Abstract
Involvement of the contact system of coagulation in the pathogenesis of various inflammatory diseases is suggested by reduced plasma levels of factor XII (Hageman factor) and prekallikrein generally considered to result from activation of the contact system. However, in many of these diseases patients develop an acute-phase response and, therefore, an alternative explanation for the decreased levels of factor XII could be the downregulation of factor XII gene expression in the liver as described for negative acute-phase proteins. We report here that interleukin-6 (IL-6), the principal cytokine mediating the synthesis of most acute-phase proteins in the liver, downregulates the production of factor XII by the human hepatoma cell line HepG2 by up to 75%. The decrease in protein secretion correlated with an equivalent decrease of factor XII mRNA likely indicating a pretranslational control of factor XII gene expression by IL-6. Downregulation of factor XII production by IL-6 in vitro parallelled that of transthyretin, a known negative acute-phase protein. Moreover, we show that, in patients developing an acute-phase response after immunotherapy with IL-2, plasma levels of factor XII correlate (r = .76, P < .0001) with those of transthyretin. Taken together, these results suggest that factor XII behaves as a negative acute-phase protein.
THE PLASMA CONTACT system is involved in the maintenance of homeostasis of the organism; indeed, activated contact system proteins trigger the activation of the intrinsic pathways of coagulation and fibrinolysis, the activation of the complement system, and the production of kinins.1-5
The activation of the contact system is initiated by factor XII (FXII), a serine protease synthesized by the liver6 as a single-chain inactive zymogen. Upon binding to a negatively charged surface, FXII is converted to activated FXII (FXIIa), which, in turn, activates prekallikrein to kallikrein and FXI to activated FXI. Kallikrein activates additional FXII and cleaves high molecular weight kininogen generating the vasoactive peptide bradykinin.
FXII and prekallikrein plasma levels are low in sepsis, which has been ascribed to increased consumption resulting from activation of the contact system. However, evidence for such an activation process is often lacking. For example, circulating levels of FXIIa- and kallikrein-C1-inhibitor complexes, which reflect activation of the contact system in vivo, are also low in most patients with sepsis.7
Mammalian liver responds to acute systemic injury by a dramatic change in the synthesis of various plasma proteins. This phenomenon is known as hepatic acute-phase response.8,9 The rate and amplitude of changes in plasma levels of the acute-phase proteins reflect the induction of their synthesis by various cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor α (TNFα), and IL-6. In particular, the latter cytokine has been shown to regulate the entire set of acute-phase proteins. IL-6 increases the expression of genes coding for the positive acute-phase proteins and downregulates that of the so-called negative acute-phase proteins.10-13 Decreased plasma levels of a given protein in human diseases may therefore be due to a negative acute-phase behavior of that protein.
Thus far, there are no data on the regulation of FXII synthesis by liver cells during the acute-phase reactions. We, therefore, investigated the effect of cytokines on the synthesis of FXII by the human hepatoma cell line HepG2, which is generally considered to be a suitable model for studies of acute-phase protein regulation. Our results indicate that, in HepG2 cells, FXII synthesis is downregulated by IL-6. Furthermore, we show that, in patients who develop an acute-phase response during IL-2 therapy, decreased plasma levels of FXII correlated with decreased levels of transthyretin (TTR; prealbumin), a well-known negative acute-phase protein.
MATERIALS AND METHODS
Cell culture.HepG2 cells were cultured in Dulbecco's modified Eagles medium supplemented with 10% (vol/vol) fetal calf serum (FCS), penicillin-streptomycin, and glutamine (all purchased from GIBCO BRL, Paisley, UK). For the stimulation experiments, cells were plated at a density of 1 × 105 cells/cm2 in 25-cm2 flasks; after 24 hours, the medium was replaced with fresh medium with or without 10% (vol/vol) FCS and with or without added cytokines (0 time). After 24 hours, culture media were harvested and replaced with fresh medium containing the appropriate cytokines and cells cultured for an additional 24 hours. This procedure was repeated on 3 successive days. Cells and culture fluids were harvested at 24-hour intervals for RNA analysis by Northern blot and for protein (FXII, TTR, and fibrinogen) quantification by enzyme-linked immunoassorbent assays (ELISAs), respectively. Cell supernatants were centrifuged at 4,000g for 20 minutes at 4°C to remove detached cells and stored at −20°C until analysis. All stimulation experiments were repeated at least three times, with duplicate incubations and duplicate determination of the secreted proteins.
Cytokines.Human recombinant IL-6 (rIL-6) produced in Escherichia coli was purified to homogeneity by gel filtration and ion exchange high-performance liquid chromatography as described.14,15 The specific activity of rIL-6 was 109 U per milligram as measured in the B9 bioassay.16 Endotoxin content was less than 3.5 pg/μg as measured in the Limulus assay. A second preparation of rIL-6 was kindly provided by G. Ciliberto (IRBM, Pomezia, Rome, Italy). rIL-1β was kindly provided by Dr P.T. Lomedico (Hoffmann-La Roche, Nutley, NJ) and had a specific activity of 5 × 106 U/mg, as measured in D10 bioassay, and an endotoxin content of 6 pg/μg.16 rTNFα, produced in E coli, was generously provided by W. Haas (Hoffmann-La Roche, Basel, Switzerland). The specific activity was 5.9 × 107 U/mg as measured in the WEHI 164 bioassay.17
Immunoassays.Proteins secreted in the culture media were quantified by sandwich-type ELISA. The ELISAs used to quantify fibrinogen and TTR were performed using a first polyclonal antibody for coating and a second polyclonal antibody labeled with biotin. The rabbit polyclonal antibodies used were separated from antiserum (Rabbit-antihuman fibrinogen and rabbit-anti-TTR; both from the Department of Immune Reagents, Central Laboratory of The Netherlands Red Cross Transfusion Service, Amsterdam, The Netherlands) by affinity chromatography on protein-G-Sepharose (Pharmacia Fine Chemicals, Uppsala, Sweden) according to the manufacturer's instructions. FXII was measured using two monoclonal antibodies, both directed against the catalytic region of FXII, as previously described.18 The amount of proteins present in the culture media was calculated by reference to a standard curve of pooled normal human plasma. By comparing dose-response curves of the latter with those of purified FXII, the pooled plasma was estimated to contain 35.3 ± 2.9 μg of FXII per milliliter. The interassay coefficient of variation of all assays was less than 10%.
Results were corrected for cell number by assessing the total amount of RNA extracted from the flasks and were expressed as the amount of protein secreted into the culture medium during 24 hours by 106 HepG2 cells. Data are presented as the mean ± SD from four to six replicate cultures.
Preparation of RNA and Northern blot analysis.Cells were washed with ice-cold phosphate-buffered saline and lysed in guanidinium thiocyanate solution (4 mol/L guanidinium thiocyanate, 25 mmol/L sodium citrate, and 0.5% sodium lauryl sarcosylate, pH 7). Total RNA was isolated by phenol-chloroform extraction.19 Twenty-five micrograms of total RNA, as determined spectrophotometrically, was fractionated on 1% agarose-formaldehyde gel and transferred onto nylon membranes (Hybond-N; Amersham, Buckinghamshire, UK). Hybridization, washing, and rehybridization were performed according to the manufacturer's instructions. Filters were hybridized with 32P-labeled 0.5-kb FXII cDNA probe. The FXII probe was the HindIII-Pst I fragment of the pBFXII plasmid20 containing nucleotides 1-543 of hFXII cDNA. Each filter was rehybridized with a 0.3-kb human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe to perform a normalization for mRNA loading. Filters were washed and exposed to Kodak X-Omat film (Eastman Kodak, Rochester, NY) at −70°C with intensifying screens. The abundance of specific FXII transcripts in each lane was directly quantified on filters by a Phosphoimager Analyser (Canberra Packard, Meriden, CT).
Analysis of FXII and TTR levels in IL-2–treated patients.Four patients who received high doses of recombinant IL-2 as a treatment for metastatic melanoma or renal cell carcinoma malignant diseases were studied with regard to the changes occurring in the contact system proteins.21 Further details regarding the patients, the IL-2 treatment, and the blood sampling are given elsewhere.22 FXII levels in plasma samples were determined by radioimmunoassay as described.7 21 TTR levels were measured by ELISA, as described above.
Data analysis.All statistical analyses were performed by Graphpad Instat (San Diego, CA). Intergroups and intragroups differences were assessed by the Mann-Whitney U test and the Wilcoxon test, respectively. The Spearman rank correlation analysis was used to assess the correlation between protein levels in plasma samples. A difference was considered significant, using a two-tailed P, at P < .05.
RESULTS
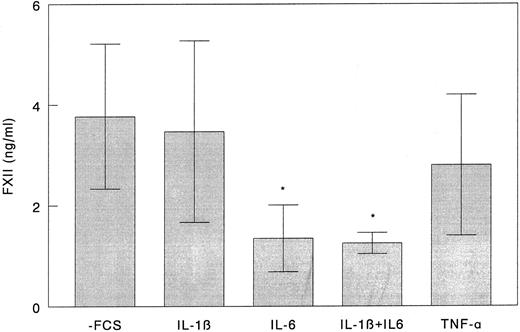
FXII production by HepG2 cells.FXII was constitutively synthesized and secreted by HepG2 cells in the absence of any stimulus. In initial experiments, we studied the effects of rIL-1β, rIL-6, and rTNFα on the production of FXII. Cells were cultured under serum-free conditions in the presence of rIL-1β, rIL-6, rTNFα, and combinations of rIL-1β and rIL-6. As shown in Fig 1, the amount of FXII present in the culture media of HepG2 cells stimulated for 72 hours with rIL-6 was three times lower than in the controls (P = .022), whereas changes induced by rIL-1β and rTNFα were not statistically significant. Moreover, rIL-1β did not significantly modify the effect of rIL-6 on FXII production.
Effect of rIL-1β, rIL-6, and rTNFα on FXII synthesis by HepG2 cells. HepG2 cells were grown to 80% confluence in 24-well plates and incubated under serum-free conditions with 50 ng/mL of rIL-1β, rIL-6, rTNFα, and a combination of rIL-1β and rIL-6 for 72 hours. Culture medium was refreshed every 24 hours. FXII concentration in culture media obtained after the third 24-hour period was measured by ELISA as described in the Materials and Methods. Results represent the mean ± SD of three independent experiments. *P < .05 as compared with unstimulated cells.
Effect of rIL-1β, rIL-6, and rTNFα on FXII synthesis by HepG2 cells. HepG2 cells were grown to 80% confluence in 24-well plates and incubated under serum-free conditions with 50 ng/mL of rIL-1β, rIL-6, rTNFα, and a combination of rIL-1β and rIL-6 for 72 hours. Culture medium was refreshed every 24 hours. FXII concentration in culture media obtained after the third 24-hour period was measured by ELISA as described in the Materials and Methods. Results represent the mean ± SD of three independent experiments. *P < .05 as compared with unstimulated cells.
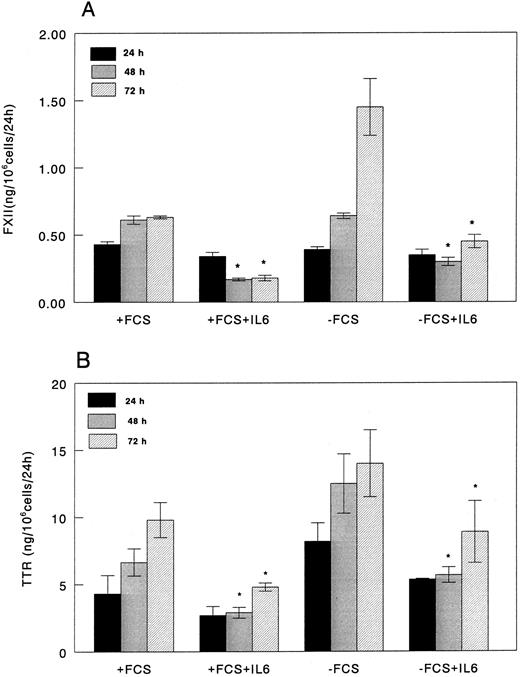
Based on the initial results, we focused our attention on the effect of IL-6 on FXII production by HepG2 cells cultured in the presence or absence of FCS. Dose-response experiments using rIL-6 concentrations ranging from 0.1 to 50 ng/mL indicated that rIL-6 was already effective at a concentration of 0.5 ng/mL (data not shown) and reached a plateau at concentrations less than 5 ng/mL. Therefore, all subsequent experiments were performed using 5 ng of rIL-6 per milliliter of culture medium. Under these conditions, rIL-6 significantly downregulated the production of FXII by HepG2 cells cultured both in the presence and absence of FCS (Fig 2A). The amount of FXII in culture media after 24 hours of exposure to rIL-6 was 75% of that in medium from cells cultured in absence of rIL-6 and decreased to 25% at 48 and 72 hours. The amount of FXII in culture media from unstimulated cells cultured with or without FCS was comparable at 24 and 48 hours, whereas it was twofold increased in HepG2 cells maintained without FCS for 72 hours.
FXII and TTR production in control and rIL-6–treated HepG2 cells cultured in the presence or in the absence of 10% FCS. HepG2 cells were plated at a density of 1 × 105 cells/cm2 in 25-cm2 flasks. After 24 hours, the medium was replaced with fresh medium with or without 10% (vol/vol) FCS with or without rIL-6 (0 time). After 24 hours of culture, media were harvested and replaced with fresh medium (±rIL-6) and the cells were cultured for an additional 24 hours. FXII (A) and TTR (B) concentrations in the culture media were determined by ELISA. Results were corrected for cell number by assessing the total amount of RNA extracted from the flasks and are expressed as the amount of protein secreted in the culture medium in 24 hours by 106 HepG2 cells. Results (mean ± SD) are the average of three independent experiments in duplicate. * P < .05 as compared with corresponding control.
FXII and TTR production in control and rIL-6–treated HepG2 cells cultured in the presence or in the absence of 10% FCS. HepG2 cells were plated at a density of 1 × 105 cells/cm2 in 25-cm2 flasks. After 24 hours, the medium was replaced with fresh medium with or without 10% (vol/vol) FCS with or without rIL-6 (0 time). After 24 hours of culture, media were harvested and replaced with fresh medium (±rIL-6) and the cells were cultured for an additional 24 hours. FXII (A) and TTR (B) concentrations in the culture media were determined by ELISA. Results were corrected for cell number by assessing the total amount of RNA extracted from the flasks and are expressed as the amount of protein secreted in the culture medium in 24 hours by 106 HepG2 cells. Results (mean ± SD) are the average of three independent experiments in duplicate. * P < .05 as compared with corresponding control.
Synthesis of fibrinogen, studied as positive control of acute-phase reaction, was threefold to fourfold increased in the presence of rIL-6 (data not shown). As control for negative acute-phase protein, we evaluated the production of TTR, whose levels decreased by 37% after 24 hours and by 70% after 72 hours after rIL-6 stimulation of HepG2 cells (Fig 2B). Although TTR levels were generally higher in culture media from cells maintained without FCS, the extent of the decrease induced by rIL-6 was comparable with that observed in cells cultured in the presence of 10% FCS (Fig 2B).
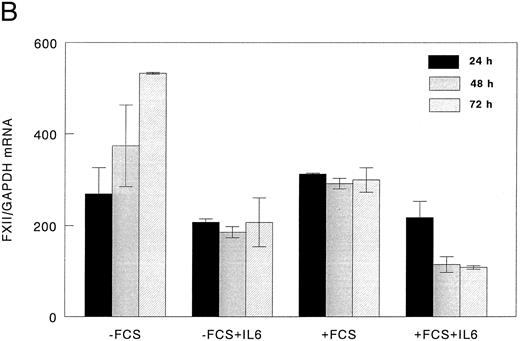
FXII mRNA levels in HepG2 cells.Northern blot analysis showed that FXII mRNA levels were decreased by 23% after 24 hours and by 66.5% after 48 and 72 hours of rIL-6 stimulation (Fig 3A and B). An increase of FXII mRNA was observed at 72 hours in unstimulated HepG2 cells cultured without FCS.
Downregulation of FXII mRNA in HepG2 cells by rIL-6. HepG2 cells (1 × 105 cells/cm2 in 25-cm2 flasks) were incubated for the indicated times with or without rIL-6 (5 ng/mL) in the presence or absence of 10% (vol/vol) FCS. RNA was extracted and analyzed by Northern blot as indicated in the Materials and Methods. The filters were hybridized with 32P-human FXII cDNA, cleaned, and rehybridized with 32P-human GAPDH cDNA. (A) Time course of the FXII mRNA decrease after rIL-6 addition in the presence or in the absence of FCS; the autoradiograms of a representative Northern blot experiment are shown. (B) Quantitative analysis of the inhibitory effect of rIL-6 on FXII mRNA concentration in HepG2 cells. The intensity of the bands corresponding to FXII mRNA and to GAPDH mRNA, used as an internal control, was derived from direct radioactivity measurement on filters by Phosphoimager Analyser (Canberra Packard). Data are expressed as the FXII/GAPDH ratio and represent the mean ± SEM of two experiments.
Downregulation of FXII mRNA in HepG2 cells by rIL-6. HepG2 cells (1 × 105 cells/cm2 in 25-cm2 flasks) were incubated for the indicated times with or without rIL-6 (5 ng/mL) in the presence or absence of 10% (vol/vol) FCS. RNA was extracted and analyzed by Northern blot as indicated in the Materials and Methods. The filters were hybridized with 32P-human FXII cDNA, cleaned, and rehybridized with 32P-human GAPDH cDNA. (A) Time course of the FXII mRNA decrease after rIL-6 addition in the presence or in the absence of FCS; the autoradiograms of a representative Northern blot experiment are shown. (B) Quantitative analysis of the inhibitory effect of rIL-6 on FXII mRNA concentration in HepG2 cells. The intensity of the bands corresponding to FXII mRNA and to GAPDH mRNA, used as an internal control, was derived from direct radioactivity measurement on filters by Phosphoimager Analyser (Canberra Packard). Data are expressed as the FXII/GAPDH ratio and represent the mean ± SEM of two experiments.
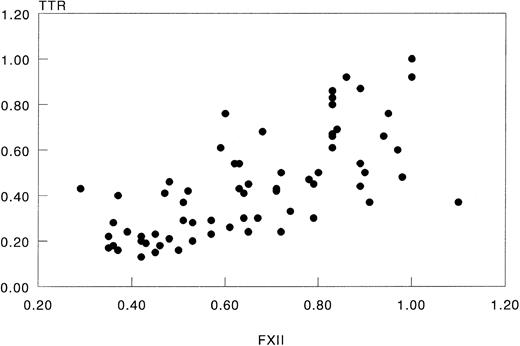
FXII and TTR levels in IL-2–treated patients.The experiments described above showed that rIL-6 downregulated FXII production by a human hepatoma cell line and that the decrease resembled that of TTR. These results raised the question as to whether FXII might behave as a negative acute-phase protein during the acute-phase reaction in vivo. We therefore evaluated a possible correlation between plasma levels of FXII and TTR in patients treated with high doses of rIL-2 who develop an acute-phase reaction after high plasma levels of IL-6. Similarly to FXII,21 TTR also significantly decreased during the treatment with rIL-2 (P < .0001), and this decrease correlated with that of FXII (r = .76, P < .0001; Fig 4).
Relationship of plasma levels of FXII to those of TTR in IL-2–treated patients. Data were those obtained in 7 cycles of escalating doses of rIL-2 administered to 4 patients. In each patient, initial levels of FXII and TTR were arbitrally set at 1 and protein levels at subsequent days were related to these. Data points represent the relative concentrations measured in plasma samples that were taken daily (r = .76, P < .0001).
Relationship of plasma levels of FXII to those of TTR in IL-2–treated patients. Data were those obtained in 7 cycles of escalating doses of rIL-2 administered to 4 patients. In each patient, initial levels of FXII and TTR were arbitrally set at 1 and protein levels at subsequent days were related to these. Data points represent the relative concentrations measured in plasma samples that were taken daily (r = .76, P < .0001).
DISCUSSION
The results presented here show that the synthesis of FXII in the human hepatoma cell line HepG2 is downregulated by IL-6. The hepatoma cell line HepG2 is very similar to hepatocytes in terms of biologic responsiveness23-25 and is widely used as model system for studying the regulation of acute-phase protein synthesis in human liver.26-29
The decrease in FXII production by HepG2 cells was significant after 24 hours of rIL-6 stimulation and reached a nadir at 48 hours. Neither rIL1-β nor rTNFα had a significant effect on FXII levels. In fact, IL1-β and TNFα directly control the synthesis of only a limited subset of acute-phase proteins,27,30-35 and their effect appears to be mediated, at least in part, by the induction of IL-6 synthesis.10,11,36-38 In vitro studies using hepatocyte cultures and correlations of serum IL-6 concentrations with acute-phase protein levels in various inflammatory states in vivo indicate that IL-6 may be the principal regulator for most acute-phase protein genes.11,26,33,34 38
Hepatocyte activation with IL-6 can result in both positive and negative regulation of acute-phase proteins. Positive acute-phase proteins are represented by a large number of proteins, including various protease inhibitors, clotting factors, and transport plasma proteins, whose plasma levels may increase from twofold to 1,000-fold. Concomitant to the increase of positive acute-phase protein concentrations, a decrease in plasma levels of negative acute-phase proteins, which include albumin, α2-HS-glycoprotein, TTR, transferrin, and retinol-binding protein, has been observed. Decreased levels have generally been thought to be due to transfer to the extravascular space or to increased metabolism; nevertheless, recent studies indicate that IL-1, TNF, and IL-6 are all capable of decreasing transcription of genes for albumin29,39-41 and that IL-6 decreases transcription of the TTR gene in HepG2 cells.29
FXII and TTR levels were decreased in the culture media of rIL-6–stimulated HepG2 cells cultured either in the presence or in the absence of FCS, thus ruling out the possibility that the observed decreases were due to negative regulators present in the FCS. However, higher levels of FXII protein and specific mRNA as well as of TTR were found in the medium from cells cultured without FCS especially after 72 hours. This my have been due to the presence of IL-6 in the FCS, but the finding that this FCS was negative in a bioassay for IL-6, ie, the B9 assay,16 allowed us to exclude this possibility. Possibly, the higher levels of proteins and mRNA found in the FCS-free cultures, compared with the FCS cultures, reflected either the presence of unidentified negative regulators in the FCS or a higher expression of liver-specific genes by nonproliferating hepatoma cells.
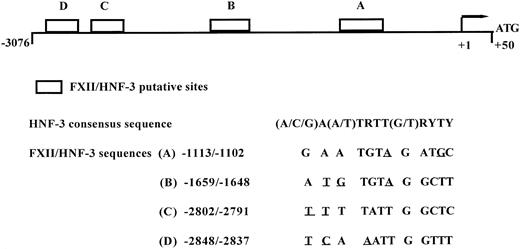
Modulation of acute-phase gene expression by cytokines has been shown to be mostly at pretranslational level.9,10,31,39,42,43 The quantitative changes in secreted FXII and steady-state mRNA levels during rIL-6 stimulation were comparable, suggesting that rIL-6 control on FXII synthesis was indeed at a pretranslational level. However, more experiments are needed to clarify whether the inhibitory effect of IL-6 on accumulation of FXII transcripts is mediated by a decrease of FXII gene transcriptional rate and/or a decrease in FXII mRNA stability. Recently, the hepatocyte nuclear factor-3 (HNF-3) proteins have been shown to regulate the transcription of a number of negative acute-phase genes, such as albumin, transferrin, and TTR,44-46 and it has been proposed that the decreased HNF-3α expression during the acute-phase response may, indeed, mediate the reduced transcription of HFN-3 target genes that respond negatively to cytokine signalling.44 Using computer-assisted analysis of the sequence of the 5′-flanking region of the human FXII gene,47 we have identified 4 putative binding consensus for HNF-3 proteins (Fig 5), which supports the hypothesis that IL-6 modulation of FXII gene occurs at the transcriptional level. The 4 putative consensus sequences found in FXII gene promoter show 2 or 3 nucleotide substitutions when compared with the canonical consensus sequence48; nevertheless, subtle nucleotide changes at the 3′ and 5′ ends of the consensus binding site sequence provide a different HNF-3 binding affinity and allow for discrimination between various HNF-3 family members.
Schematic diagram of FXII gene promoter and the location of four putative binding site for HNF-3. Location of HNF-3 putative binding sites on the 5′-flanking region of FXII gene. Abbreviations for nucleotides are as follows: R = G or A; Y = C or T. The mismatches from the consensus sequence deduced for HNF-3 binding sites48 are underlined.
Schematic diagram of FXII gene promoter and the location of four putative binding site for HNF-3. Location of HNF-3 putative binding sites on the 5′-flanking region of FXII gene. Abbreviations for nucleotides are as follows: R = G or A; Y = C or T. The mismatches from the consensus sequence deduced for HNF-3 binding sites48 are underlined.
The finding that in vitro IL-6 downregulates the hepatocyte synthesis of FXII to the same extent as the synthesis of TTR suggests that FXII may act as a negative acute-phase protein during the acute-phase reaction. This was corroborated in vivo in patients who develop an acute-phase response during therapy with rIL-2. In these patients, plasma levels of FXII have been shown to decrease disproportionally to albumin.21 We show here that the FXII decrease correlated very significantly with that of TTR. This correlation, together with the lack of evidence of products of contact system activation,21 suggests that the observed low levels of FXII can be, at least in part, ascribed to the modulation of FXII synthesis by IL-6.
It has been proposed that decreased levels of negative acute-phase proteins are necessary to allow for an increased synthesis of positive acute-phase protein without changing the overall rate of total protein synthesis in the liver. Alternatively, the downregulation of the synthesis of negative acute-phase proteins may serve biologic goals. In the case of FXII, the biologic goal of diminished synthesis during acute-phase responses is difficult to comprehend, because the precise physiologic role of FXII is not known. Considering the increased risk of FXII-deficient persons for thromboembolic processes,49-51 it is tempting to speculate that a diminished synthesis of FXII may favor clotting possibly via reduced activation of the fibrinolytic system.52-54 Indeed, the tendency to favor a procoagulant state by enhanced expression of clotting factors and fibrinolysis inhibitor seems to be a general feature of acute-phase responses.55-67
In conclusion, we show that the synthesis of FXII by HepG2 cells is downregulated by IL-6. This finding, together with the observation that FXII levels closely correlate with those of TTR in patients undergoing an acute-phase reaction during immunotherapy with rIL-2, led us to propose that, in humans, FXII is a negative acute-phase protein.
Supported by Human Capital and Mobility Award Contract No. ERBCHBGCT920109, by Fondazione Istituto Pasteur-Fondazione Cenci Bolognetti, and by Ministero dell'Università e Ricerca Scientifica.
Address reprint requests to Franca Citarella, Central Laboratory of the Netherlands Red Cross Transfusion Service, Department of Pathophysiology of Plasma Proteins, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal