Abstract
We recently demonstrated that the gene encoding the peripheral cannabinoid receptor (Cb2) may be a proto-oncogene involved in murine myeloid leukemias. We show here that Cb2 may have a role in hematopoietic development. RNAse protection analysis showed that Cb2 is normally expressed in spleen and thymus. Cb2 mRNA is also expressed in 45 of 51 cell lines of distinct hematopoietic lineages, ie, myeloid, macrophage, mast, B-lymphoid, T-lymphoid, and erythroid cells. The effect of the fatty acid anandamide, an endogenous ligand for cannabinoid receptors, on primary murine marrow cells and hematopoietic growth factor (HGF )-dependent cell lines was then investigated. In vitro colony cultures of normal mouse bone marrow cells showed anandamide to potentiate interleukin-3 (IL-3)–induced colony growth markedly. Whereas HGFs alone stimulate proliferation of the various cell lines in serum-free culture only weakly, anandamide enhances the proliferative response of the cell lines to HGFs profoundly. This was apparent for responses induced by IL-3, granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and erythropoietin. Anandamide was already effective at concentrations as low as 0.1 to 0.3 μmol/L and plateau effects were reached at 0.3 to 3 μmol/L. The addition of anandamide as single growth factor had no effect. The costimulatory effect of anandamide was not evident when cells were cultured with fetal calf serum (FCS), suggesting that FCS contains anandamide or another ligand capable of activating the peripheral cannabinoid receptor. Other cannabinoid ligands did not enhance the proliferative responsiveness of hematopoietic cells to HGFs. Transfection experiments of Cb2 in myeloid 32D cells showed that anandamide specifically activates proliferation through activation of the peripheral cannabinoid receptor. Anandamide appears to be a novel and synergistic growth stimulator for hematopoietic cells.
PROLIFERATION and differentiation of hematopoietic precursor cells is regulated by hematopoietic growth factors (HGFs).1,2 These cytokines bind and activate receptors that belong to the hematopoietin receptor superfamily.3 Receptors of this superfamily are single transmembrane proteins that, upon binding to their specific ligands, form heterodimeric or homodimeric complexes.3 Ligands that bind hematopoietin receptors are small glycoproteins, eg, interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF ), granulocyte colony-stimulating factor (G-CSF ), erythropoietin (Epo), or macrophage colony-stimulating factor (M-CSF ). Another family of surface membrane receptors is the G-protein–coupled receptors (GPCRs).4,5 This superfamily of receptor molecules is characterized by seven hydrophobic stretches of 20 to 25 amino acids that form seven transmembrane-helices connected by alternating extracellular and intracellular loops. Up to now, more than 300 GPCRs have been identified.4,5 In contrast to receptors of the hematopoietin family, GPCRs are in most cases not activated by glycoproteins. Ligands of heptahelical GPCRs include amines, amino acids, peptides or proteins, nucleosides or nucleotides, fatty acid derivates, and phospholipid derivates.4,5 Little is known about the role of GPCRs in hematopoietic growth and development. We recently identified the peripheral cannabinoid receptor (Cb2), which encodes a GPCR, in a common ecotropic virus integration site (Evi11).6,7 In vitro transfection studies in 32D cells supported the hypothesis that Cb2 is a proto-oncogene that is involved in leukemogenesis.6 In the present study, we investigated by RNAse protection analysis the expression pattern of the Cb2 gene in comparison to the gene that encodes the central cannabinoid receptor (Cb1)8,9 in different murine tissues and a panel of murine hematopoietic cell lines. We show that Cb2 encodes a hematopoietic receptor that is expressed in myeloid, macrophage, erythroid, lymphoid, and mast cells. In contrast, Cb1 is mainly expressed in brain and in testis.8 9 The effects of the fatty acid N-arachidonylethanolamide or anandamide, an endogenous ligand for cannabinoid receptors, on the proliferative abilities of HGF-dependent hematopoietic cell lines were investigated. The results of this study demonstrate that anandamide is a ligand that stimulates proliferation of hematopoietic cell lines in synergy with IL-3, Epo, GM-CSF, and G-CSF under serum-free conditions.
MATERIALS AND METHODS
Mouse hematopoietic cell lines.A list of murine hematopoietic cell lines used in this study is presented in Table 1. Macrophage cell lines were cultured in Dulbecco's modified Eagle's medium (GIBCO, Ghent, Belgium) plus supplements (100 IU/mL penicillin, 100 ng/mL streptomycin, and 10% fetal calf serum [FCS]). The other cell lines were cultured in RPMI-1640 medium plus supplements. Myeloid cell lines were cultured with 10 ng murine IL-3 and CTLL cells were cultured with 10 IU/mL IL-2 (Cetus, Emmeryville, CA). The pro-B–cell line BAF3 and myeloid 32D cell line both transfected with the human G-CSF receptor gene (BAF-G and 32D [G-CSF-R], respectively) were donated by Dr I.P. Touw (Erasmus University Rotterdam, Rotterdam, The Netherlands).36
Cb2 and Cb1 mRNA Expression in Hematopoietic Cell Lines
| (A) Cb2 +/Cb1 − | |||
| Myeloid | Ref. | Macrophage | Ref. |
| 32D | 10 | RAW 264.7 | 22 |
| 32Dcl3 | 11 | RAW 309. | 22 |
| DA-1 | 12 | WR19M.1 | 22 |
| DA-3 | 12 | Pu5-1.8 | 23 |
| DA-13 | 12 | J774 | 24 |
| DA-24 | 12 | WEHI 3 | 25 |
| DA-28 | 12 | ||
| DA-29 | 12 | Erythroid | |
| DA-31 | 12 | RED5 | 26 |
| DA-33 | 12 | RED8 | 26 |
| NFS-22 | 13 | 32D-Epo | 11 |
| NFS-36 | 13 | ||
| NFS-56 | 13 | B-lymphoid | |
| NFS-58 | 13 | DA-25 | 12 |
| NFS-60 | 13 | WEHI 231 | 27 |
| NFS-61 | 13 | WEHI 279 | 28 |
| NFS-78 | 13 | BAF3 | 29 |
| NFS-107 | 13 | DA-8 | 12 |
| NFS-124 | 13 | ||
| BXH2-43 | 14 | T-lymphoid | |
| BXH2-115 | 14 | DA-2 | 12 |
| 14-122 | 15 | RL12 | 30 |
| C6 | 16 | WEHI 22 | 31 |
| RMB1 | 17 | ||
| RMB3 | 18 | Mast cells | |
| ABPL-4 | 19, 20 | ABFTL-1 | 21 |
| ABFTL-2 | 21 | ||
| (B) Cb2 −/Cb1 − | |||
| Myeloid | Ref. | Macrophage | Ref. |
| M1 | 32 | P388-D1 | 33 |
| 14-166 | 15 | ||
| 14-259 | 15 | T-lymphoid | Ref. |
| EL4 | 34 | ||
| (C) Cb2 −/Cb1 + | |||
| T-lymphoid | Ref. | ||
| CTLL | 35 |
| (A) Cb2 +/Cb1 − | |||
| Myeloid | Ref. | Macrophage | Ref. |
| 32D | 10 | RAW 264.7 | 22 |
| 32Dcl3 | 11 | RAW 309. | 22 |
| DA-1 | 12 | WR19M.1 | 22 |
| DA-3 | 12 | Pu5-1.8 | 23 |
| DA-13 | 12 | J774 | 24 |
| DA-24 | 12 | WEHI 3 | 25 |
| DA-28 | 12 | ||
| DA-29 | 12 | Erythroid | |
| DA-31 | 12 | RED5 | 26 |
| DA-33 | 12 | RED8 | 26 |
| NFS-22 | 13 | 32D-Epo | 11 |
| NFS-36 | 13 | ||
| NFS-56 | 13 | B-lymphoid | |
| NFS-58 | 13 | DA-25 | 12 |
| NFS-60 | 13 | WEHI 231 | 27 |
| NFS-61 | 13 | WEHI 279 | 28 |
| NFS-78 | 13 | BAF3 | 29 |
| NFS-107 | 13 | DA-8 | 12 |
| NFS-124 | 13 | ||
| BXH2-43 | 14 | T-lymphoid | |
| BXH2-115 | 14 | DA-2 | 12 |
| 14-122 | 15 | RL12 | 30 |
| C6 | 16 | WEHI 22 | 31 |
| RMB1 | 17 | ||
| RMB3 | 18 | Mast cells | |
| ABPL-4 | 19, 20 | ABFTL-1 | 21 |
| ABFTL-2 | 21 | ||
| (B) Cb2 −/Cb1 − | |||
| Myeloid | Ref. | Macrophage | Ref. |
| M1 | 32 | P388-D1 | 33 |
| 14-166 | 15 | ||
| 14-259 | 15 | T-lymphoid | Ref. |
| EL4 | 34 | ||
| (C) Cb2 −/Cb1 + | |||
| T-lymphoid | Ref. | ||
| CTLL | 35 |
Cb2 and Cb1 mRNA expression was determined by RNAse protection (Fig 2). References are the original reports describing the cell lines.
RNA isolation.Mouse tissues were homogenized using an Ultratarax T25 shearing device (IKA Labortechnique, Heiterheim, Germany). Total RNA was extracted from murine hematopoietic cell lines with guanidinium thiocyanate followed by phenol extraction.37 RNA samples of several cell lines were kindly donated by Dr J. Cleveland (St Jude Children's Hospital, Memphis, TN).
RNAse protection.RNAse protection experiments were performed as described.37 cDNA fragments were cloned into Bluescript II SK+ (Stratagene, La Jolla, CA) linearized using the proper enzymes and RNA probes synthesized with T3 or T7 polymerase (Promega, Leiden, The Netherlands). For each incubation, 10 μg of RNA and radiolabeled probe (25,000 cpm) were suspended in 30 μL hybridization buffer (80% deionized formamide, 40 mmol/L PIPES, pH 6.4, 0.4 mol/L NaAc, 1 mmol/L EDTA). The samples were heated to 85°C for 5 minutes and then incubated for 16 hours at 50°C anealing temperature. To these mixtures, 300 μL RNAse digestion buffer (10 mmol/L Tris-HCl, pH 7.5, 5 mmol/L EDTA, and 200 mmol/L NaAc) and 1 U RNAse One (Promega) was added. After 1 hour at 37°C, the reaction was stopped by the addition of 3.3 μL 10% sodium dodecyl sulfate and 20 μg carrier tRNA. The reactions were precipitated with ethanol and fractionated by electrophoresis on a 6% polyacrylamide/7 mol/L urea gel and analyzed by autoradiography. A radiolabeled GAPDH RNA fragment was used as a control.
Serum-free culture medium.A serum-free culture medium was used to study responses to HGFs and cannabinoid ligands.38-40 Iscove's modified Dulbecco's medium (GIBCO) was supplemented with 15 mg/mL bovine serum albumin (Cohn fraction V; Sigma, Bornem, Belgium), 10−7 mol/L sodium selenite (Merck, Darmstad, Germany), 7.7 × 10−6 mol/L iron-saturated human transferrin (Behring Institute, Marburg, Germany), 7.8 μg/mL cholesterol (Sigma), and 2.8 μg/mL linoleic acid (Merck). In normal bone marrow colony cultures, 10 ng/mL insulin (Sigma), 10−4 mol/L β-mercaptoethanol (Merck), and 50 μg/mL of the nucleosides, adenosine, thymidine, guanosine, cytidine, uridine, 2′deoxycytidine, 2′deoxyadenosine, and 2′deoxyguanosine (Sigma) were added to the culture medium.
HGFs and cannabinoid ligands.IL-3 (10 ng/mL; donated by Dr J.N. Ihle, St Jude Children's Research Hospital, Memphis, TN), G-CSF (100 ng/mL; Amgen, Thousand Oaks, CA), GM-CSF (50 ng/mL; Genetics Institute, Cambridge, MA), and Epo (2 IU/mL; Boehringer Mannheim, Mannheim, Germany) were added to the cultures at optimal concentrations, which had been determined with normal bone marrow colony cultures.39 40 Cannabinoid ligands included anandamide, Δ-8-tetrahydrocannabinol (Δ8-THC), WIN55212-2, Cannabinol, and Cannabidiol (Sigma) and CP 55,940 (Pfizer, Groton, CT). They were added at final concentrations between 0.1 and 10 μmol/L.
Tritiated thymidine (3H-TdR) incorporation.DNA synthesis was measured essentially as described.38 Five thousand cells from various cell lines were cultured in 100 μL of serum-free medium, with or without the addition of HGFs or cannabinoid ligands in 96-well round-bottom microtiter trays (Greiner, Nurtingen, Germany) for 90 hours. Four hours before harvesting, 0.1 μCi tritiated thymidine (2 Ci/mmol 3H-TdR; Amersham International, Amersham, UK) was added. Cells were harvested on nitrocellulose using a filtermate 196 harvester (Packerd Instrument Co, Meriden, CT). Radioactivity was determined with a Topcount, microscintillation counter (Packerd).
Colony formation by normal mouse bone marrow.Normal bone marrow cells isolated from femora and tibae from BCBA mice were collected in Hank's Balanced Salt Solution. Fifty thousand cells were cultured in 1 mL serum-free medium with 1.2% methylcellulose. Dishes were incubated with or without 10 ng/mL IL-3 in the presence or absence of 10 μmol/L anandamide at 37°C and 100% humidity and 5% CO2. Colonies containing 50 cells or more were scored at day 14.
RESULTS
Cb2 and Cb1 mRNA expression in murine tissues.The expression of Cb2 and Cb1 transcripts in murine tissues was determined by RNAse protection (Fig 1B). Using Cb2 cDNA probe I (Fig 1A), an expected mRNA fragment of 185 bp was protected in spleen, thymus, and heart (Fig 1B). No Cb2 transcripts were identified in any of the other tissues examined. RNAse protection experiments using a 460-bp Cb1 probe (Fig 1A) demonstrated Cb1 transcripts in brain and to a lesser extent in testis (Fig 1B). No detectable levels of Cb1 mRNA were identified in any of the other organs investigated.
Cb2 and Cb1 mRNA expression in murine organ tissues. (A) Schematic representation of murine Cb2 and Cb1 mRNA (noncoding exon 1 and protein coding exon 2) and the cDNA probes used for RNAse protection experiments. The shaded boxes represent open reading frames. H, HincII; B, BamHI; S, Stu I; N, Nco I; (E), EcoRI in vector. (B) RNAse protection on 10 μg of total RNA of different mouse organs using Cb2 probe I and Cb1 probe I (see [A]). The protected fragments were 185 bp (Cb2) and 460 bp (Cb1). Sp, spleen; Th, thymus; H, heart; S, stomach; G, gut; K, kidney; B, bladder; L, liver; Br, brain; T, testis.
Cb2 and Cb1 mRNA expression in murine organ tissues. (A) Schematic representation of murine Cb2 and Cb1 mRNA (noncoding exon 1 and protein coding exon 2) and the cDNA probes used for RNAse protection experiments. The shaded boxes represent open reading frames. H, HincII; B, BamHI; S, Stu I; N, Nco I; (E), EcoRI in vector. (B) RNAse protection on 10 μg of total RNA of different mouse organs using Cb2 probe I and Cb1 probe I (see [A]). The protected fragments were 185 bp (Cb2) and 460 bp (Cb1). Sp, spleen; Th, thymus; H, heart; S, stomach; G, gut; K, kidney; B, bladder; L, liver; Br, brain; T, testis.
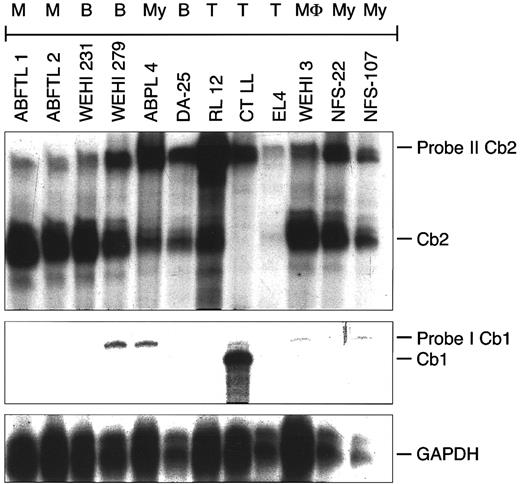
Cb2 and Cb1 mRNA expression in murine hematopoietic cell lines.To examine in which hematopoietic lineages the Cb2 gene may be expressed, RNAse protection experiments were performed using mRNA samples from a large panel of murine hematopoietic cell lines (see the Materials and Methods). Using a 187-bp Cb2 cDNA fragment representing exon-1 (137 bp) and exon-2 (50 bp; Fig 1A), Cb2 transcripts of the correct size were identified in 26 of 29 myeloid, 6 of 7 macrophage, 5 of 5 B-lymphoid, 3 of 5 T-lymphoid, 2 of 2 mast cell, and 3 of 3 erythroid cell lines (Table 1). An example of an RNAse protection experiment is presented in Fig 2. Interestingly, a 460-bp Cb1 transcript was shown in a murine CTLL cell line (Fig 2 and Table 1). In the CTLL cell line, no Cb2 transcripts were identified (Fig 2). No detectable Cb1 mRNA levels were found in any of the other cell lines (Table 1 and Fig 2). No Cb1 or Cb2 transcripts were found in 3 myeloid, 1 macrophage, and 1 T-lymphoid cell lines. These results show that the peripheral cannabinoid receptor is a blood cell receptor that may be expressed in all hematopoietic lineages. The central cannabinoid receptor is only incidentally expressed in hematopoietic cell lines.
Cb2 and Cb1 mRNA expression in murine hematopoietic cell lines. RNAse protection on 10 μg of total RNA of different murine hematopoietic cell lines, using Cb2 probe II and Cb1 probe I (see Fig 1A). The hematopoietic phenotype of the cell lines is indicated at the top of the figure. M, mast cell; B, B lymphoid; My, myeloid; T, T lymphoid; MΦ, macrophage.
Cb2 and Cb1 mRNA expression in murine hematopoietic cell lines. RNAse protection on 10 μg of total RNA of different murine hematopoietic cell lines, using Cb2 probe II and Cb1 probe I (see Fig 1A). The hematopoietic phenotype of the cell lines is indicated at the top of the figure. M, mast cell; B, B lymphoid; My, myeloid; T, T lymphoid; MΦ, macrophage.
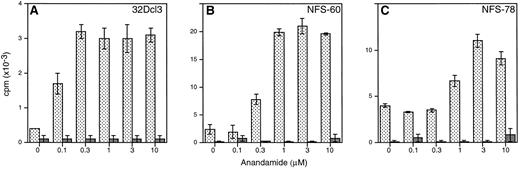
Anandamide potentiates the proliferative response of myeloid cells to IL-3.The effect of a natural ligand of cannabinoid receptors, anandamide, on the proliferation of an IL-3–dependent myeloid cell line 32Dcl3 was studied in vitro. When cultured with FCS, anandamide did not alter IL-3–induced proliferation of 32D cells (Fig 3A). However, in serum-free medium, anandamide significantly enhanced the proliferative response of 32D cells to IL-3 (Fig 3B). Anandamide as a single factor did not induce a measurable proliferative effect in 32Dcl3 cells. Tritiated thymidine incorporation (3H-TdR) showed that as little as 0.1 to 0.3 μmol/L anandamide was sufficient to augment DNA synthesis in synergy with IL-3 in 32Dcl3, NFS-60, and NFS-78 cells (Fig 4). The maximal stimulative effect was reached at a concentration of 0.3 to 3 μmol/L anandamide. 3H-TdR experiments with other IL-3–dependent myeloid cell lines, ie, DA-13, DA-28, DA-29, DA-31, NFS-36, NFS-56, and NFS-107, showed similar synergistic dose-response relationships between IL-3 and anandamide (data not shown).
The effect of anandamide on the proliferation of the myeloid cell line 32Dcl3. Growth curves of 32Dcl3 cells cultured in the presence of fetal calf serum (A) or serum-free (B). Cells were cultured with no stimulus (○), with 10 μmol/L anandamide alone (▵), with 10 ng/mL IL-3 alone (□), or with IL-3 plus anandamide (▪). Mean cell numbers (±1 × SD) of triplicate experiments are plotted against the number of days in culture. The doubling time in serum-free culture without anandamide was 75 hours and with anandamide was 29 hours. 3H-TdR incorporation data (cpm ±1 × SD) of 32Dcl3 cells cultured in serum containing medium (C) or in serum-free medium (SFM) (D).
The effect of anandamide on the proliferation of the myeloid cell line 32Dcl3. Growth curves of 32Dcl3 cells cultured in the presence of fetal calf serum (A) or serum-free (B). Cells were cultured with no stimulus (○), with 10 μmol/L anandamide alone (▵), with 10 ng/mL IL-3 alone (□), or with IL-3 plus anandamide (▪). Mean cell numbers (±1 × SD) of triplicate experiments are plotted against the number of days in culture. The doubling time in serum-free culture without anandamide was 75 hours and with anandamide was 29 hours. 3H-TdR incorporation data (cpm ±1 × SD) of 32Dcl3 cells cultured in serum containing medium (C) or in serum-free medium (SFM) (D).
The effects of different concentrations of anandamide on IL-3–induced 3H-TdR incorporation of three murine myeloid cell lines. Cells of 32Dcl3 (A), NFS-60 (B), and NFS-78 (C) were cultured with titrated concentrations of anandamide (0 to 10 μmol/L) in the presence (10 ng/mL; ▧) or absence of IL-3 (▪). The mean values ±1 × SD (cpm × 10−3) of triplicate experiments are shown.
The effects of different concentrations of anandamide on IL-3–induced 3H-TdR incorporation of three murine myeloid cell lines. Cells of 32Dcl3 (A), NFS-60 (B), and NFS-78 (C) were cultured with titrated concentrations of anandamide (0 to 10 μmol/L) in the presence (10 ng/mL; ▧) or absence of IL-3 (▪). The mean values ±1 × SD (cpm × 10−3) of triplicate experiments are shown.
Anandamide enhances colony growth of normal bone marrow progenitors induced with IL-3.Normal mouse bone marrow colony cultures were performed to investigate whether anandamide enhances IL-3–stimulated colony growth. In two independent experiments, a twofold elevation of colony formation was observed when the cells were cultured with IL-3 plus anandamide as compared with IL-3 alone (Table 2). Anandamide not only increased colony numbers, but also showed an effect on the size of the colonies (Fig 5). More colonies containing 250 cells or more were found in cultures with IL-3 plus anandamide. Mega-size colonies of 1,000 cells or more were found in IL-3 plus anandamide cultures, but none in colony cultures with IL-3 alone.
Effect of Anandamide on IL-3–Stimulated Colony Formation of Normal Murine Bone Marrow CFU-C (Day 14)
| Stimulus . | CFU-C per 105 Cells . | |||||
|---|---|---|---|---|---|---|
| . | Experiment No. 1 . | Experiment No. 2 . | . | . | ||
| . | >50 Cells . | >250 Cells . | >50 Cells . | >250 Cells . | . | . |
| No | 0 | 0 | 0 | 0 | ||
| AN | 0 | 0 | 0 | 0 | ||
| IL-3 | 20 | 6 | 37 ± 8 | 11 ± 4 | ||
| IL-3 + AN | 37 | 17 | 47 ± 7 | 23 ± 5 | ||
| Stimulus . | CFU-C per 105 Cells . | |||||
|---|---|---|---|---|---|---|
| . | Experiment No. 1 . | Experiment No. 2 . | . | . | ||
| . | >50 Cells . | >250 Cells . | >50 Cells . | >250 Cells . | . | . |
| No | 0 | 0 | 0 | 0 | ||
| AN | 0 | 0 | 0 | 0 | ||
| IL-3 | 20 | 6 | 37 ± 8 | 11 ± 4 | ||
| IL-3 + AN | 37 | 17 | 47 ± 7 | 23 ± 5 | ||
In experiment no. 1, mean colony numbers of duplicate experiments are shown. In experiment no. 2, the mean and ± 1 SD of quaduplicate CFU-C counts are shown.
Abbreviations: No, no stimulus; AN, 10 μmol/L anandamide; IL-3, 10 ng/mL IL-3.
Effect of ananadamide on the size of IL-3–induced normal bone marrow colonies. Examples of representative IL-3–induced normal bone marrow colonies after 14 days of culture with (IL-3+AN) or without anandamide (IL-3).
Effect of ananadamide on the size of IL-3–induced normal bone marrow colonies. Examples of representative IL-3–induced normal bone marrow colonies after 14 days of culture with (IL-3+AN) or without anandamide (IL-3).
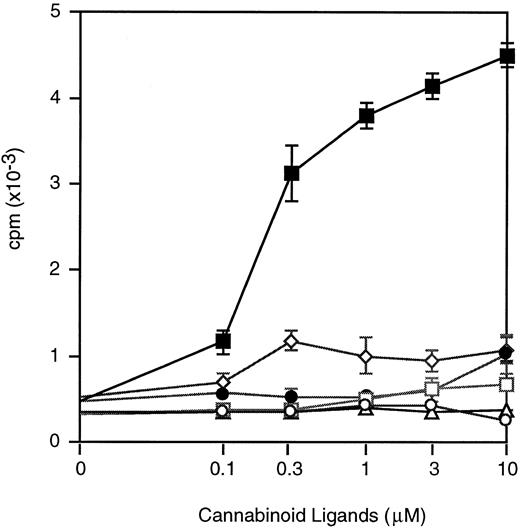
Effects of different cannabinoid ligands on IL-3–induced proliferation of myeloid cells.To investigate whether various synthetic molecules all capable of binding cannabinoid receptors would be capable of enhancing the proliferative effects of IL-3, 32Dcl3 cells were cultured with IL-3 plus the cannabinoid agonists CP-55,940, WIN 55212-2, Δ8-THC, Cannabinol, and Cannabidiol. Whereas DNA synthesis of 32Dcl3 cells was augmented significantly when costimulated with IL-3 and anandamide, no enhancement of thymidine uptake was apparent when 32Dcl3 cells were stimulated with IL-3 plus any of the other cannabinoid ligands (Fig 6).
Dose effects of ananadamide and other cannabinoid ligands on IL-3–induced 3H-TdR incorporation of 32Dcl3 cells. Cells were cultured serum-free with IL-3 (10 ng/mL) and titrated concentrations (0 to 10 μmol/L) of anandamide (▪), WIN 55212-2 (○), Δ8-THC (□), Cannabinol (•), Cannabidiol (▵), and CP55,940 (⋄).
Dose effects of ananadamide and other cannabinoid ligands on IL-3–induced 3H-TdR incorporation of 32Dcl3 cells. Cells were cultured serum-free with IL-3 (10 ng/mL) and titrated concentrations (0 to 10 μmol/L) of anandamide (▪), WIN 55212-2 (○), Δ8-THC (□), Cannabinol (•), Cannabidiol (▵), and CP55,940 (⋄).
Anandamide enhances DNA synthesis of hematopoietic cell lines in synergy with GM-CSF, Epo, and G-CSF.3H-TdR incorporation experiments were performed with three selected GM-CSF–responsive (NFS-36), Epo-responsive (32D-Epo), and G-CSF–responsive (BAF-G) cell lines to investigate whether anandamide would potentiate proliferation in synergy with hematopoietic growth factors other than IL-3. NFS-36 cells showed a weak response to GM-CSF alone or anandamide alone, but a significant increase of thymidine incorporation was evident when both ligands were added (Fig 7A). 32D cells cultured with Epo alone, ie, in the absence of anandamide, did not stimulate DNA synthesis at all. 32D-Epo cells became responsive to Epo in the presence of anandamide (Fig 7B). Finally, whereas G-CSF stimulated some DNA synthesis of BAF-G cells, ananadamide supplemented to the culture medium augmented the G-CSF effect by twofold (Fig 7C). These experiments indicate that anandamide may enhance the stimulative activity of various HGFs.
Synergistic activity of anandamide on GM-CSF–, Epo-, or G-CSF–stimulated thymidine incorporation. Cells were cultured with optimal concentrations of GM-CSF (50 ng/mL; [A] NFS-36), Epo (2 IU/mL; [B] 32D-Epo), or G-CSF (100 ng/mL; [C] BAF-G) in the presence of 10 μmol/L anandamide (AN). The mean values ± 1 × SD (cpm × 10−3) of triplicate experiments are shown.
Synergistic activity of anandamide on GM-CSF–, Epo-, or G-CSF–stimulated thymidine incorporation. Cells were cultured with optimal concentrations of GM-CSF (50 ng/mL; [A] NFS-36), Epo (2 IU/mL; [B] 32D-Epo), or G-CSF (100 ng/mL; [C] BAF-G) in the presence of 10 μmol/L anandamide (AN). The mean values ± 1 × SD (cpm × 10−3) of triplicate experiments are shown.
Effects of anandamide and other cannabinoid ligands on Cb2-overexpressing myeloid cells.To verify whether the stimulatory effect of anandamide is mediated through activation of the peripheral cannabinoid receptor, we overexpressed Cb2 cDNA in 32D cells that express the G-CSF receptor (G-CSF-R), but do not respond to G-CSF.6Cb2-transfected cells show high tritiated thymidine incorporation when cultured with G-CSF plus anandamide (Fig 8A). In comparison, anandamide alone or G-CSF alone did not induce DNA synthesis. Furthermore, anandamide plus G-CSF did not induce proliferation in control vector-transfected 32D (G-CSF-R) cells. These data indicate that anandamide stimulates DNA synthesis after the specific activation of Cb2. In addition, 3H-TdR incorporation experiments using various concentrations of the other cannabinoid ligands showed that DNA synthesis of 32D (G-CSF-R/Cb2) cells was not influenced by any of the other ligands (Fig 8B). Anandamide is apparently selectively capable of stimulating hematopoietic cells through activation of the peripheral cannabinoid receptor among several cannabinoid ligands.
Effects of anandamide and other cannabinoid ligands on G-CSF–induced thymidine incorporation of Cb2-transfected 32D/G-CSF-R cells. (A) 32D/G-CSF-R were transfected with Cb-2 cDNA (32D/G-CSF-R/Cb2) or with pBabe control vector (32D/G-CSF-R/pB). Cells were cultured without G-CSF with different concentrations anandamide ([○] 32D/G-CSF-R/pB; [•] 32D/G-CSF-R/Cb2 ) or with G-CSF plus anandamide ([□] 32D/G-CSF-R/pB; [▪] 32D/G-CSF-R/Cb2). (B) 32D/G-CSF-R-Cb2 cells were cultured with G-CSF plus different concentrations of the distinct cannabinoid ligands, anandamide (▪), WIN 55212-2 (○), Δ8-THC (□), Cannabinol (•), Cannabidiol (▵), and CP55,940 (⋄).
Effects of anandamide and other cannabinoid ligands on G-CSF–induced thymidine incorporation of Cb2-transfected 32D/G-CSF-R cells. (A) 32D/G-CSF-R were transfected with Cb-2 cDNA (32D/G-CSF-R/Cb2) or with pBabe control vector (32D/G-CSF-R/pB). Cells were cultured without G-CSF with different concentrations anandamide ([○] 32D/G-CSF-R/pB; [•] 32D/G-CSF-R/Cb2 ) or with G-CSF plus anandamide ([□] 32D/G-CSF-R/pB; [▪] 32D/G-CSF-R/Cb2). (B) 32D/G-CSF-R-Cb2 cells were cultured with G-CSF plus different concentrations of the distinct cannabinoid ligands, anandamide (▪), WIN 55212-2 (○), Δ8-THC (□), Cannabinol (•), Cannabidiol (▵), and CP55,940 (⋄).
DISCUSSION
In this study, we show that the gene encoding the peripheral cannabinoid receptor is expressed in hematopoietic cells and appears to be important for the efficiency of stimulation of growth by a variety of HGFs.
Several studies had previously demonstrated the presence of cannabinoid binding sites on hematopoietic cells,41-43 but these studies had not distinguished between the central and the peripheral cannabinoid receptor. Cb2 expression was shown in spleen and in thymus. The Cb2 mRNA expression in heart tissue might be the result of residual blood that was not eliminated before extraction. Others demonstrated Cb2 mRNA expression in spleen, in the myeloid cell line HL60, and in mast cell lines.7,44 We show here that Cb2 may be expressed in myeloid, erythroid, B-lymphoid, T-lymphoid, macrophage, and mast cells. This finding indicates that Cb2 encodes a hematopoietic receptor that may have a function in a broad scale of hematopoietic lineages. By applying reverse transcriptase-polymerase chain reaction, several groups demonstrated Cb1 transcripts in hematopoietic cells.41,43,45 46 RNAse protection studies presented here show that Cb1 could be detected in brain and testis but not in spleen and thymus. Futhermore, Cb1 could be detected in 1 of 51 cell lines only. These data suggest that Cb1 may occasionally be expressed in hematopoietic cells, whereas Cb2 is commonly expressed. These results enforce the notion that Cb2 rather than Cb1 encodes a hematopoietic cannabinoid receptor.
In vitro studies with murine hematopoietic cell lines and normal bone marrow precursors showed that the peripheral cannabinoid receptor has a function in the regulation of proliferation by HGFs. Stimulatory effects of anandamide on hematopoietic cell proliferation had not been reported. Anandamide enhanced the cellular proliferation induced with IL-3, GM-CSF, G-CSF, and Epo when the cells were cultured in serum-free medium. Whether anandamide synergizes with other cytokines remains open to future investigation. Most of the cell lines analyzed for Cb2 mRNA expression in this study are HGF-independent and therefore did not allow for an extended analysis of the stimulative effects of anandamide with other HGFs. However, interestingly, when a panel of HGF independent cell lines were cultured in a serum-free medium, ie, DA-2 (T-lymphoid), DA-25 (B-lymphoid), RED-5 (erythroid), and J774 cells (macrophage), anandamide was required to induce proliferation in vitro (data not shown). This indicates that the Cb2 may have a role in stimulation of growth of several, if not all, hematopoietic lineages. An important modification of the culture conditions used in this study is the elimination of FCS and the use of a serum-free culture system. The effects of anandamide are not evident when cells are cultured with FCS and therefore other investigators may have missed the effects of anandamide stimulation. The results of this study would suggest that anandamide or another ligand for the cannabinoid receptor is present in FCS. In fact, four other fatty acids have recently been identified to bind and activate cannabinoid receptors.44,47,48 The role and presence of anandamide or any of these fatty acids may explain at least in part the serum dependence of various hematopoietic cell lines and primary hematopoietic cells. The in vitro experiments show that, among a selected number of cannabinoid ligands studied, only anandamide was capable of stimulating the proliferation of hematopoietic cells synergistically with HGFs. This is remarkable, because, according to other investigators, WIN 55212-2, CP55,940, and THC bind and activate the peripheral cannabinoid receptors more efficiently than anandamide.7,49,50 Our findings thus suggest strongly that, in hematopoietic cells, anandamide acts as the most potent agonist through the latter receptors. Up to 1 to 10 μmol/L anandamide was needed for optimal activation of proliferation, which is within the concentration range required for optimal binding.7,42,44,45,49 The transfection studies of Cb2 (Fig 8) confirm that anandamide specifically activates proliferation of 32D (G-CSF-R/Cb2) cells through activation of the peripheral cannabinoid receptor. In contrast, the other cannabinoid ligands fail to stimulate these Cb2-transfected cells. In fact, when cultured with serum, the other Cb ligands inhibit proliferation (data not shown). Because the cannabinoid agonists CP55,940 and THC have been shown to stimulate an additional nonreceptor-mediated signal transduction pathway,51 it is perhaps possible that the alternative nonreceptor-mediated signals suppress proliferation. In any case, the current available data would suggest that only anandamide is capable of stimulating proliferation synergistically with HGFs. Whether the other fatty acids are capable of stimulating proliferation will be investigated.
We have recently identified the Cb2 gene in a common virus integration site (Evi11)6 and Cb2 was suggested to be a proto-oncogene. Transfection of Cb2 in 32D (G-CSF-R) cells generated G-CSF–dependent cell lines that could be maintained serum free when cultured with anandamide.6 The studies presented here show that activation of the Cb2 receptor has a profound effect on the proliferation of cells. This adds further support to the notion that this receptor, when aberrantly expressed, may alter the proliferative response of hematopoietic cells and contribute to the development of leukemia. Cb2 transgenic animals are currently being studied to investigate whether abnormal Cb2 expression might contribute to the development of leukemia in vivo.
Cb2 receptor-mediated signal transduction by anandamide has been observed by others.49 How activated cannabinoid receptors may stimulate proliferation synergistically with HGFs is unresolved. The costimulatory effects may occur at different levels of HGF receptor signalling. Stimulation of the peripheral cannabinoid receptor may have the following effects: (1) transactivation of the HGF receptors; (2) potentiation of HGF receptor-mediated signal transduction, eg, JAK/STAT or the p21ras/MAP kinase pathways52-54; and (3) activation of signalling pathways that, in parallel with the HGF receptor signalling routes, enhances cell cycling. In fact, examples in support of each of those possibilities exist for seven other transmembrane receptors.55-62 Whether these alternative mechanisms are activated by Cb2 requires verification in future studies.
The results presented in Table 2 and Fig 5 show that the synergism between IL-3 and anandamide is also evident in normal bone marrow colony formation. A greater number of IL-3–dependent colonies were formed in the presence of anandamide. These results indicate that, in the presence of anandamide, IL-3 stimulates the outgrowth of additional populations of precursor cells. IL-3 plus anandamide also stimulated colonies of greater size, which would indicate that the combination of the two factors augments the production of progeny from individual precursor cells as well. Whether anandamide synergizes with other HGFs in the stimulation of normal marrow precursor cells is currently under investigation.
ACKNOWLEDGMENT
The authors thank Dr J. Cleveland (St Jude Children's Hospital, Memphis, TN) for providing RNA samples, Dr T.I. Bonner (National Institute of Mental Health, Bethesda, MD) for donating the Cb1 cDNA, Dr I.P. Touw (Erasmus University Rotterdam, Rotterdam, The Netherlands) for donating BAF-G and 32D (G-CSF-R) cells, and K. van Rooyen for preparation of the figures.
Supported by the Dutch Cancer foundation “Koningin Wilhelmina Fonds,” the Netherlands Organisation for Scientific Research “NWO,” and the Royal Dutch Academy of Sciences “KNAW.”
Address reprint requests to Ruud Delwel, PhD, Erasmus University Rotterdam, Institute of Hematology, PO Box 1738, 3000 DR Rotterdam, The Netherlands.

![Fig. 1. Cb2 and Cb1 mRNA expression in murine organ tissues. (A) Schematic representation of murine Cb2 and Cb1 mRNA (noncoding exon 1 and protein coding exon 2) and the cDNA probes used for RNAse protection experiments. The shaded boxes represent open reading frames. H, HincII; B, BamHI; S, Stu I; N, Nco I; (E), EcoRI in vector. (B) RNAse protection on 10 μg of total RNA of different mouse organs using Cb2 probe I and Cb1 probe I (see [A]). The protected fragments were 185 bp (Cb2) and 460 bp (Cb1). Sp, spleen; Th, thymus; H, heart; S, stomach; G, gut; K, kidney; B, bladder; L, liver; Br, brain; T, testis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1448/3/m_bl_0031f1.jpeg?Expires=1769308858&Signature=G6bmKdG0fqFONDa3KljxJLiBKFhMmrpIFGOPkDngqqy-rHqhMzBZk2tSdUHdn5WIoTS~2nbPNxvjvO23ZvIi4AeG2we3viDPN1xCbfqmTJF5QcWfxUF77WZ3Vk5xXlpzKeYWx9dDz4nzKMnzO6Fn0ltoE--3RL1~Qp1dnD~lCKWghBNGKnizQ7EjjqbjdIUgGOyTkQifHs0BZ9HuiNmuMcl4eINkiHZWe3YaYPhBtfUBHcXQ2qFj10X8MTmtkqqxrh7KpCVunWTlxT~UjyGM6pxUJ9okE0t5-AvPzRJKOZ7mRX4u~94UJ88keGvUCqEnBvxdcoA~-whUHpTOK6SuFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 7. Synergistic activity of anandamide on GM-CSF–, Epo-, or G-CSF–stimulated thymidine incorporation. Cells were cultured with optimal concentrations of GM-CSF (50 ng/mL; [A] NFS-36), Epo (2 IU/mL; [B] 32D-Epo), or G-CSF (100 ng/mL; [C] BAF-G) in the presence of 10 μmol/L anandamide (AN). The mean values ± 1 × SD (cpm × 10−3) of triplicate experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1448/3/m_bl_0031f7.jpeg?Expires=1769308858&Signature=ONY5Y7BqhVWKTb2ASl798C8B~DyfOmQiczfntYEMRU2NFl2F6Q1rE7ZjywCR2fD7rVDHGJdg0zZ762luDtlCV0RuzQ~iUFpd2vo0q4kmq~E3KPguzkX84rAmCIUTXvPXDVounvkoo3CXHhyRyT8Eebf2FCndqhkyeD5Dftj8brlc5ww-0rPX9odeHLaK6KhGsnGmvqj0UjO6cjg~zMhjY1Og6NpYJhmGQ5tD~4vuPNVgnpXPE5PwXDjGm5qg2rlUr0N-S7BWufiLwrqqh-GDvjfiOT9Sj4La3IM22z9YYxmpx-G2QL81PvHThsv1391j02Q1uCiCbgjw9qw4x8KfxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effects of anandamide and other cannabinoid ligands on G-CSF–induced thymidine incorporation of Cb2-transfected 32D/G-CSF-R cells. (A) 32D/G-CSF-R were transfected with Cb-2 cDNA (32D/G-CSF-R/Cb2) or with pBabe control vector (32D/G-CSF-R/pB). Cells were cultured without G-CSF with different concentrations anandamide ([○] 32D/G-CSF-R/pB; [•] 32D/G-CSF-R/Cb2 ) or with G-CSF plus anandamide ([□] 32D/G-CSF-R/pB; [▪] 32D/G-CSF-R/Cb2). (B) 32D/G-CSF-R-Cb2 cells were cultured with G-CSF plus different concentrations of the distinct cannabinoid ligands, anandamide (▪), WIN 55212-2 (○), Δ8-THC (□), Cannabinol (•), Cannabidiol (▵), and CP55,940 (⋄).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1448/3/m_bl_0031f8.jpeg?Expires=1769308858&Signature=RmfBA1RK-KpRX1s0X4uPfbVjZqSN-G706PlxVcTTNXtHMsaKqjj46W7wtQqJ1JB-uJHBzlSLDUPxD-QzktQXeGbApFMWooHInv-bWpIbeH31Wxtn1fHdvNmQzcZpwYmLP~h~GV2~E6y54fGNv6-DvesTmfeXvgleyoxhocOdu0aIjax1AwTltSuXXMLPPwMx1af8TQYT1BKqVVjgUBrpPZMP8H~a2SUQ81Gk1yeGQJ3HTD8lR1QJZ9OKQJ16mA2dRJvgJM8ZcS43YL~gyA6Uvy7FpBMiWws1gBledMQo9BnxhsC5XHeh4ip59pRTaqinnga-JOd5O7iym2GZehfhHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal