar flow, indicating that shear-induced vWF release reflected enhanced secretion without de novo protein synthesis. Immunofluorescence studies showed that the release of vWF is due to exocytosis from Weibel-Palade bodies, the storage organelles of vWF. These data indicate a novel mechanism by which local hemodynamic shear forces modulate endothelial cell function and may play a role in development of arterial thrombotic events.
ALTHOUGH A CORRELATION between atherosclerosis and thrombus formation was recognized many years ago,1 cellular and molecular determinants of the complex interaction among endothelial cells, circulating cells, and soluble factors have been defined only recently and the process of thrombus formation has been at least in part clarified. It has been consistently established that von Willebrand factor (vWF ), a glycoprotein of protomeric subunits circulating as multimers of a wide size range (from 0.45 to >12 million daltons),2 is a key molecule involved in the acute occlusion of atherosclerotic vessels. Plasma vWF derives primarily from endothelial cells. Largest multimers of the molecule are more thrombogenic due to their property of enhancing platelet adhesion and aggregation. Endothelial-derived vWF mediates endothelial cell adhesion to the vessel wall in physiologic condition and promotes platelet adhesion to the subendothelium in case of endothelial cell detachment.3-5 Mature vWF protein elaborated by endothelial cells is either secreted through a constitutive pathway or stored in Weibel-Palade bodies and released upon stimulation. Potent secretagogues include thrombin, fibrin, and histamine.6-8 Thrombin, promoting disruption of the endothelial cell monolayer, enables vWF release from both sides of the cell, towards circulating plasma and extracellular matrix. By contrast, regulated secretory pathway from Weibel-Palade bodies is predominantly towards basolateral compartment,9 where the molecule binds to the matrix and acquires a remarkably high affinity for platelet receptors.10 11 Early events that determine platelet-vWF interaction depend on platelet glycoprotein (GP) Ib-IX-V receptor complex, whereas subsequent events are mediated by GP IIb-IIIa receptor complex suddenly exposed on the surface of activated platelets.
That changes in fluid shear forces induced by blood flow in damaged arteries may promote a role for vWF in thrombus formation is suggested by the observation that high shear rates, such as those developing in stenotic vessels, are known to favor vWF-GP Ib interaction with platelet activation and subsequent GP IIb-IIIa–dependent platelet spreading and aggregation.12-14
No information exists at the moment on whether local vWF concentrations are modulated by physical stimulation of the endothelium deriving from blood motion. Direct measurements of vWF concentrations are technically impossible, but the possibility exists that shear forces increase local vWF concentrations modulating vWF release by vascular endothelial cells. In line with this hypothesis, it has been shown that there is a correlation between vascular resistance in pulmonary bed and circulating levels of vWF.15 Fluid shear stress acting on endothelial cell surface is known to affect endothelial behavior by eliciting complex biochemical and gene regulatory responses. Data are available showing that magnitude of laminar shear stresses influences cell shape and orientation and cytoskeleton protein distribution and induces a variety of cell processes that include protein synthesis and secretion.16,17 In vitro studies established in recent years show that fluid shear stress governs biochemical pathways of several intracellular signaling.18-20 The present study was then designed to investigate whether shear forces, induced on cultured endothelial cells by laminar flow, modulate vWF release in cell supernatant and in the extracellular matrix. We investigated also whether this physical stimulation of the endothelial cells can affect the synthesis of vWF and/or its release.
MATERIALS AND METHODS
Endothelial cell culture.Endothelial cells were obtained from human umbilical veins (HUVEC) by collagenase digestion according to the method of Jaffe et al.21 The cells were first plated in tissue culture plates (Falcon Labware Division, Becton Dickinson, Milan, Italy) precoated with 0.2% bovine gelatin. The growth medium consisted of Medium 199 (GIBCO, Grand Island, NY) supplemented with 10% newborn calf serum (GIBCO) and 10% human serum, 20 mmol/L N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES; Sigma Chemical, St Louis, MO), 100 U/mL penicillin, 100 μg/mL streptomycin, 250 ng/mL fungizone, 2 mmol/L glutamine (GIBCO), 15 U/mL heparin (Parke-Davis, Milan, Italy), and 50 μg/mL endothelial cell growth factor. Cultures were grown at 37°C in 5% CO2 -95% air. Confluent HUVEC were passaged with 0.025% trypsin-0.02% EDTA (GIBCO) and were routinely used between the first and fifth passage. Cultured cells were identified as endothelial by their morphology and the presence of vWF antigen, using indirect immunofluorescence microscopy. For shear stress experiments, HUVEC were plated on 145-cm2 plastic dishes and used 1 day after attaining confluence in Medium 199 plus 5% newborn calf serum and 5% of human serum (test medium).
Shear exposure experiments.Confluent monolayers of HUVEC were exposed to laminar flow in a cone-and-plate apparatus described in detail elsewhere.22,23 Briefly, the cell culture dishes coated by HUVEC were inserted in the apparatus and laminar flow over the cell surface was induced using a rotating cone with an angle of 0.5°. The apparatus was designed to maintain constant speed of rotation of the cone, temperature (37°C), and humidified air with 5% CO2 . Cone rotation speed was varied to expose endothelial cells to constant shear stress levels of 2, 8, and 12 dynes/cm2. Shear stress values were extrapolated from previous measurements and theoretical analysis of fluid motion in the cone-plate geometry.24-26 Two culture plates were used in each experiment; one plate was placed in the flow apparatus and the other was kept in a normal incubator under static conditions. Culture medium (22 mL) was used to completely fill the space between the plate and the cone, and cells maintained in static conditions were incubated with the same amount of medium. After 6 and 15 hours of exposure to flow or static conditions, HUVEC incubation medium was collected for vWF measurement and cells were washed with phosphate-buffered saline and then detached with 0.025% trypsin-0.02% EDTA (GIBCO). Cells were then counted and used for mRNA vWF expression evaluation. After HUVEC were removed, the matrix deposited on the plates was solubilized with 2.5 mL enzyme-linked immunosorbent assay (ELISA) washing solution (phosphate-buffered saline, pH 7.4, with 0.5% Tween 20) for 45 minutes on a rotating platform. The solubilized matrix was collected, and the amount of vWF was measured by ELISA. For immunofluorescence studies, cells were grown on plastic coverslips (Thermanox; Nunc Inc, Naperville, IL) and subjected to fluid shear stress in a parallel-plate perfusion chamber as previously described.27 Culture medium was perfused inside the chamber using computer controlled syringe pumps. The perfusion system was maintained under constant condition of temperature (37°C) and in an atmosphere of 5% CO2 . Exposure of cell monolayers using both experimental equipments did not induce endothelial cell alignment. This is not in contrast with previous reports because cell alignment has been shown to take place only after more than 24 hours to shear stress levels higher than 8 dynes/cm2.
vWF measurements.Levels of vWF antigen were determined by sandwich ELISA. Briefly, 50-μL test samples were incubated for 2 hours in microplate wells coated with mouse antihuman vWF antibodies (kindly provided by Dr Z.M. Ruggeri, The Scripps Research Institute, La Jolla, CA). Subsequently, rabbit anti-vWF polyclonal antibodies (Dakopatts, Glostrup, Denmark) were added to the wells followed by peroxidase-conjugated antirabbit IgG (Sigma Immunochemicals). The final step consisted of incubation with the substrate 1,2-phenylendiamine (Dakopatts) in the presence of hydrogen peroxide. The absorbance was read at 492 nm on a Flow Titertek Multiscan (ICN Biochemicals, Costa Mesa, CA) and the concentration of vWF was calculated by extrapolation from a standard curve obtained with purified human vWF. The amount of vWF in culture medium containing human serum was subtracted from vWF concentration measured in cell supernatants. Results, which were normalized for the number of cells, were expressed as the percentage changes in respect to static control.
vWF mRNA expression.Total cellular RNA was isolated from HUVEC by the guanidium isothiocyanate/cesium chloride procedure.28 Ten micrograms of RNA was then fractionated on 1.2% agarose gel with 6% formaldehyde and blotted onto synthetic membranes (Gene Screen Plus; New England Nuclear, Boston, MA).29 Gels were stained with ethidium bromide to visualize 28S and 18S ribosomal RNA bands. These bands were used to confirm that approximately equivalent amounts of RNA were loaded in each gel lane and that there was not obvious degradation of RNA. Plasmid containing vWF probe was kindly provided by Dr D.C. Lynch (Dana-Farber Cancer Institute, Boston, MA). vWF mRNA was detected by using the 1.9-kb fragment of a vWF cDNA. The cDNA fragment of vWF was labeled with α 32P-dCTP using the random-primed method.30 Membranes were hybridized for 20 hours at 60°C with 1.5 × 106 cpm/labeled probe and the filters were washed as previously described.29 Membranes were subsequently rehybridized with a GAPDH cDNA31 to determine an internal standard of total RNA content. vWF mRNA optical density was normalized to that of the constituently released GAPDH gene expression.
Immunofluorescence and confocal microscopy studies.For each experiment, endothelial cells grown on plastic coverslips were maintained for 6 hours as follows: static conditions with control medium, static conditions plus 100 U/mL interleukin-1β (IL-1β; Boehringer Mannheim, Mannheim, Germany) that is known to induce vWF release32 or subjected to flow (8 dynes/cm2 ). After exposure to shear or to static conditions, cultured cells on coverslips were fixed in 4% paraformaldehyde and permeabilized with Triton X-100 in phosphate-buffered solution. vWF was visualized by sequential incubation with rabbit anti-vWF antibodies (Dakopatts) at a concentration of 2.5 μg/mL and with fluorescein-conjugated swine antirabbit IgG (Dakopatts) diluted 1:20. Coverslips were washed, mounted, and examined under a confocal inverted laser microscope (InSight plus; Meridian Instruments, Inc, Okemos, MI). Argon laser emission filter at 488 nm was used to excite specimens at that wavelength. A band-pass 530/30 filter was used to suppress the undesirable fluorescent beams other than the green emitted one at 525 nm. Representative fields of controls or stimulated HUVEC were digitized with 256 gray levels and printed using a film printer (Montage, FR2 film recorder; Presentation Technologies, Sunnyvale, CA).
Statistical analysis.Results are expressed as the mean ± SE. Statistical analysis was performed using ANOVA and the Tukey-Cicchetti test for multiple group comparisons. Statistical significance was defined as P < .05.
RESULTS
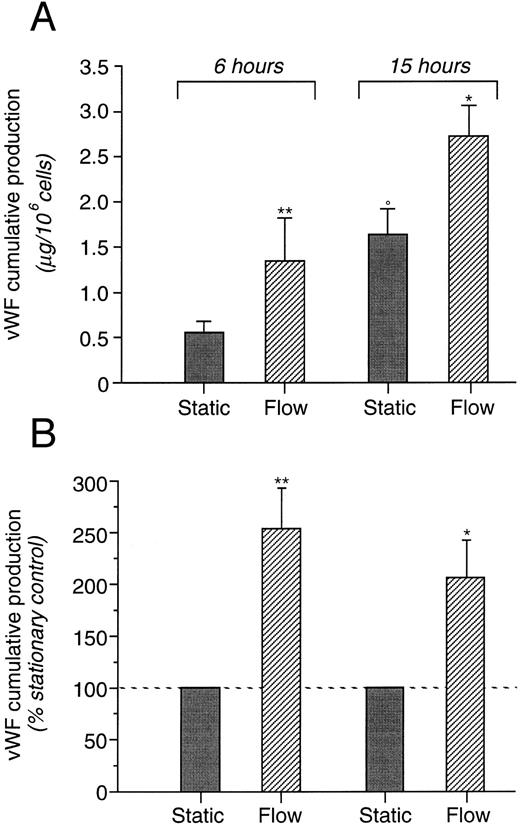
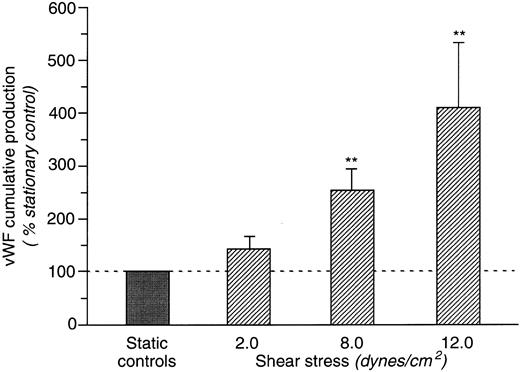
vWF production.Cumulative production of vWF in culture media of HUVEC maintained in static conditions or subjected to shear stress of 8 dynes/cm2 is reported in Fig 1A. vWF release during 6- and 15-hour incubations was significantly higher (P < .01 and P < .05, respectively) in HUVEC exposed to shear stress as compared with static cultures. As shown in Fig 1B, the actual amount of vWF released in cell supernatant, after 6 hours of exposure to shear stress, as compared with static controls, was more than 250% and more than 200% after 15 hours of incubation of that of static controls. HUVEC monolayers were also exposed to different shear stress levels, 2, 8, and 12 dynes/cm2, for 6 hours. Percentage increases in vWF production induced by different levels of shear are reported in Fig 2. At 2 dynes/cm2, vWF production was numerically increased over values measured in static controls (1.23 ± 0.66 v 0.72 ± 0.28 μg/106 cells) but the difference did not reached statistical significance. vWF production was significantly higher in cells exposed to 8 dynes/cm2 (1.35 ± 0.45 v 0.54 ± 0.14 μg/106 cells) and 12 dynes/cm2 (1.69 ± 0.51 v 0.49 ± 0.10 μg/106 cells) than in static cultures.
Cumulative production of vWF released into the supernatant from cultured HUVEC exposed to fluid shear stress (8 dynes/cm2 ) for 6 and 15 hours (n = 6 and 5, respectively). Data are expressed as the mean ± SE of vWF released from cells exposed to shear and to static condition (A) and as the percentage increase of cumulative production by cells exposed to flow versus static controls (B). *P < .05 flow versus static; **P < .01 flow versus static; °P < .05 static at 15 hours versus static at 6 hours.
Cumulative production of vWF released into the supernatant from cultured HUVEC exposed to fluid shear stress (8 dynes/cm2 ) for 6 and 15 hours (n = 6 and 5, respectively). Data are expressed as the mean ± SE of vWF released from cells exposed to shear and to static condition (A) and as the percentage increase of cumulative production by cells exposed to flow versus static controls (B). *P < .05 flow versus static; **P < .01 flow versus static; °P < .05 static at 15 hours versus static at 6 hours.
vWF release in cell supernatant by HUVEC exposed for 6 hours to shear stress level of 2, 8, and 12 dynes/cm2 (n = 4, 6, and 6, respectively). Cumulative production of vWF is expressed as the percentage increase of corresponding cumulative production of stationary cultures. Data are the mean ± SE. **P < .01 flow versus static.
vWF release in cell supernatant by HUVEC exposed for 6 hours to shear stress level of 2, 8, and 12 dynes/cm2 (n = 4, 6, and 6, respectively). Cumulative production of vWF is expressed as the percentage increase of corresponding cumulative production of stationary cultures. Data are the mean ± SE. **P < .01 flow versus static.
vWF production towards basolateral surface of HUVEC exposed to flow or to static conditions was evaluated measuring the amount of vWF in cell matrix. As shown in Fig 3, after 6 hours of exposure of HUVEC to shear stress no significant difference was observed in vWF production in the matrix of cells maintained in static conditions or exposed to 12 dynes/cm2. At variance, after 15 hours of exposure to shear (12 dynes/cm2 ) vWF deposition in the cell matrix was importantly increased as compared with control cultures.
vWF release in basolateral direction from HUVEC exposed for 6 (n = 3) and 15 hours (n = 3) to shear stress of 12 dynes/cm2. Cumulative production of vWF in cell matrix is expressed as the percentage increase of corresponding stationary cultures.
vWF release in basolateral direction from HUVEC exposed for 6 (n = 3) and 15 hours (n = 3) to shear stress of 12 dynes/cm2. Cumulative production of vWF in cell matrix is expressed as the percentage increase of corresponding stationary cultures.
To verify that shear exposure did not induce disruption of the cell monolayer, we measured lactate dehydrogenase (LDH) activity in cell supernatants in a separate series of experiments. LDH activity was comparable in supernatants derived from cells maintained in static conditions and from cells exposed to flow (<1% cell lysis for static and flow cultures).
vWF mRNA expression.To test whether enhanced release of vWF was due to a secretion from storage sites or was the result of new protein synthesis, possibly regulated by shear, we measured vWF mRNA expression in cells exposed to shear and in corresponding static cultures. Shear exposure did not enhance vWF mRNA expression. As reported in Fig 4, the expression of vWF mRNA in HUVEC exposed to flow (8 dynes/cm2 ) for 3 and 6 hours were comparable with those of cells maintained in static conditions. Densitometric analysis (data not shown) failed to detect significant differences between cells exposed to flow and to static conditions.
Northern blot analysis of RNA obtained from HUVEC exposed to laminar shear stress of 8 dynes/cm2 and to static conditions for 3 and 6 hours. After electrophoresis on agarose/formaldehyde gels, RNA was transferred to nylon membranes and hybridized sequentially with [α32P]-labeled vWF (top) and GAPDH (bottom) cDNA probes. The optical density of the autoradiography signals was quantitated using densitometric analysis and calculated as the ratio of vWF to GAPDH mRNA. The mRNA levels of laminar flow were calculated assuming optical density of static controls (unsheared cells) as a unit. As shown in the right side, the amount and integrity of RNA were checked by ethidium bromide staining.
Northern blot analysis of RNA obtained from HUVEC exposed to laminar shear stress of 8 dynes/cm2 and to static conditions for 3 and 6 hours. After electrophoresis on agarose/formaldehyde gels, RNA was transferred to nylon membranes and hybridized sequentially with [α32P]-labeled vWF (top) and GAPDH (bottom) cDNA probes. The optical density of the autoradiography signals was quantitated using densitometric analysis and calculated as the ratio of vWF to GAPDH mRNA. The mRNA levels of laminar flow were calculated assuming optical density of static controls (unsheared cells) as a unit. As shown in the right side, the amount and integrity of RNA were checked by ethidium bromide staining.
Immunofluorescence studies.The results of the immunofluorescent staining are represented in Fig 5. The cell culture monolayer appeared uniformly stained, without predominant localization in individual cells or groups of cells. In HUVEC maintained in control conditions, the immunofluorescent staining was localized throughout the thickness of the cytoplasm in the Weibel-Palade bodies (see Fig 5-1a and 5-1b), the rod-shaped endothelial cell-specific organelles. In contrast, after exposure to flow (see Fig 5-2a and 5-2b) or incubation with IL-1 β (see Fig 5-3a and 5-3b), there was the appearance of irregular patches typical of extracellular vWF and the depletion in Weibel-Palade bodies. These results suggest that shear stress exposure, like IL-1β, induces release of vWF by exocytosis from Weibel-Palade bodies.
Representative images of two experiments showing shear stress-induced vWF release by immunofluorescence. Confluent HUVEC were maintained in static conditions (1a and 1b), subjected to shear stress of 8 dynes/cm2 for 6 hours (2a and 2b), or stimulated with IL-1β (100 U/mL) for 6 hours (3a and 3b). After fixation and permeabilization, the cells were stained by sequential incubation with rabbit anti-vWF antibodies and fluorescein-conjugated antirabbit IgG. The rod-shaped Weibel-Palade bodies are found in unstimulated controls, whereas cells exposed to flow and stimulated with IL-1β showed a depletion of Weibel-Palade bodies and the appearance of extracellular patches of staining.
Representative images of two experiments showing shear stress-induced vWF release by immunofluorescence. Confluent HUVEC were maintained in static conditions (1a and 1b), subjected to shear stress of 8 dynes/cm2 for 6 hours (2a and 2b), or stimulated with IL-1β (100 U/mL) for 6 hours (3a and 3b). After fixation and permeabilization, the cells were stained by sequential incubation with rabbit anti-vWF antibodies and fluorescein-conjugated antirabbit IgG. The rod-shaped Weibel-Palade bodies are found in unstimulated controls, whereas cells exposed to flow and stimulated with IL-1β showed a depletion of Weibel-Palade bodies and the appearance of extracellular patches of staining.
DISCUSSION
The present results show that vWF release by endothelial cells, which are the principal source of this protein, is influenced by fluid shear stress. Our data show that laminar shear stress greater than 8 dynes/cm2 acting on the cell monolayer for at least 6 hours induces a significant increase in the amount of vWF released in culture media. The increase in vWF released from HUVEC into the culture medium is dependent on the level of shear. Shear stress exposure of HUVEC monolayer also importantly increases vWF deposition in extracellular matrix. Recent observations from Thoumine et al33 have shown that exposure of endothelial cells to laminar flow importantly changes the composition of extracellular proteins such as fibronectin, type IV collagen, and laminin. This evidence indicates that shear forces acting on vascular endothelium modulate the final composition of extracellular matrix with important implications for endothelial and smooth muscle cell function.
vWF mRNA levels in HUVEC exposed to laminar flow were comparable with those derived from cells maintained in static conditions. This indicates that the enhancement of vWF release induced by shear exposure was not the consequence of de novo protein synthesis but rather was derived from an enhanced secretion of this protein. Endothelial cells release vWF by two different pathways. One is a constitutive pathway of synthesis and secretion of vWF that serves to maintain physiologic levels in circulating plasma. The other is the exocytosis from Weibel-Palade bodies, the storage sites of this protein, after endothelial cell stimulation.3 Our results indicate that shear stress causes the release of vWF by exocytosis from Weibel-Palade bodies. Immunofluorescence microscopy showed extracellular vWF deposits and the depletion of Weibel-Palade bodies from endothelial cells exposed to shear stress. Thus, in line with a previous report, vWF released by exocytosis appears as large irregular patches associated with the external surface of the cells.34 The explanation for this phenomenon is that the protein released is extremely large, because Weibel-Palade bodies contain vWF multimers larger than those circulating in plasma. Some vWF molecules may be trapped nonspecifically between adhesion plaques of the cells. The same results were here obtained by cell stimulation with IL-1β. It has been shown that also IL-1β induces vWF release without de novo protein synthesis.32 It has been suggested that this release derives from granular stores, because evaluation of 51Cr release failed to evidence a nonspecific endothelial cell injury. Our present study confirmed that IL-1β induces depletion of Weibel-Palade bodies in cells treated with IL-1β. Cell morphology was unaltered after IL-1β incubation or shear stress exposure, indicating that vWF release did not result from cell lysis.
An essential signaling in the agonist-induced secretory response is the variation of intracellular ionized calcium concentration. Evidence that exocytosis of Weibel-Palade bodies in response to histamine, thrombin, and xanthine oxidase8,35,36 is mediated by an increase in intracellular calcium and that vWF secretion can be induced by calcium ionophore37 suggest that the increase in ionized calcium concentration may be both necessary and sufficient to induce vWF release by exocytosis. One of the effects produced by fluid shear stress on endothelial cells is the capacity to increase the intracellular ionized calcium concentration,38 also in the absence of an agonist,39 indicating that mechanical transduction is a component of the signaling process.
The present study describes a previously unrecognized modulation of vWF secretion induced by mechanical forces. Our results indicate that, when cultured endothelial cells grown under static conditions are suddenly exposed to shear stress, they increase vWF secretion into circulating medium within a few hours and subsequently in the extracellular matrix. As a general view, these results would suggest that changes in shear forces induce cell adaptation that enhance vWF secretion. Changes in local hemodynamic conditions may develop in damaged arterial segments as a consequence of partial occlusion of vessel lumen by arterial thrombus formation. In these conditions, blood velocity can greatly increase rising wall shear stress. A recent study by Senis et al40 also indicates that local hemodynamic conditions may play an important role in vWF distribution along the aortic endothelial layer. These investigators reported that vWF localization is particularly prominent around the orifices of the intercostal arteries, sites of the vasculature that are characterized by nonuniform distribution of shear stresses.
Other conditions that lead to changes in wall shear stress are related to changes in blood flow rate. It has been suggested that, under physiologic conditions, vascular wall adapts to changes in blood flow rate in such a way to maintain constant wall shear stress.41 The vascular wall seems to react to acute and chronic changes in blood flow with two distinct mechanisms, both mediated by the endothelium. Acute changes in blood flow induce release of vasoactive molecules by the endothelium,42,43 whereas more chronic changes induce vascular remodeling.44 One can speculate that alterations in these peculiar functions of the endothelial cells can lead to changes in wall shear stress and, in these circumstances, secretion of vWF molecules from endothelial cells into flowing blood may change. As mentioned previously, data are available that increased vascular resistance in pulmonary circulation is associated with enhanced endothelial release of vWF, which may play an important role in thrombotic complications in these patients.15 Additional evidence is available showing that major conditions associated with vascular dysfunction in humans (ie, hypercholesterolemia, diabetes, hypertension, and smoking45-48 ) are all consistently characterized by an increase in circulating vWF levels, suggesting an important role of vWF in mediating thrombotic complications of atherosclerosis. That vWF is directly implicated in these pathologic processes rests on experimental data that pigs with von Willebrand disease49 or made vWF-deficient by monoclonal antibodies50 had no occlusive coronary thrombi despite an atherogenic diet. In addition, in humans, high levels of circulating vWF predict reinfarction and mortality after an acute myocardial infarction.51
In conclusion, our study indicates that changes in hemodynamically induced shear forces influence vWF secretion by vascular endothelium on either sides of the cell. This phenomenon may be involved in the development of arterial thrombosis. It derives that new antithrombotic strategies oriented to inhibit vWF binding to GP Ib may prevent shear-dependent platelet aggregation and thrombus formation.
ACKNOWLEDGMENT
The authors thank Dr Annalisa Perna for help in statistical analysis, Dr Marina Noris for helpful discussion, Dr Barbara Imberti for immunofluorescence studies, and Dr Pio Zilio for LDH measurements.
Dedicated to Prof Alfredo Leonardi.
Supported in part by the CNR (National Research Council, Rome, Italy), Contract No. 88.00426.04..
Presented in part at the annual meeting of the American Society of Nephrology (November 1995, San Diego, CA) as a poster.
Address reprint requests to Andrea Remuzzi, EngD, Biomedical Engineering Laboratory, Mario Negri Institute for Pharmacological Research, Via Gavazzeni, 11-24125 Bergamo, Italy.


![Fig. 4. Northern blot analysis of RNA obtained from HUVEC exposed to laminar shear stress of 8 dynes/cm2 and to static conditions for 3 and 6 hours. After electrophoresis on agarose/formaldehyde gels, RNA was transferred to nylon membranes and hybridized sequentially with [α32P]-labeled vWF (top) and GAPDH (bottom) cDNA probes. The optical density of the autoradiography signals was quantitated using densitometric analysis and calculated as the ratio of vWF to GAPDH mRNA. The mRNA levels of laminar flow were calculated assuming optical density of static controls (unsheared cells) as a unit. As shown in the right side, the amount and integrity of RNA were checked by ethidium bromide staining.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1558/3/m_bl_0007f4.jpeg?Expires=1767734283&Signature=VV5BOrPCmID-zD5UmJHESdyKkkQvUY0QTfgrqrlekbGaXs35KmeXc79OljWw8gPbWN0NXxkf7GcjI6La2IdthdJ7kHqeq~0VRWmhVi8F155u5eilngLAVbmtED0UFzS8GhsRRjpilZF-XgAoMclqS3tCWXTk13e2GvQIhSYWyKJ0uFrAMJR9zsvEZWEEavj7KQ0ETkaGRH-H8JFxkjVsLPFKuJ~Y0LjyCRAe4tH1JtsMpqVyXYAcvtITzi5-spjpY~50xHuQ28GsfTep81vusPzJlao4pGAkGSQHejXbQ51SKjVNlO6hWFqq20V0d6O7z5kC14Gb-P-guBZw2OOS5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal