Abstract
Impaired fibrinolysis, resulting from increased plasminogen activator inhibitor-1 (PAI-1) or reduced tissue-type plasminogen activator (t-PA) plasma levels, may predispose the individual to subacute thrombosis in sepsis and inflammation. The objective of these studies was to show that adenovirus-mediated gene transfer could increase systemic plasma t-PA levels and thrombolytic capacity in animal model systems. Recombinant adenovirus vectors were constructed that express either human wild type or PAI-1–resistant t-PA from the cytomegalovirus (CMV) promoter. Both t-PA-deficient (t-PA−/−) and PAI-1–overexpressing transgenic mice were infected by intravenous injection of these viruses. Intravenous injection of recombinant adenovirus resulted in liver gene transfer, t-PA synthesis, and secretion into the plasma. Virus dose, human t-PA antigen, and activity concentrations in plasma and extent of lysis of a 125I-fibrin–labeled pulmonary embolism were all closely correlated. Plasma t-PA antigen and activity were increased approximately 1,000-fold above normal levels. Clot lysis was significantly increased in mice injected with a t-PA–expressing virus, but not in mice injected with saline or an irrelevant adenovirus. Comparable levels of enzyme activity and clot lysis were obtained with wild type and inhibitor-resistant t-PA viruses. Adenovirus-mediated t-PA gene transfer was found to augment clot lysis as early as 4 hours after infection, but expression levels subsided within 7 days. Adenovirus-mediated transfer of a t-PA gene can effectively increase plasma fibrinolytic activity and either restore (in t-PA–deficient mice) or augment (in PAI-1–overexpressing mice) the thrombolytic capacity in simple animal models of defective fibrinolysis.
DEFICIENT FIBRINOLYTIC activity, resulting from increased plasma plasminogen activator inhibitor-1 (PAI-1) levels or reduced plasma tissue-type plasminogen activator (t-PA) or plasminogen levels, is associated with thromboembolic disease,1-5 whereas increased fibrinolytic activity due to excess plasminogen activator or decreased PAI-1 levels predisposes to bleeding.6-8 In mice, gene targeting studies have shown that deficiencies of t-PA, urokinase-type plasminogen activator (u-PA),9,10 and plasminogen11,12 and overexpression of PAI-113 increases the susceptibility to spontaneous or endotoxin-induced vascular thrombosis, whereas the deficiency of PAI-1 reduces it.14,15 In humans, elevated PAI-1 levels correlate with increased risk for vascular thrombosis in the settings of disseminated intravascular coagulation,16 sepsis,17 surgery,18 and trauma.19 Elevated PAI-1 levels have also been variably identified in patients with coronary artery disease20 and unstable myocardial ischemia21 and correlate with the risk for recurrent myocardial infarction.22
Currently available antithrombotic therapy includes the use of anticoagulant and/or thrombolytic agents. For life-threatening acute thrombotic syndromes, eg, acute myocardial infarction, stroke, or pulmonary embolism, short-term administration of a thrombolytic agent in combination with anticoagulation is an effective mode of therapy, although recurrent vascular thrombosis following successful thrombolytic therapy remains an important problem.4,23 24 Protracted administration of these agents for subacute episodes of thrombosis during sepsis, trauma, or surgery, however, is impractical.
In theory, transient overexpression of a gene encoding a thrombolytic (or antithrombotic) protein might provide an alternative approach to achieving a more potent and sustained antithrombotic state. Conceivably, gene transfer to treat vascular thrombosis could be directed either locally or systemically. In the setting of an identifiable at-risk vessel, eg, in syndromes of acute myocardial ischemia, direct genetic modification of cells in the wall of the target vessel segment might be preferable, as it could limit the potential for deleterious systemic effects.25-29 In the setting of a systemic hypercoagulable state, however, effective prophylaxis or therapy is likely to require a systemic approach. Intravenous administration of recombinant adenovirus results in preferential and efficient expression of a transgene in mammalian liver and the accumulation of physiologically significant concentrations of recombinant protein in plasma.30-34 Based on these observations, we hypothesized that intravenous administration of a recombinant adenovirus carrying a gene encoding t-PA could transiently augment the systemic fibrinolytic potential.
The capacity to increase plasma fibrinolytic activity through overexpression of a t-PA gene is potentially limited by the high concentration of PAI-1 in blood.2-5 For treatment of thrombotic occlusion, large quantities of t-PA are administered, in part to overwhelm the inhibitory capacity of plasma serpins. Variants of human t-PA resistant to serpin inhibition have been engineered,35 which show clot lysis activity substantially greater than that of the native enzyme in the presence of physiologic concentrations of PAI-1.36-38
In the present study, experiments were undertaken to determine whether adenovirus-mediated transfer of either the wild type t-PA gene or a gene encoding a serpin-resistant variant of t-PA could have a beneficial effect on clot lysis in vivo. Using a pulmonary plasma clot lysis model in mice, adenovirus-mediated t-PA gene transfer was found to augment circulating t-PA antigen levels and to restore the fibrinolytic capacity in mice homozygous for a targeted disruption of the endogenous t-PA gene (t-PA−/−) in a dose-dependent manner. Transgenic mice overexpressing murine PAI-1 also showed large increases in circulating t-PA activity and an augmentation of their thrombolytic potential. Overexpression of both forms of t-PA was found to be highly effective even in the presence of elevated quantities of PAI-1.
MATERIALS AND METHODS
Production of recombinant adenovirus.The recombinant adenoviruses AdCMVt-PAWT and AdCMVt-PACB were generated by homologous recombination in 293 cells essentially as previously described.39 Briefly, an Xba I-fragment of the plasmid pSTEt-PA encoding wild type human t-PA or pSTEt-PA (K296,R298,R299 → E,E,E) encoding the serpin-resistant variant of human t-PA in which the lysine residue at position 296 and the arginine residues at positions 298 and 299 are replaced by glutamic acid,35 were ligated into XbaI-digested pACCMVpLpA40 to produce pACCMVt-PAWT and pACCMVt-PACB, respectively. In these plasmids, the t-PA cDNA is positioned between the human cytomegalovirus immediate-early enhancer/promoter and the SV40 t-antigen intron/polyadenylation signal to form a complete transcriptional unit. Monolayer cultures of 293 cells41 were cotransfected with either 10 μg of pACCMVt-PAWT or 10 μg of pACCMVt-PACB and 5 μg of pJM17,39 a plasmid containing a full-length adenovirus 5 dl309 genome. Homologous recombination between these plasmids results in the formation of recombinant viral genomes in which the adenovirus E1 region is replaced by the respective t-PA transgenes. Replication of the recombinant viruses in cultured 293 cells is supported by E1A gene products supplied in trans from a copy of E1 integrated into the 293 cell genome.
After transfection, recombinant viral plaques were harvested and amplified as described.42 The identity of recombinant viruses was determined by restriction analysis and Southern blotting of viral DNA prepared from productively infected 293 cells. The recombinant adenovirus AdRR5, which lacks an inserted gene in the E1 position, was generated from pACRR5 and pJM17 in the same manner and was used as a control adenovirus.31,43 Recombinant viruses were replaqued to ensure clonal identity before further use. Large scale production of recombinant adenovirus was performed as described.42 Purified virus was supplemented with 0.1 mg/mL sterile bovine serum albumin (BSA), snap frozen in liquid nitrogen and stored at −80°C until use. The titer of infectious viral particles in purified stocks was determined by plaque assay on monolayers of 293 cells with 1 hour of adsorption at 37°C. Purified viral stocks of >1010 plaque forming units (pfu) per mL were routinely obtained.
Infection of cultured cells.Primary human umbilical vein endothelial cells (HUVEC, passage 3) were propagated as previously described44 in 6 cm culture dishes. Cells (approximately 2.5 × 106 per dish) were infected for 1 hour with recombinant adenovirus stocks at a multiplicity of infection of 20 pfu per cell and subsequently cultured in complete endothelial cell growth medium for 48 hours. Medium was removed and the cells washed with 1:1 Dulbecco's Modified Eagle Medium (DMEM): Medium 199 supplemented with 0.5% BSA. Medium (2 mL DMEM:199 containing 0.5% BSA) was then conditioned for 4 hours at 37°C and t-PA activity was determined using an indirect assay for fibrin-dependent plasminogen activation.45 The activity of t-PA was quantified by comparison with a standard curve generated by the addition of known concentrations of purified human recombinant t-PA to DMEM:199 containing 0.5% BSA.
Animals and blood collection.Mice containing a single copy of the murine cDNA for PAI-1 under control of the cytomegalovirus (CMV) promoter were generously provided by Dr David Ginsburg (University of Michigan, Ann Arbor, MI). Such mice express 108 ± 17 ng/mL total PAI-1 by comparison to 6.2 ± 0.3 ng/mL for controls, of which approximately 50% is present in active form46 and can inhibit both human and murine t-PA. Wild type (C57Bl/6), t-PA+/−, and t-PA−/− mice were obtained from breeding stocks.9 10 Mice were kept in microisolation cages on a 12-hour day/night cycle and fed regular chow. Unless otherwise stated, the mice were anesthetized by intraperitoneal injection of 60 mg/kg Nembutal (Abbott Laboratories, North Chicago, IL), and blood was collected by vena cava puncture with a 24-gauge needle. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) determinations were performed in the hospital clinical laboratory. All procedures involving experimental animals were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee of KU Leuven.
Pulmonary embolism model.Anesthetized 8 to 12 week-old mice received the indicated dose of control or recombinant adenovirus diluted into saline supplemented with 0.1 mg/mL BSA (Boehringer, Mannheim, Germany) to a final volume of 200 μL. Virus was delivered by intravenous injection into either the jugular vein using a 2 French catheter (Portex Green, Hythe, UK) or into the tail vein. No differences in liver gene transfer were observed using the two routes of intravenous injection. Radiolabeled autologous murine platelet-poor plasma clots were produced as previously described.47 A 25-μL 125I-fibrin labeled plasma clot, containing ≈70,000 cpm human 125I-fibrinogen (corresponding to 0.07 μCi 125I), was injected into the jugular vein either immediately after administration of virus or at a later time as indicated, following a second anesthesia.
To assess the efficacy of exogenously administered t-PA in t-PA–deficient mice, groups of animals were treated with either saline or a bolus intravenous injection of 2 mg/kg body weight recombinant human t-PA (Activase, Genentech, Inc, San Francisco, CA), dissolved in 200 μL saline immediately after injection of the 125I-fibrin–labeled plasma clots. The rate of lysis of the 125I-fibrin–labeled plasma clots, which embolize to the pulmonary arteries after injection into the jugular vein, was monitored as previously described.47 At varying times after injection of the plasma clots, mice were killed and clot lysis measured from the residual radioactivity in the heart and lungs. Clot lysis was quantified as the fraction of radioactivity disappearing from the heart and lungs over the interval between clot injection and death.
t-PA, plasminogen, and fibrinogen measurements.At the time of death, blood was collected for assessment of plasma t-PA antigen and activity, and liver was harvested for immunohistochemical staining for t-PA antigen. Plasma levels of t-PA antigen were determined using enzyme-linked immunosorbent assays (ELISA)48 specific for the human enzyme. For plasma t-PA activity measurements, 0.05 mL of blood was collected from the retroorbital plexus and immediately acidified with 0.1 volume of 0.3 mol/L Na acetate pH 5.2 to inhibit t-PA:PAI-1 complex formation. t-PA activity was measured using an indirect plasminogen activation assay and comparison to a standard curve of known t-PA concentration as previously described.35 Plasma fibrinogen was determined by a coagulation rate assay as previously described.49 Immunohistochemical staining for t-PA used an indirect immunoperoxidase method employing polyclonal human t-PA specific antibodies (American Diagnostica, Greenwich, CT), as previously described.10 Plasminogen antigen levels were quantified by capture ELISA using affinity-purified rabbit polyclonal antibody to murine plasminogen and comparison with a standard curve, as previously described.11
Statistical analysis.Data are presented as mean ± standard error of mean (SEM) of the indicated number of animals for each treatment group. The data were examined by analysis of variance (ANOVA) unless otherwise stated, with P < .05 assumed to indicate a significant difference between experimental groups.
RESULTS
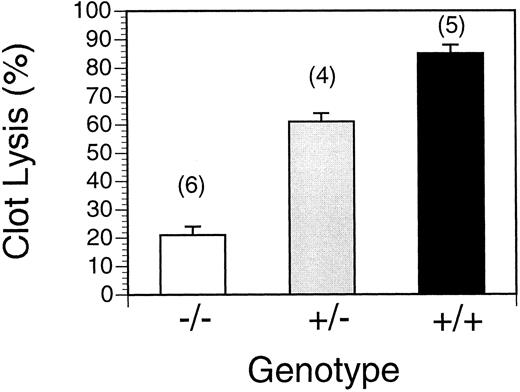
Efficiency of thrombolysis as a function of the number of t-PA alleles in the murine genome.Mice homozygous for a targeted disruption of the endogenous t-PA gene have no detectable tissue plasminogen activator antigen in plasma.10 As shown in Fig 1, clot lysis, defined as the disappearance of radioactivity from the heart and lungs of a 25 μL radiolabeled plasma clot injected in the jugular vein, was dependent on the number of functional t-PA alleles in the mouse genome. Wild type mice almost completely lysed (85% ± 4%, n = 5) the embolized clot within 16 hours. By comparison, t-PA−/− mice were highly defective in clot lysis, showing a dramatically reduced rate of dissolution of pulmonary platelet-poor 125I-fibrin labeled plasma clots (21% ± 3%, n = 6). Heterozygotes showed an intermediate level of lysis (61% ± 4%, n = 4), which was correlated with their expression of intermediate levels of t-PA.10
Clot lysis in wild type, t-PA+/− and t-PA−/− mice. Spontaneous lysis of pulmonary emboli 16 hours after clot injection. +/+: wild type mice; +/−, heterozygous t-PA deficient; −/−, homozygous t-PA deficient. Values represent mean ± SEM of the indicated number of mice in parentheses.
Clot lysis in wild type, t-PA+/− and t-PA−/− mice. Spontaneous lysis of pulmonary emboli 16 hours after clot injection. +/+: wild type mice; +/−, heterozygous t-PA deficient; −/−, homozygous t-PA deficient. Values represent mean ± SEM of the indicated number of mice in parentheses.
Effect of bolus injection of recombinant human t-PA on thrombolysis in t-PA−/− mice.To determine whether administration of therapeutic quantities of recombinant t-PA would restore clot lysis activity in this animal model, an intravenous bolus of 2 mg/kg or an equal volume of saline was given to t-PA−/− mice following injection of the clot into the jugular vein. The extent of lysis of the plasma clot was determined 1 hour later. Control (saline injected) t-PA−/− mice (n = 9) showed 6% ± 2% lysis of the plasma clot in this assay. Administration of rt-PA significantly increased the extent of lysis to 71% ± 9% (n = 4, P < .001 v control).
t-PA expression following infection of endothelial cells with adenovirus expressing human t-PA.Endothelial cells, because they express an excess of PAI-1, are a good test system for the ability of t-PA expression vectors to augment their thrombolytic potential. Previous results have shown the utility of recombinant retroviral vectors expressing t-PA to enhance the local antithrombotic activity of endothelial cells in vivo.50 HUVEC were therefore infected with AdCMVt-PAWT, AdCMVt-PACB, or control AdRR5 virus at a multiplicity of infection of 20 plaque forming units per cell. After 48 hours, the growth medium was replaced and the infected cells allowed to condition fresh medium for 4 hours. The synthesis of catalytically active t-PA in medium conditioned by HUVECs infected with the control adenovirus, AdRR5, was ≤ 0.002 pmol per hour. Medium conditioned by HUVECs infected with AdCMVt-PAWT and AdCMVt-PACB synthesized and secreted 17 pmol and 40 pmol of active enzyme per hour, respectively. The increased quantity of active t-PA enzyme present in AdCMVt-PACB-infected cell culture medium is likely a consequence of its resistance to inhibition by PAI-1 secreted by the endothelial cells.
Expression of t-PA in mice injected with AdCMVt-PACB virus.Intravenous injection of mice with a recombinant adenovirus carrying a reporter gene encoding Escherichia coli β-galactosidase results in efficient infection and transgene expression in both endothelial cells and hepatocytes of the liver.30 At a dose of 4 × 109 pfu, most cells within the liver of an adult mouse are transduced. In addition, mice infected with a recombinant adenovirus carrying a firefly luciferase reporter gene show preferential expression of the transgene (>99% of total body luciferase activity) in the liver.30 Four days after injection of t-PA−/− mice with 200 μL saline, either with or without 2 × 109 pfu of AdRR5 or AdCMVt-PACB, liver tissue was harvested and immunostained for t-PA antigen. Immunoreactive t-PA was observed in approximately 25% of hepatocytes in the livers of t-PA−/− mice that received AdCMVt-PACB, but not in uninfected control animals or animals infected with AdRR5 (data not shown).
Whereas a transient lymphocytic hepatitis was previously observed in mice after intravenous administration of high doses (>1010 pfu per mouse) of recombinant adenovirus,30,31,51 no histologic evidence of liver inflammation 4 days after infection was observed at any of the doses used in this study. In addition, the liver enzymes AST and ALT were only slightly elevated by virus injection, in agreement with previous observations.51 Uninjected control mice had AST values of 166 ± 26 U/L (n = 10) and ALT values of 109 ± 33 U/L (n = 10), whereas mice injected with the most frequently used dose in this study, 5 × 108 pfu, had AST levels of 261 ± 30 U/L (P = .047, n = 28) and ALT levels of 151 ± 19 U/L (P = .035, n = 29). There were no significant differences in liver enzyme levels in plasma from animals injected with different viruses containing either the two t-PA cDNAs or no foreign gene. Thus, the expression of t-PA per se was not deleterious to the liver and thus apparently did not result in plasminogen activation within the hepatocytes.
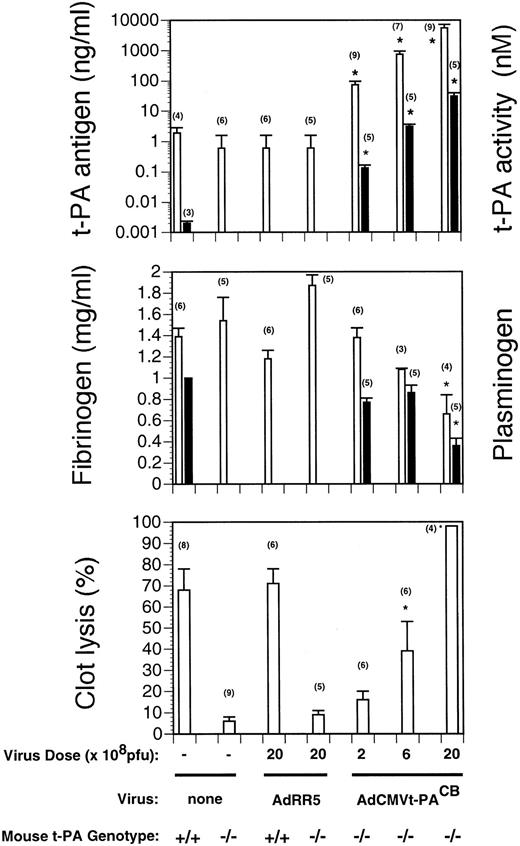
To determine whether t-PA synthesized in hepatocytes was secreted into the plasma, plasma samples obtained from uninfected animals or from mice 4 days after infection with AdCMVt-PACB or AdRR5 were assayed. As shown in the top panel of Fig 2, high plasma levels of t-PA antigen were observed in mice that received AdCMVt-PACB virus, while plasma from either uninfected animals or from mice infected with the control adenovirus showed no immunoreactive human t-PA above background (< 0.6 ng/mL). The levels of t-PA antigen were dose-dependent, although the correlation was nonlinear. Injection of 2 × 108 pfu of AdCMVt-PACB into t-PA−/− mice (n = 9) resulted in the synthesis and secretion of 73 ± 23 ng/mL human t-PA, 6 × 108 pfu produced 744 ± 209 ng/mL (n = 7), and 2 × 109 pfu produced 8,360 ± 1,757 ng/mL (n = 9). Injection of irrelevant AdRR5 vector per se did not result in changes in the circulating levels of t-PA antigen in either wild type or t-PA−/− mice. For comparative purposes, normal t-PA levels in wild type mice are approximately 2 ng/mL.
Circulating t-PA, plasminogen, and fibrinogen levels and clot lysis in virus-injected mice. Measurements were performed 4 days after virus injection with the indicated dose of AdRR 5 or AdCMVt-PACB. Values are expressed as mean ± SEM of the indicated number of mice in parentheses. (Top panel) t-PA antigen and activity levels as a function of virus dose. Human t-PA antigen levels (open bars) in plasma of saline- and AdRR5-injected mice were not significantly different from background (<0.6 ng/mL). For reference purposes, t-PA antigen levels in t-PA+/+ mice are ∼2 ng/mL. t-PA activity levels are shown by the solid bars. (Middle panel) Fibrinogen and plasminogen levels as a function of virus dose in AdRR5 and AdCMVt-PACB-injected t-PA−/− mice. *, P < .005 v AdRR5-injected mice. Fibrinogen levels are shown by open bars and plasminogen levels (expressed as the fraction of plasminogen present in pooled plasma of untreated mice) are shown as solid bars. (Bottom panel) Lysis of emboli in uninfected or virus-infected t-PA−/− mice. Three days after infection of mice with the indicated dose (pfu) of control AdRR5 or AdCMVt-PACB virus, a clot was injected, and the residual radioactivity measured after 16 hours. *, P = .01 v saline- and AdRR5-injected t-PA−/− mice.
Circulating t-PA, plasminogen, and fibrinogen levels and clot lysis in virus-injected mice. Measurements were performed 4 days after virus injection with the indicated dose of AdRR 5 or AdCMVt-PACB. Values are expressed as mean ± SEM of the indicated number of mice in parentheses. (Top panel) t-PA antigen and activity levels as a function of virus dose. Human t-PA antigen levels (open bars) in plasma of saline- and AdRR5-injected mice were not significantly different from background (<0.6 ng/mL). For reference purposes, t-PA antigen levels in t-PA+/+ mice are ∼2 ng/mL. t-PA activity levels are shown by the solid bars. (Middle panel) Fibrinogen and plasminogen levels as a function of virus dose in AdRR5 and AdCMVt-PACB-injected t-PA−/− mice. *, P < .005 v AdRR5-injected mice. Fibrinogen levels are shown by open bars and plasminogen levels (expressed as the fraction of plasminogen present in pooled plasma of untreated mice) are shown as solid bars. (Bottom panel) Lysis of emboli in uninfected or virus-infected t-PA−/− mice. Three days after infection of mice with the indicated dose (pfu) of control AdRR5 or AdCMVt-PACB virus, a clot was injected, and the residual radioactivity measured after 16 hours. *, P = .01 v saline- and AdRR5-injected t-PA−/− mice.
To confirm that the t-PA secreted into the plasma was in an enzymatically active form, plasminogen activation assays were performed on plasma of t-PA−/− mice injected with the recombinant adenoviruses. The results are shown in the top panel of Fig 2. Active t-PA levels in mice injected with 2 × 108 pfu of AdCMVt-PACB were 0.135 ± 0.032 nmol/L (n = 5), 6 × 108 pfu gave 3.11 ± 0.52 nmol/L (n = 5) and 2 × 109 pfu gave 31.6 ± 8.5 nmol/L (n = 5). Thus, t-PA antigen and activity levels were closely correlated (r = 0.99 by linear regression). In a separate experiment, the expression of active t-PA by AdCMVt-PAWT and AdCMVt-PACB were compared. Active t-PA enzyme levels following injection of 5 × 108 pfu AdCMVt-PAWT were 2.5 ± 0.99 nmol/L (n = 8) and those in mice injected with AdCMVt-PACB were 2.6 ± 1.6 nmol/L (n = 8). These values, while not significantly different from each other, represent very large increases in circulating activity because t-PA−/− mice lack detectable t-PA and wild type mice express approximately 1.5 pmol/L active t-PA.
Adenovirus-mediated transfer of the t-PA gene reduced the levels of other circulating components of the fibrinolytic system. Plasma fibrinogen levels declined to approximately 30% of those in AdRR5-treated t-PA−/− mice at the highest dose used (Fig 2, middle panel). However, this depletion of fibrinogen was not associated with spontaneous bleeding. Lower doses of virus also slightly reduced circulating fibrinogen levels in a dose-dependent manner. Plasminogen antigen levels were 36% of those found in uninjected controls at the highest dose of AdCMVt-PACB used. However, significant decreases in plasminogen levels were not seen with lower doses of recombinant adenovirus.
Restoration of thrombolysis in t-PA−/− mice by t-PA gene transfer.Intravenous injection of AdCMVt-PACB, at doses ranging from 2 × 108 to 2 × 109 pfu, restored thrombolysis in t-PA−/− mice in a dose-related manner at 4 days after infection (Fig 2, bottom panel). Half-maximal lysis was obtained with a dose of approximately 6 × 108 pfu AdCMVt-PACB, which is over one order of magnitude lower than the toxic dose of adenovirus (>1010 pfu per mouse). A highly significant correlation was observed between plasma t-PA antigen and activity levels and the percent of plasma clot lysis. The restoration of thrombolysis in t-PA−/− mice after adenovirus-mediated gene transfer was highly efficient. At 16 hours, clot lysis in AdCMVt-PACB virus-infected t-PA−/− mice injected with 2 × 109 pfu was 98% ± 0% (n = 4) compared with 71% ± 7% (n = 6) in AdRR5 virus-infected wild type mice (P = .015). Injection of control AdRR5 virus per se did not result in any changes in the extent of clot lysis in either wild type or t-PA−/− mice.
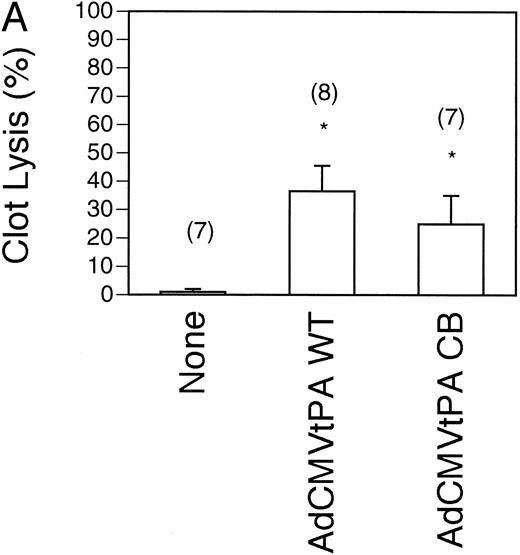
Restoration of thrombolysis in t-PA−/− mice by wild type and inhibitor-resistant t-PAs.To test whether the wild type t-PA was as effective at restoring the thrombolytic capacity to t-PA−/− mice as the inhibitor-resistant t-PA, comparison of the rate of clot lysis was performed in mice injected with submaximal doses (5 × 108 pfu) of the two recombinant adenoviruses. An early time point of 4 hours after injection of the radiolabelled clot was also chosen to show potential differences between the viruses. As shown in Fig 3A, the AdCMVt-PAWT–treated mice (n = 8) lysed 37% ± 9% of the clot, which was not significantly different from that observed in the AdCMVt-PACB–treated mice (25% ± 10%, n = 7). Both values were significantly increased over the 1% ± 0% lysis seen in uninjected t-PA−/− mice (n = 7).
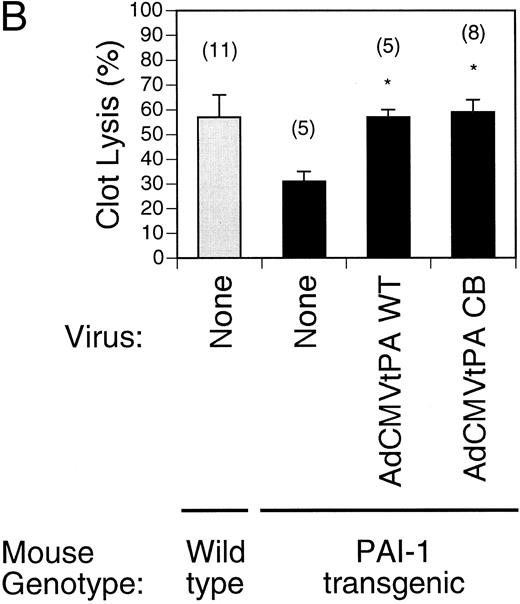
Comparison of pulmonary plasma clot lysis in mice injected with wild type versus inhibitor-resistant t-PA-expressing adenoviruses. Clot lysis experiments were performed 4 days after gene transfer. The residual radioactivity in clots was measured after 4 hours of lysis. Values represent mean ± SEM of the indicated number of mice in parentheses. *P < .05 v lysis in uninjected mice (none) of the same genotype. (A) Clot lysis in t-PA−/− mice. (B) Wild type (shaded bars) and PAI-1–overexpressing transgenic mice (solid bars) were injected intravenously with 5 × 108 pfu recombinant viruses AdCMVt-PAWT and AdCMVt-PACB.
Comparison of pulmonary plasma clot lysis in mice injected with wild type versus inhibitor-resistant t-PA-expressing adenoviruses. Clot lysis experiments were performed 4 days after gene transfer. The residual radioactivity in clots was measured after 4 hours of lysis. Values represent mean ± SEM of the indicated number of mice in parentheses. *P < .05 v lysis in uninjected mice (none) of the same genotype. (A) Clot lysis in t-PA−/− mice. (B) Wild type (shaded bars) and PAI-1–overexpressing transgenic mice (solid bars) were injected intravenously with 5 × 108 pfu recombinant viruses AdCMVt-PAWT and AdCMVt-PACB.
Augmentation of thrombolysis in PAI-1–overexpressing mice by t-PA gene transfer.Mice harboring a single copy of a CMV/murine PAI-1 transgene46 overexpress murine PAI-1 and are a suitable test system in which to compare the adenoviruses expressing wild type and inhibitor-resistant t-PA enzymes. Endogenous levels of t-PA activity in the plasma of these mice was low (5.6 ± 1.6 pmol/L, n = 5) and murine PAI-1 serum antigen levels were significantly elevated (56 ± 7 ng/mL, n = 5), as previously reported. Intravenous injection of 5 × 108 pfu AdCMVt-PAWT or AdCMVt-PACB increased t-PA antigen levels from 13 ± 4 ng/mL (n = 5) in control animals to 1,700 ± 580 ng/mL (n = 5) and 2,600 ± 470 ng/mL (n = 8), respectively. This 30-fold to 50-fold overexpression of t-PA by comparison to PAI-1 was reflected in the net t-PA activity in plasma, which increased over 1,000-fold to 5.0 ± 2.3 nmol/L (n = 7) and 5.0 ± 1.8 nmol/L (n = 8), respectively, for the two viruses.
As shown in Fig 3B, PAI-1–overexpressing transgenic mice partially lysed pulmonary clots by 4 hours (31% ± 4%, n = 5) and were not significantly different from wild type controls (57% ± 9%, n = 11, P = .083). However, after adenovirus injection into PAI-1 transgenic mice, clot lysis was increased to 57% ± 3% for AdCMVt-PAWT–treated mice (n = 5) and 59% ± 5% (n = 8) for AdCMVt-PACB–treated mice. The extent of clot lysis in both virus-treated groups, although not statistically different from each other, was significantly augmented in comparison to the uninjected group (P < .001 and P = .003, respectively).
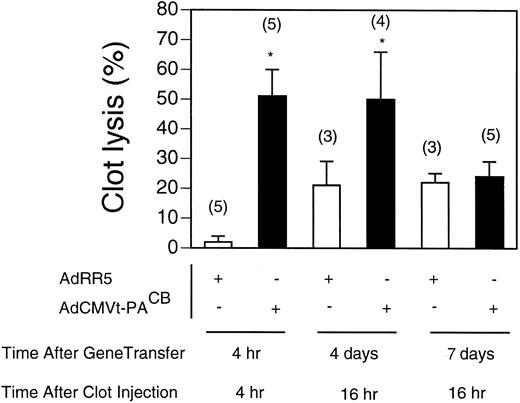
Kinetics of AdCMVt-PACB virus-induced thrombolysis.Coinjection of AdCMVt-PACB or AdRR5 with the 125I-fibrin–labeled plasma clot showed that AdCMVt-PACB increased lysis of pulmonary plasma clots above that obtained by injection of the control AdRR5 virus as early as 4 hours after intravenous injection in t-PA−/− mice (Fig 4). Although the increased thrombolytic potential following AdCMVt-PACB virus-injection persisted for at least 4 days, no significant increase in thrombolytic potential was observed in AdCMVt-PACB virus-infected t-PA−/− mice after 7 days.
Time course and onset of clot lysis. Lysis of 125I-fibrin–labeled pulmonary clots in t-PA−/− mice over the course of 4 or 16 hours. Clot injection was performed at the indicated time or day after injection with either 2 × 109 pfu AdRR5 (open bars) or AdCMVt-PACB virus (solid bars). Clot lysis after 4 hours was measured after coinjection of the plasma clot and virus; clot lysis 4 and 7 days after virus delivery was determined by measuring the extent of lysis 16 hours after injection of the clot. Values are expressed as percent of total radioactivity injected and represent mean ± SEM of the indicated number of mice in parentheses. *P < .05 v lysis in AdRR5 infected mice.
Time course and onset of clot lysis. Lysis of 125I-fibrin–labeled pulmonary clots in t-PA−/− mice over the course of 4 or 16 hours. Clot injection was performed at the indicated time or day after injection with either 2 × 109 pfu AdRR5 (open bars) or AdCMVt-PACB virus (solid bars). Clot lysis after 4 hours was measured after coinjection of the plasma clot and virus; clot lysis 4 and 7 days after virus delivery was determined by measuring the extent of lysis 16 hours after injection of the clot. Values are expressed as percent of total radioactivity injected and represent mean ± SEM of the indicated number of mice in parentheses. *P < .05 v lysis in AdRR5 infected mice.
DISCUSSION
The present study shows that adenovirus-mediated transfer of the human t-PA gene into mice results in (1) efficient expression of the transgene in the liver, (2) a dose-dependent increase in plasma t-PA antigen concentration and restoration of plasma plasminogen activator activity, and (3) dose-dependent normalization of the rate of lysis of pulmonary plasma clots. In fact, t-PA−/− mice carrying a targeted disruption of the endogenous murine t-PA gene that received the highest tested doses of recombinant adenovirus expressing human t-PA showed significantly greater plasma clot lysis activity than did wild type control animals.
In most settings, the conditions producing disordered fibrinolysis (eg, sepsis, surgery, inflammation) are transient, suggesting that intervention strategies to temporarily augment plasma fibrinolytic activity might convey protection against clinically significant thromboembolic events. It is possible that a modest increase in plasma fibrinolytic activity sustained over several days might protect against thrombotic occlusion during the period of greatest risk. The present observations suggest that transient overexpression of t-PA by gene transfer might be a useful strategy to accomplish this goal. The relatively brief kinetics of t-PA gene expression following gene transfer with first-generation adenovirus vectors may, from this perspective, be an attractive characteristic. After adenoviral injection, significant increases in plasma fibrinolytic activity were observed within 4 hours, persisted through 4 days, but returned to baseline by day 7. Adenovirus-mediated gene transfer of t-PA might thus serve as a useful adjunct to conventional antithrombotic strategies.
Tissue-type plasminogen activator is normally synthesized primarily by vascular endothelial cells. Mice infected by intravenous injection of AdCMVt-PACB expressed human t-PA antigen in a high percentage of hepatocytes and accumulated very high levels of both t-PA antigen and enzymatic activity in plasma, which was sufficient to restore systemic fibrinolytic potential to normal or supranormal levels. The efficient expression and secretion of the t-PA enzyme into the plasma by the liver is in agreement with earlier observations on another secreted protein, apolipoprotein A1.31 The lack of t-PA specific hepatotoxicity and spontaneous bleeding in these experiments, which is in contrast to that observed following u-PA gene transfer,52 is perhaps not surprising because plasminogen activation by t-PA is tightly regulated by the interaction with fibrin. Further, the human t-PA enzyme is only a slow activator of murine plasminogen (kcat = 0.0013 s−1v 0.12 s−1 for human plasminogen),53 which may also contribute to the lack of plasminogen activation in the liver.
A related issue is that there was less clot lysis observed in the t-PA–deficient mice after t-PA gene transfer than would be expected considering the circulating enzyme levels of the human enzyme that were achieved. That is, several hundred times the level of murine t-PA normally present in plasma of wild type mice was required to obtain equivalent levels of clot lysis with the human t-PA enzyme. However, this can be at least partly explained by the 40-fold difference in the catalytic efficiency of the human t-PA enzyme on mouse plasminogen by comparison to murine t-PA, which render the human enzyme relatively ineffective in murine clot lysis.53
Surprisingly, the efficacy of the virus expressing the wild type t-PA was equivalent to that of the inhibitor-resistant version. Previous data indicates that an inhibitor-resistant form of the enzyme is a superior thrombolytic agent,36-38 at least at relatively low doses. It appears that when t-PA is overexpressed such that there are large quantities of active enzyme present in the circulation, then clot lysis proceeds efficiently, even if there are increased circulating levels of the murine PAI-1, which can efficiently inhibit the wild type human t-PA enzyme.52 We did not observe a significant decrease in circulating PAI-1 antigen in the AdCMVt-PAWT–injected mice (data not shown) as has previously been observed in rabbits injected with high doses of wild type t-PA,38 although this does not eliminate the possibility of rapid complex formation between PAI-1 and wild type t-PA.
Whether the results of the present study using gene disruption and gene replacement in mice can be extrapolated to more complex physiologic imbalances of the hemostatic system in man remains uncertain. The most common clinically encountered disorder of endogenous fibrinolysis results from an elevation of the plasma concentration of PAI-1, which behaves as an acute phase reactant. In addition, many clinically significant thrombi are platelet (and presumably PAI-1) rich, in contrast to the platelet-poor plasma thrombi used in these experiments. A gene encoding a variant of human t-PA with substantial resistance to PAI-1 inactivation was used, which showed significantly greater thrombolytic activity than the native enzyme in lysis of platelet-rich thrombi in previous animal studies.36 38 We have shown that overexpression of both the inhibitor-resistant variant and wild type t-PA enzymes can overcome the deficient fibrinolysis due to elevated plasma PAI-1 levels in vivo and efficiently dissolve clots in a murine pulmonary embolism model. Because the increased risk of thrombotic and thromboembolic events associated with sepsis and inflammation presumably results from a multitude of biochemical alterations in the coagulation and fibrinolytic systems, whether augmenting plasma fibrinolytic potential alone can materially alter this risk in a clinical setting would likely require direct experimental evaluation in human subjects.
In summary, adenovirus-mediated transfer of a t-PA gene has the potential to increase plasma fibrinolytic activity and to restore (in t-PA–deficient mice) and augment (in PAI-1–overexpressing mice) the thrombolytic capacity in simple animal models of defective fibrinolysis. The results appear sufficiently encouraging to warrant further investigation.
ACKNOWLEDGMENT
The authors gratefully acknowledge the technical assistance of B. Amarneh, A. Bouché, O. Cooper, I. Cornelissen, M. De Mol, H. Moreau, and I. Vanlinthout. We also thank L. Moons and L. Schoonjans for help with immunostaining and the preparation of Fig 1. Ed Madison provided the t-PA mutant cDNA used in these studies.
Supported by Grant No. SCOR HL17669 from the National Institutes of Health (Bethesda, MD). R.D.G. was the recipient of an American Heart Association-Genentech, Inc Established Investigator award.
Address reprint requests to Désiré Collen, MD, PhD, Center of Transgene Technology and Gene Therapy, Vlaams Interuniversitair Instituut voor Biotechnologie, Campus Gasthuisberg, Herestraat 49, B-3000 Leuven, Belgium.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal