Abstract
Two major causes of the anemia in β-thalassemia are a deficiency in hemoglobin (Hb) β-subunit (and consequently HbA) synthesis and, due to the resulting excess of Hb α-subunits, erythroid cell hemolysis. The hemolytic component might be ameliorated by increasing the intracellular proteolysis of the excess α-subunits. Isolated 3H-labeled α-chains are known to be degraded primarily by the adenosine triphosphate (ATP)- and ubiquitin (Ub)-dependent proteolysis pathway in unfractionated β-thalassemic hemolysates. Our objective was to increase this degradation by targeted intervention. Ub aldehyde (Ubal), a synthetic inhibitor of isopeptidases (proteases that hydrolyze the bond between the Ub polypeptide and its protein adduct), was added to reaction mixtures containing a hemolysate from the blood cells of one of four β-thalassemic donors and 3H-α-chains or 3H-α-globin as a substrate. Optimum enhancement of ATP-dependent degradation occurred at 0.4 to 1.5 μmol/L Ubal and ranged from 29% to 115% for 3H-α-chains and 47% to 96% for 3H-α-globin among the four hemolysates. We suggest that Ubal stimulates 3H-α-subunit proteolysis by inhibition of an isopeptidase(s) that deubiquitinates, or “edits,” Ub-3H-α-subunit conjugates, intermediates in the degradative pathway. In control studies, similarly low Ubal concentrations did not enhance the degradation of 3H-α2β2 (HbA) tetramers or inhibit the activities of methemoglobin reductase and four selected glycolysis pathway enzymes. These and other results may be the basis for a therapeutic approach to β-thalassemia.
BETA-THALASSEMIA is a severe anemia caused by a deficiency in the synthesis of β-subunits of adult human hemoglobin (HbA) with a concomitant accumulation of newly synthesized α-subunits in the developing red blood cell.1 These excess α-subunits, unable to serve effectively in oxygen transport, are only poorly degraded. A substantial fraction of them become denatured and form intracellular inclusion bodies, a process that impairs membrane function by both chemical and physical means. These events result in eventual cell lysis that exacerbates the anemia caused by the deficiency in Hb β-subunit synthesis. The relative contribution of β-subunit deficiency and hemolysis to the pathophysiology of an individual with β-thalassemia remains unknown. However, if a way could be found to remove the deleterious, excess α-subunits by enhancing their intracellular proteolysis, the anemia of these individuals might be improved.
Early studies from several laboratories2-7 suggested that some of the excess Hb α-subunits in developing β-thalassemic red blood cells are destroyed by intracellular proteolysis. However, the biochemical mechanism of this proteolysis and a possible basis for its inefficiency in α-subunit degradation were unknown. In 1977, Etlinger and Goldberg8 showed that a proteolytic system in the soluble phase of a rabbit reticulocyte lysate was responsible for the rapid adenosine triphosphate (ATP)-dependent degradation of globins made abnormal by the incorporation of an amino acid analog. Further investigations by several groups9 showed that the ATP-dependent proteolysis of many abnormal or foreign proteins added to the cytoplasm or cytoplasmic extracts of rabbit reticulocytes and other mammalian cells was mediated by ubiquitin (Ub), a polypeptide of 8565 Da. More recently, the ATP- and Ub-dependent proteolysis pathway was shown to degrade rapidly not only specific short-lived natural proteins in various eukaryotic cells,10,11 but also, at least in mouse lymphoblasts,12 a significant fraction of the long-lived proteins. One of us13 showed that the degradation of excess, newly synthesized 3H-α-chains (ie, Hb α-subunits with associated heme groups) in intact β-thalassemic reticulocytes was dependent on ATP. In subsequent studies with whole hemolysates of β-thalassemic reticulocytes, the degradation of added 3H-α-chains was found to depend not only on ATP but also on the presence of Ub.14
In the ATP- and Ub-dependent proteolytic pathway, the carboxyl terminus of Ub is activated in an ATP-dependent reaction, and through a series of enzymatic steps, activated Ub molecules are covalently linked to the ε-amino group(s) of one or more lysine residues of the protein destined for proteolysis.9 These Ub-protein conjugates are recognized through their Ub “tag” by the 26S proteasome, a multisubunit protease complex, and the protein substrate moiety is then degraded in a process that also uses ATP.15-17 Ub components of the Ub-protein conjugates are recycled to the pool of monomeric Ub.
The cytoplasmic fractions that catalyzed the degradation of ubiquitinated proteins in the above studies also contain Ub-protein hydrolases or “isopeptidases.” These enzymes cleave the isopeptide bond between the carboxyl terminus of Ub and ε-amino group(s) of the conjugated protein and thus deubiquitinate or “disassemble” the conjugate.18 Sokolik and Cohen19 showed that the addition of submicromolar amounts of Ub aldehyde (Ubal), a potent inhibitor of many isopeptidases,20 greatly increased the levels of Ub-cytochrome c conjugates in a crude ubiquitination reaction mixture. Likewise, addition of this synthetic inhibitor, a Ub derivative with the C-terminal carboxyl replaced by an aldehyde group, enhanced levels of Ub conjugates of 125I-lysozyme20 and of modified forms of ribonuclease A.21 With β-thalassemic hemolysates, Ubal greatly increased the concentration of the Ub-125I-α-globin intermediates observed during proteolysis of 125I-α-globin,22 the heme-depleted apoprotein of hemoglobin α chains. This finding suggested that isopeptidases in unfractionated β-thalassemic lysates may prevent the accumulation of ubiquitinated α-globin, thereby reducing the overall proteolysis of α-globin.
Recently, we showed that low concentrations (≤1 μmol/L) of Ubal significantly enhanced the total degradation of 125I-α-globin in an unfractionated rabbit reticulocyte lysate.23 However, increased levels of Ubal resulted in less stimulation, and with sufficiently high levels (>3 μmol/L), there was a slight inhibition of total degradation. We proposed that enhancement of 125I-α-globin degradation occurred because Ubal inhibited isopeptidase activity in the lysate that “edited” or disassembled Ub-125I-α-globin conjugate intermediates. At high levels, Ubal also blocked another isopeptidase activity that normally disassembles polyubiquitin chains, natural inhibitors of the conjugate-degrading 26S protease complex. In the current study, we show that Ubal enhances the degradation of 3H-α-chains and 3H-α-globin in hemolysates of blood cells from several individuals with β-thalassemia. We also explore the possibility that these findings may be the basis for a therapeutic approach to this disorder.
SUBJECTS AND METHODS
Preparation of blood cell hemolysates and 3H-labeled α-subunit proteolysis substrates.Blood was obtained, using EDTA anticoagulant, from four unrelated individuals with β-thalassemia intermedia in 1988. The blood Hb composition and presumed genotype of the donors (S.O., C.G., P.C., and S.D.) were described previously.13,24 Crude hemolysates were isolated from the chilled (4°C) washed blood cells, dialyzed against 20 mmol/L Tris HCl, pH 7.6 (4°C), 0.5 mmol/L dithiothreitol, 1 mmol/L MgCl2 , 25 μmol/L EGTA, and 5% (vol/vol) glycerol, and concentrated to 150 mg/mL in Hb as described previously.14 The dialyzed and concentrated hemolysates were stored in several aliquots frozen in liquid N2 or at −80°C until current (1996) use. Identical procedures were used to prepare fresh hemolysates from rabbit reticulocytes (Green Hectares, Oregon, WI); these were stored unfrozen at 4°C and used within 1 week.
3H-α-chains of adult human HbA were prepared with associated heme groups from the [3H]-leucine–labeled blood cells (∼40% reticulocytes) of an individual with unstable Hb Sabine (α2β91 leu→pro2) and stored frozen at 5 mg/mL in 10 mmol/L PO4 (K+), pH 7.0, as described previously.25 The buffers used during isolation and for the storage of these 3H-α-chains were routinely saturated with carbon monoxide (CO); however, in one experiment (see Fig 1), 3H-α-chains were isolated without exposure to CO. A batch of the 3H-α-chains was precipitated with acidified acetone26 to prepare 3H-α-globin, which was stored frozen in aliquots at 5 mg/mL in 10 mmol/L HCOO−(Na+), pH 4.0.
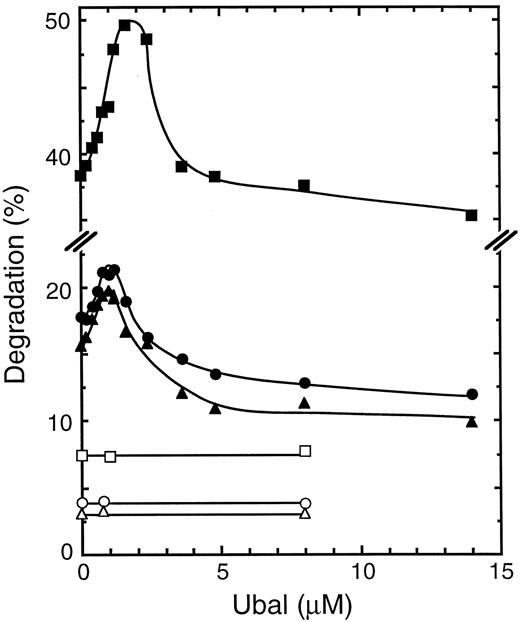
Effect of Ubal on the proteolysis of human Hb α-subunits in a β-thalassemic lysate. Each reaction mixture was incubated at 37°C for 2 hours with a dialyzed hemolysate from the blood cells of a β-thalassemic donor (S.O.) and either 3H-α-globin (□, ▪) or 3H-α-chains, stabilized with CO (▵, ▴) or unexposed to CO (○, •), as a proteolysis substrate. Protein degradation values are plotted for mixtures without (unfilled symbols) or with ATP, an ATP-regenerating system, and supplementary Ub (filled symbols).
Effect of Ubal on the proteolysis of human Hb α-subunits in a β-thalassemic lysate. Each reaction mixture was incubated at 37°C for 2 hours with a dialyzed hemolysate from the blood cells of a β-thalassemic donor (S.O.) and either 3H-α-globin (□, ▪) or 3H-α-chains, stabilized with CO (▵, ▴) or unexposed to CO (○, •), as a proteolysis substrate. Protein degradation values are plotted for mixtures without (unfilled symbols) or with ATP, an ATP-regenerating system, and supplementary Ub (filled symbols).
Construction, incubation, and analysis of the proteolysis reaction mixtures.Each proteolysis reaction mixture was prepared in a 0.5-mL plastic microcentrifuge tube and contained the following in a total volume of 25 μL: 50 mmol/L Tris HCl, pH 7.5 (23°C), 3 mmol/L dithiothreitol, 5 mmol/L MgCl2 , 1.25 mg dialyzed hemolysate as a source of proteolysis enzymes, and 3.75 μg (∼2.5 × 104 cpm) 3H-α-chains or 3H-α-globin as the substrate. Some reaction mixtures (designated “+ATP”) also contained 0.5 mmol/L ATP (Na+), 10 mmol/L creatine phosphate, 24 U/mL creatine phosphokinase (Sigma C3755), and 2 U/mL inorganic pyrophosphatase (Sigma I1643). The +ATP mixtures usually were supplemented with 47 μmol/L Ub (Sigma U6253). Some mixtures also contained Ubal, which was prepared by a modification (Cohen RE, et al, manuscript in preparation) of the procedure described previously.21 In these experiments, an aliquot of a stock solution of Ubal in H2O was transferred to a microcentrifuge tube and evaporated to dryness in vacuo. The residue was redissolved with the hemolysate, and this mixture was preincubated at 37°C for 5 minutes before addition of the other components.
Each tube containing a reaction mixture was sealed and then incubated at 37°C for the time (usually 2 hours) specified in the figures or tables. Proteolysis reactions were stopped by chilling on ice and by addition of 0.3 mL 10% (wt/vol) trichloracetic acid (TCA) to precipitate the proteins. After incubation at 4°C for 60 minutes, the soluble phase was separated by centrifugation (10,000g for 5 minutes) and transferred to a 20-mL liquid scintillation vial. Water (2.7 mL) and 12 mL Econo-Safe counting fluid (Research Products International, Mount Prospect, IL) were added, and 3H-radioactivity was determined in a Beckman liquid scintillation counter. The fraction (percentage) of substrate proteolyzed was calculated by dividing the acid-soluble 3H-radioactivity by the total initial 3H-radioactivity determined by counting, without acid treatment, a sample of the 3H-α-subunit substrate identical to that included in the reaction mixture. These protein degradation values were corrected for those (≤0.8%) obtained in a similar manner from unincubated reaction mixtures.
RESULTS
Effect of Ubal on Hb α-subunit proteolysis.Reaction mixtures containing an unfractionated hemolysate from the blood cells of a β-thalassemic donor were incubated with various concentrations of Ubal and with either 3H-α-chains or 3H-α-globin as a proteolysis substrate. Figure 1 shows that, in the absence of Ubal, there was an approximately fourfold stimulation of the degradation of either substrate by ATP (and an ATP-regenerating system). These results confirm those obtained previously in similar experiments in which this ATP-dependent degradation of 3H-α-chains was shown to be also largely Ub-dependent.14 When Ubal was added to the reaction mixtures, proteolysis of 3H-α-globin increased with increasing concentrations of this Ub derivative in the presence but not in the absence of ATP (Fig 1). An optimal stimulation of this ATP-dependent degradation of 36% was obtained at about 1.5 μmol/L Ubal. With higher Ubal concentrations, 3H-α-globin degradation decreased until a plateau was observed where the degradation was slightly less than in the absence of Ubal. This pattern of the effect of different Ubal concentrations on ATP-dependent proteolysis of 3H-α-globin is identical to that observed previously by us23 during analogous experiments with human 125I-α-globin in rabbit reticulocyte lysates. This identity confirms that the previous pattern of proteolysis is not an artifact resulting from iodination of the α globin molecule. These patterns might be explained by the simultaneous inhibition by Ubal of two different isopeptidase activities, one that decreased and another that increased 3H-α-subunit degradation (see the Discussion).
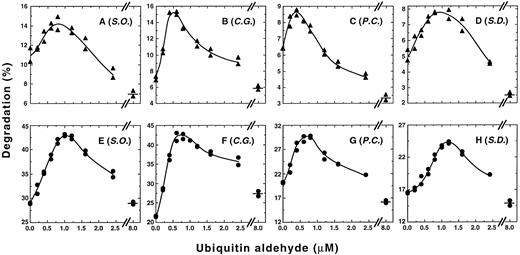
Low Ubal concentrations enhance ATP-dependent proteolysis of α-subunits. Each reaction mixture was incubated at 37°C for 2 hours with 3H-α-chains (A through D) or with 3H-α-globin (E through H) as a substrate and with a dialyzed hemolysate from the blood cells of either β-thalassemic donor S.O. (A and E), C.G. (B and F), P.C. (C and G), or S.D. (D and H). 3H-α-chain degradation values obtained in the absence of ATP (2.5%, 2.2%, 1.1%, and 1.7% for mixtures with lysates from S.O., C.G., P.C., and S.D., respectively) or 3H-α-globin degradation values obtained in the absence of ATP (6.9%, 5.3%, 4.7%, and 3.9% for mixtures with lysates from S.O., C.G., P.C., and S.D., respectively) remained essentially constant with the different Ubal concentrations (eg, see Fig 1) and were subtracted from each value obtained in the presence of ATP, an ATP-regenerating system, and supplementary Ub. Data points shown for each Ubal concentration represent ATP-dependent degradation values from duplicate reaction mixtures.
Low Ubal concentrations enhance ATP-dependent proteolysis of α-subunits. Each reaction mixture was incubated at 37°C for 2 hours with 3H-α-chains (A through D) or with 3H-α-globin (E through H) as a substrate and with a dialyzed hemolysate from the blood cells of either β-thalassemic donor S.O. (A and E), C.G. (B and F), P.C. (C and G), or S.D. (D and H). 3H-α-chain degradation values obtained in the absence of ATP (2.5%, 2.2%, 1.1%, and 1.7% for mixtures with lysates from S.O., C.G., P.C., and S.D., respectively) or 3H-α-globin degradation values obtained in the absence of ATP (6.9%, 5.3%, 4.7%, and 3.9% for mixtures with lysates from S.O., C.G., P.C., and S.D., respectively) remained essentially constant with the different Ubal concentrations (eg, see Fig 1) and were subtracted from each value obtained in the presence of ATP, an ATP-regenerating system, and supplementary Ub. Data points shown for each Ubal concentration represent ATP-dependent degradation values from duplicate reaction mixtures.
ATP-dependent proteolysis of 3H-α-chains, stabilized routinely with CO gas during their preparation to prevent oxidation, was affected by Ubal in a manner similar to that of the 3H-α-globin substrate (Fig 1). At a low Ubal concentration (∼1 μmol/L) there was a 33% stimulation of this degradation, whereas at high Ubal concentrations (≥5 μmol/L) the plateau level of ATP-dependent degradation was 66% of that found in the absence of this inhibitor. In one experiment, 3H-α-chains isolated without CO and therefore presumably in the oxygenated state were used as the proteolysis substrate. Figure 1 shows little difference in their pattern of degradation with increasing Ubal concentration versus that of 3H-α-chains exposed to CO. We conclude that use of the stabilizing CO ligand did not significantly change the effect of Ubal on ATP-dependent degradation, and therefore, in all subsequent experiments, only 3H-α-chains prepared with CO were used.
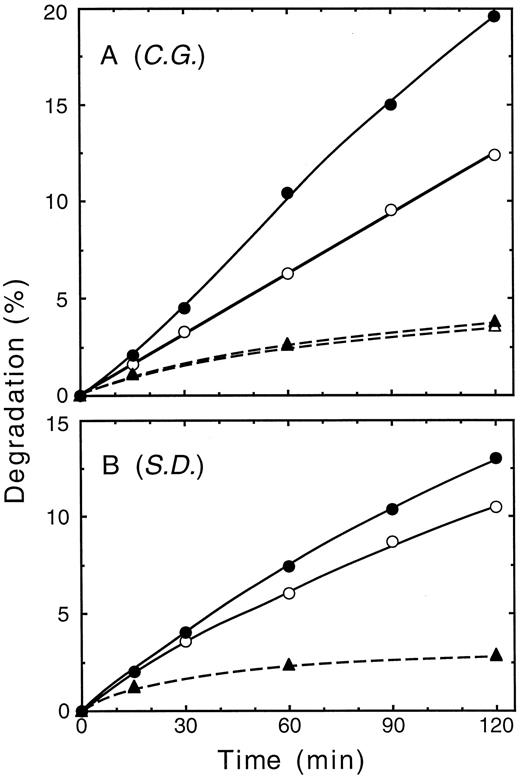
The effect of addition of low concentrations of Ubal on ATP-dependent proteolysis of 3H-α-chains catalyzed by blood cell hemolysates from each of four unrelated β-thalassemic individuals was investigated. Figure 2A to D shows that the pattern of 3H-α-chain degradation in each of four hemolysates, corrected for the ATP-independent component, was similar to that of Fig 1. Maximal stimulation of ATP-dependent proteolysis by Ubal ranged from 29% (blood donor S.O.; Fig 2A) to 115% (C.G.; Fig 2B) and occurred at concentrations of 0.4 μmol/L (P.C.; Fig 2C) to about 1 μmol/L (S.D.; Fig 2D). Comparable results (Fig 2E to H) were obtained with the four hemolysates when 3H-α-globin was used as the proteolysis substrate instead of 3H-α-chains. Optimal stimulation of ATP-dependent degradation of 3H-α-globin by Ubal varied from 47% (P.C. and S.D.; Fig 2G and H) to 96% (C.G.; Fig 2F) and was found at concentrations of 0.8 μmol/L (C.G. and P.C.; Fig 2F and G) to 1.2 μmol/L (S.D.; Fig 2H) Ubal. Thus, low concentrations of Ubal stimulated ATP-dependent proteolysis of both types of 3H-α-subunit substrates in each of four different hemolysates. Figure 3 shows that after an initial lag time of 15 minutes, there was a steady increase in the enhancement of ATP-dependent proteolysis of 3H-α-chains by Ubal throughout the 2-hour incubation. A similar lag period was observed previously by us23 in the Ubal-promoted stimulation of 125I-α-globin degradation in a rabbit reticulocyte lysate. We suggested that the lag occurred because of a slow accumulation of the Ubn-α-subunit intermediates, or slow-binding inhibition by Ubal of isopeptidases, or both.
Time course of 3H-α-chain degradation. Replicate proteolysis reaction mixtures contained a hemolysate from the blood cells of donor C.G. (A) or S.D. (B) and were incubated at 37°C for either 0, 15, 30, 60, 90, or 120 minutes. Mixtures contained ATP, an ATP-regenerating system, supplementary Ub, and either no Ubal (○), 0.8 μmol/L Ubal (A, •), or 1.0 μmol/L Ubal (B, •). Data also are shown from the corresponding mixtures without added ATP and ± Ubal (▴ and ▵, respectively; note that unfilled symbols may be obscured by overlap with filled symbols). Each data point represents the average from duplicate reaction mixtures.
Time course of 3H-α-chain degradation. Replicate proteolysis reaction mixtures contained a hemolysate from the blood cells of donor C.G. (A) or S.D. (B) and were incubated at 37°C for either 0, 15, 30, 60, 90, or 120 minutes. Mixtures contained ATP, an ATP-regenerating system, supplementary Ub, and either no Ubal (○), 0.8 μmol/L Ubal (A, •), or 1.0 μmol/L Ubal (B, •). Data also are shown from the corresponding mixtures without added ATP and ± Ubal (▴ and ▵, respectively; note that unfilled symbols may be obscured by overlap with filled symbols). Each data point represents the average from duplicate reaction mixtures.
A substantial fraction of the free, unstable α-subunits that were in the β-thalassemic blood cells may have been removed during preparative centrifugation and dialysis of the hemolysates. Nevertheless, it was possible that residual endogenous free α-chains had diluted the added substrate 3H-α-chains, thereby affecting the observed stimulation by Ubal of their proteolysis. To investigate this, we performed two additional experiments with the blood cell lysate from one (C.G.) of the β-thalassemic donors. The amount of 3H-α-chains added to each of several proteolysis mixtures was varied from half to twice the usual amount (3.75 μg). Both the fraction of 3H-α-chains degraded in an ATP-dependent manner (∼7%) and the stimulation by 0.6 μmol/L Ubal (∼100%) were invariant with this change in substrate concentration (data not shown). In another experiment, nonradioactive α-chains were added to a proteolysis reaction mixture to mimic or enhance any endogenous pool before addition of 3H-α-chains. Ubal (0.6 μmol/L) produced a 63% stimulation of ATP-dependent degradation of 3H-α-chains added at 10% (0.37 μg) the usual amount; this was observed either with or without addition of 3.38 μg nonradioactive α-chains. These results suggest that endogenous α-chains, if present, did not significantly affect Ubal-mediated stimulation of the degradation of added 3H-α-chains.
This study is in part a continuation of previous studies13,14 that used blood cells and hemolysates from the same β-thalassemic donors. Because the hemolysates had been stored frozen for several years (see the Methods), it was conceivable that the effect of Ubal on 3H-α-subunit proteolysis was an artifact resulting from the long storage of the lysates. Unfortunately, fresh blood could not be obtained because the donors were no longer available to us. Blood from normal individuals could not be used for these studies, because the activities of several enzymes of the ATP- and Ub-dependent proteolytic pathway decline markedly during maturation of erythroid cells from reticulocytes, abundant in anemic blood, to erythrocytes.27,28 However, using fresh hemolysates from the blood of rabbits made anemic by phenylhydrazine administration, we found that low Ubal concentrations (∼0.8 μmol/L) stimulated ATP-dependent degradation of either 3H-α-chains or 3H-α-globin by 35% to 70%, whereas higher Ubal concentrations resulted in a reduction of this stimulation (data not shown). The similarities of these patterns to those observed with either rabbit23 or β-thalassemic human (this study) frozen lysates suggest that fresh human anemic blood lysates, if available, would give comparable results.
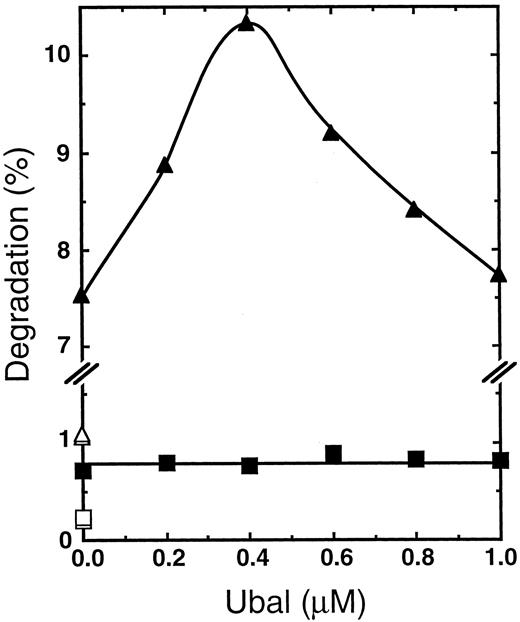
Effect of Ubal on the degradation of HbA.Isopeptidase inhibitors, such as suitable derivatives of Ubal, might be able to serve in vivo as therapeutic agents for β-thalassemia by selectively enhancing intracellular proteolysis of the excess Hb α-subunits in erythroid cells. As one test of this selectivity, we were interested in determining the effect of Ubal on other components of the β-thalassemic blood cell such as tetrameric HbA. For this purpose, some of the 3H-α-chains were incubated with a slight excess of complementary nonradioactive human Hb β-chains to prepare 3H-α2β2-tetramers,29 a form of HbA suitable for comparison to the free 3H-α-chains as a proteolysis substrate. Previous studies30,31 showed that HbA reconstituted in this manner exhibits oxygen affinity and cooperativity indicative of normal Hb tetramers. Degradation of 3H-α2β2-tetramers by a β-thalassemic hemolysate in the absence of Ubal was only about 10% of that of the free 3H-α-chains (Fig 4), a finding that confirms that reported previously from a similar experiment.14 A maximal stimulation of ATP-dependent proteolysis of free 3H-α-chains of 43% occurred at 0.4 μmol/L Ubal (Fig 4), a result similar to that observed earlier (Fig 2C) with the same β-thalassemic (donor P.C.) hemolysate. On the other hand, Fig 4 shows only minimal stimulation of degradation of the 3H-HbA construct with no apparent optimal Ubal concentration. The small enhancement of 3H-α2β2 degradation observed may occur because of the presence of residual and perhaps denatured 3H-α-chains in the preparation of tetramers. Analysis of this preparation by electrophoresis on cellulose acetate strips29 showed that about 92% of the total 3H-radioactivity migrated with HbA tetramers while 8% migrated in the free α chain region (data not shown). These results suggest that low concentrations of Ubal enhance ATP- and Ub-dependent proteolysis of free α-chains, but not that of tetrameric HbA.
Ubal enhances degradation of 3H-α-chains, but not of tetrameric 3H-HbA reconstituted from them. Each reaction mixture was incubated at 37°C for 2 hours with a dialyzed hemolysate from the blood cells of a β-thalassemic donor (P.C.) and with either the usual concentration (0.15 μg/μL) of 3H-α-chains or an equivalent concentration of 3H-α-chains in HbA (3H-α2β2-tetramer) as a proteolysis substrate. Values from mixtures with ATP, an ATP-regenerating system, and supplementary Ub are plotted for the degradation of free 3H-α-chains (▴) or 3H-α2β2 (▪). Unfilled symbols represent degradation values of the corresponding 3H-protein from otherwise duplicate mixtures without ATP. The substrates were prepared from protein stocks, each 5.0 mg/mL in a 10-mmol/L PO4 (K+), pH 7.0, buffer, by the addition of 20 μL 3H-α-chains to either 25 μL buffer alone or 25 μL nonradioactive human Hb β-chains29; these mixtures were incubated at 4°C for 2 hours to provide free 3H-α-chains or 3H-α2β2-tetramers, respectively.
Ubal enhances degradation of 3H-α-chains, but not of tetrameric 3H-HbA reconstituted from them. Each reaction mixture was incubated at 37°C for 2 hours with a dialyzed hemolysate from the blood cells of a β-thalassemic donor (P.C.) and with either the usual concentration (0.15 μg/μL) of 3H-α-chains or an equivalent concentration of 3H-α-chains in HbA (3H-α2β2-tetramer) as a proteolysis substrate. Values from mixtures with ATP, an ATP-regenerating system, and supplementary Ub are plotted for the degradation of free 3H-α-chains (▴) or 3H-α2β2 (▪). Unfilled symbols represent degradation values of the corresponding 3H-protein from otherwise duplicate mixtures without ATP. The substrates were prepared from protein stocks, each 5.0 mg/mL in a 10-mmol/L PO4 (K+), pH 7.0, buffer, by the addition of 20 μL 3H-α-chains to either 25 μL buffer alone or 25 μL nonradioactive human Hb β-chains29; these mixtures were incubated at 4°C for 2 hours to provide free 3H-α-chains or 3H-α2β2-tetramers, respectively.
Effect of Ubal on blood cell enzyme activities.Dilutions of each of the four β-thalassemic hemolysates that contained concentrations of enzymes, buffer, salt, and dithiothreitol identical to those of the proteolysis reaction mixtures (see the Methods) were prepared with or without 1.0 μmol/L Ubal. This concentration of Ubal significantly enhanced 3H-α-subunit degradation in the proteolysis studies (Fig 2). The activities of methemoglobin reductase and four enzymes of the anaerobic glycolysis pathway, chosen because of their stability during long-term storage of red blood cell lysates,32 were measured.33 Table 1 shows that there was no uniform effect of Ubal, despite a 34% to 340% variation of enzyme activities among the four hemolysates. We conclude that low concentrations of Ubal, the prototype of a potential therapeutic agent, do not inhibit the activity of at least five nonproteolytic enzymes in the β-thalassemic hemolysates. The result for methemoglobin reductase is particularly relevant, because this enzyme may reverse oxidation of the excess α chains in β-thalassemic erythroid cells and thereby reduce denaturation and inclusion-body formation (see the Discussion).
Ubal Does Not Inhibit Selected Enzymes in β-Thalassemic Hemolysates
| Enzyme . | Ubal . | Activity for β-Thalassemic Donor (IU/g Hb) . | |||
|---|---|---|---|---|---|
| . | . | S.O. . | C.G. . | P.C. . | S.D. . |
| GAPD | − | 470 | 361 | 383 | 485 |
| + | 449 | 349 | 381 | 426 | |
| PGK | − | 436 | 249 | 236 | 300 |
| + | 470 | 354 | 330 | 258 | |
| GPI | − | 176 | 130 | 135 | 144 |
| + | 165 | 126 | 156 | 139 | |
| TPI | − | 3,011 | 2,112 | 2,366 | 2,227 |
| + | 3,065 | 2,190 | 2,695 | 2,522 | |
| MetHb Red | − | 17.5 | 10.6 | 28.6 | 47.1 |
| + | 14.4 | 11.8 | 26.3 | 47.3 | |
| Enzyme . | Ubal . | Activity for β-Thalassemic Donor (IU/g Hb) . | |||
|---|---|---|---|---|---|
| . | . | S.O. . | C.G. . | P.C. . | S.D. . |
| GAPD | − | 470 | 361 | 383 | 485 |
| + | 449 | 349 | 381 | 426 | |
| PGK | − | 436 | 249 | 236 | 300 |
| + | 470 | 354 | 330 | 258 | |
| GPI | − | 176 | 130 | 135 | 144 |
| + | 165 | 126 | 156 | 139 | |
| TPI | − | 3,011 | 2,112 | 2,366 | 2,227 |
| + | 3,065 | 2,190 | 2,695 | 2,522 | |
| MetHb Red | − | 17.5 | 10.6 | 28.6 | 47.1 |
| + | 14.4 | 11.8 | 26.3 | 47.3 | |
Duplicate mixtures containing 50 mmol/L Tris HCl, pH 7.5 (23°C), 3 mmol/L dithiothreitol, 5 mmol/L MgCl2 , and 50 mg/mL of each of 4 dialyzed β-thalassemic hemolysates were prepared with or without 1.0 μmol/L Ubal. The unincubated mixtures were frozen and sent to Dr E. Beutler of the Scripps Research Institute (La Jolla, CA) for measurement of enzyme activities.33 The results, estimated to have a precision of ± a few percent, are reported in international units of activity normalized to the Hb content of the hemolysates (IU/g Hb).
Abbreviations: GAPD, glyceraldehyde phosphate dehydrogenase; PGK, phosphoglycerate kinase; GPI, glucose phosphate isomerase; TPI, triosphosphate isomerase; MetHb Red, methemoglobin reductase.
Is supplementary Ub required to observe the enhancement by Ubal of 3H-α-chain degradation?The +ATP proteolysis reaction mixtures, similar to those used previously,23 contained 47 μmol/L Ub in addition to the endogenous Ub in β-thalassemic hemolysates. In the earlier study, the supplementary Ub was used to ensure that substrate degradation was not limited by the availability of Ub. However, the necessity of addition of exogenous Ub to erythroid cells in vivo might be adverse to the development of a therapeutic approach to β-thalassemia. Therefore, we wondered if the Ub endogenous to β-thalassemic hemolysates, estimated previously14 to be 1 to 10 μmol/L, was sufficient to support Ubal-stimulated degradation of 3H-α-chains. Replicate reaction mixtures containing one of the four β-thalassemic hemolysates and 3H-α-chains as substrate were incubated with or without 47 μmol/L supplementary Ub. The patterns of degradation observed with various concentrations of Ubal either in the presence or absence of supplementary Ub were similar (data not shown) to those of Fig 2A to D. The maximum stimulation of 3H-α-chain degradation without supplementary Ub was about twice that found with 47 μmol/L Ub for three of the β-thalassemic hemolysates (Table 2; donors C.G., P.C., and S.D.). A significant increase of the basal (ie, without Ubal) level of 3H-α-chain proteolysis upon addition of Ub (Table 2) was primarily responsible for this phenomenon. The finding that the concentration of endogenous Ub in some of the β-thalassemic hemolysates was less than saturating for 3H-α-chain degradation confirms a previous observation made with similar reaction mixtures.14 We conclude that, at least with the conditions of the present in vitro reactions, supplementary Ub is not required to observe Ubal-mediated enhancement of 3H-α-chain degradation in β-thalassemic hemolysates.
Supplementary Ub Is Not Required for Stimulation of α-Chain Degradation by Ubal
| Lysate Donor . | Ub Added . | Degradation of 3H-α-Chains . | Ubal-Promoted . | |
|---|---|---|---|---|
| . | (μmol/L) . | No Ubal . | 0.6-0.8 μmol/L Ubal . | Increase (%) . |
| S.O. | 0 | 9.3 | 11.2 | 20.9 |
| 47 | 10.3 | 12.7 | 23.7 | |
| C.G. | 0 | 3.8 | 12.1 | 219.2 |
| 47 | 7.2 | 13.9 | 92.6 | |
| P.C. | 0 | 6.6 | 10.5 | 58.5 |
| 47 | 8.6 | 11.6 | 35.8 | |
| S.D. | 0 | 4.8 | 6.9 | 43.2 |
| 47 | 6.4 | 7.7 | 20.1 | |
| Lysate Donor . | Ub Added . | Degradation of 3H-α-Chains . | Ubal-Promoted . | |
|---|---|---|---|---|
| . | (μmol/L) . | No Ubal . | 0.6-0.8 μmol/L Ubal . | Increase (%) . |
| S.O. | 0 | 9.3 | 11.2 | 20.9 |
| 47 | 10.3 | 12.7 | 23.7 | |
| C.G. | 0 | 3.8 | 12.1 | 219.2 |
| 47 | 7.2 | 13.9 | 92.6 | |
| P.C. | 0 | 6.6 | 10.5 | 58.5 |
| 47 | 8.6 | 11.6 | 35.8 | |
| S.D. | 0 | 4.8 | 6.9 | 43.2 |
| 47 | 6.4 | 7.7 | 20.1 | |
Each reaction mixture was prepared with a dialyzed hemolysate from the blood cells of 1 of 4 β-thalassemic donors, 3H-α-chains as substrate, ATP, and an ATP-regenerating system with or without the usual concentration (47 μmol/L) of supplementary Ub. Replicate mixtures contained 0 to 1.0 μmol/L Ubal and were incubated at 37°C for 2 hours. Curves of ATP-dependent proteolysis similar to those shown in Fig 2 were plotted after subtraction of ATP-independent degradation values of 2.1%, 2.5%, 1.3%, and 1.8% obtained with the lysates from S.O., C.G., P.C., and S.D., respectively. Values for ATP-dependent proteolysis (% TCA-soluble radioactivity) of 3H-α-chains from mixtures without Ubal and from those with a Ubal concentration (0.6 to 0.8 μmol/L) resulting in maximum stimulation of degradation together with the corresponding calculated % increase are presented.
DISCUSSION
The excess α-subunits in β-thalassemic erythroid cells are structurally heterogeneous.34 Initially, most of the α-subunits are probably in the α-chain (α-Hb) form because the primary structure of the nascent α-globin is normal, and the heme group combines with it during or shortly after synthesis. However, the free oxygenated α-chain is highly susceptible to autoxidation, ie, conversion to the ferri (or met) hemoglobin form with attendant release of a superoxide anion. The α-methemoglobin chain, no longer able to bind a gaseous ligand, is readily further denatured either to α-globin with release of the ferriheme group or to a hemichrome form in which the heme is spuriously linked to an amino acid residue of the globin. The inclusion bodies in β-thalassemic erythroid cells contain hemichrome35 and have a peptide structure consistent with that of α-globin.36 Because of this heterogeneity in structure, it is appropriate that our study of α-subunit proteolysis involve both α-chains and α-globin, two forms that can be readily prepared. From the time the current method to isolate chains of HbA was first developed,37 gaseous CO has been used routinely to prevent autoxidation during their preparation and in subsequent studies. α-Chains prepared in the absence of CO and presumably in an oxygenated form may more closely resemble those in intact erythroid cells. However, the similarity in degradation patterns of the two 3H-α-chain types shown in Fig 1 suggests that those stabilized with CO can serve as an appropriate substitute in our cell-free studies. Our finding that ATP-dependent degradation of 3H-α-globin is about three times that of the 3H-α-chains after 2 hours of incubation (Figs 1 and 2) may reflect the relatively disordered secondary structure of the former substrate.38 Perhaps the lysine residues of α-globin are more exposed and therefore can be more readily ubiquitinated than those of the more folded, heme-containing α-chains.
The pattern of the effect of increasing Ubal concentration on ATP-dependent proteolysis of 3H-α-subunits (Fig 2) is similar to that reported previously23 for 125I-α-globin in a rabbit reticulocyte lysate. To explain this pattern, we proposed that Ubal inhibited two different isopeptidase activities, one that decreased degradation directly by disassembling Ub-protein conjugates, and another that enhanced degradation indirectly by destroying natural polyubiquitin inhibitors of the 26S proteasome. Inhibition of the first activity is the predominant effect of Ubal at low concentrations. Hb α-subunits are poor substrates, ie, they are ubiquitinated only sparsely, at a slow rate, or both compared with other proteins that are rapidly polyubiquitinated and degraded by the 26S proteasome.23 Furthermore, other laboratories39 40 showed that there is isopeptidase activity that removes Ub from Ub-protein conjugates and thus disassembles them. This activity potentially can act as a mechanism for the cell to distinguish between good and poor protein substrates by “editing” the newly formed conjugates before proteolytic degradation. This sequence of events may be the basis for the relatively poor degradation of α-subunits that, in intact β-thalassemic erythroid cells, results eventually in formation of inclusion bodies.
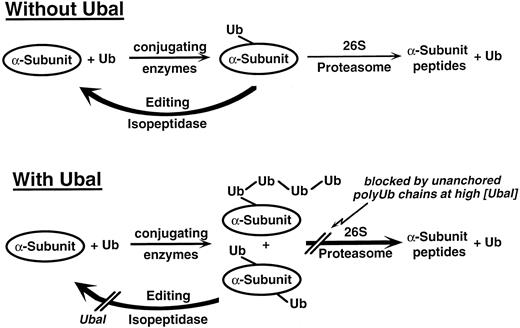
We suggest that low concentrations (≤1 μmol/L) of Ubal inhibit this editing isopeptidase activity (Fig 5). This results in an increase in multiubiquitinated or polyubiquitinated α-subunits that are more readily degraded by the 26S proteasome, and hence, the increase in ATP- and Ub-dependent proteolysis (Figs 1 and 2). We observed previously substantial increases in the amount and Ub content of conjugates of 125I-α-globin when low Ubal concentrations were included in the proteolysis reaction mixtures.22,23 Furthermore, isolated Ubn-125I-α-globin conjugates with a progressively higher Ub content (larger “n”) were degraded to a greater extent by purified 26S proteasome preparations.41 Collectively, these results are consistent with the hypothesis that Ubal inhibits an editing isopeptidase that suppresses the degradation of poorly ubiquitinated substrates such as hemoglobin α-subunits. This laboratory recently has identified a new Ubal-sensitive isopeptidase activity that is associated with the 26S proteasome and has properties consistent with such an editing function.42
Proposed scheme for the effect of Ubal on Ub-dependent proteolysis of Hb α-subunits in β-thalassemic hemolysates. Ub molecules are conjugated to the α-chain or α-globin substrates. Without Ubal, there are few conjugates and most are only sparsely ubiquitinated, in part because of ongoing deubiquitination by isopeptidase(s). These conjugates are poorly degraded by the 26S proteasome. Deubiquitination is inhibited by low concentrations (≤1 μmol/L) of Ubal. This results in more highly ubiquitinated conjugates (either from ubiquitination of multiple substrate lysines or from elaboration of polyUb chains, or both) that are more efficiently degraded by the 26S proteasome. Also, inhibition by Ubal of an “editing” isopeptidase within the 26S proteasome promotes degradation of even poorly ubiquitinated α-subunits.42 At higher levels, Ubal also blocks another isopeptidase(s) that leads to the accumulation of unanchored polyUb chains that inhibit processing of conjugates by the 26S proteasome.
Proposed scheme for the effect of Ubal on Ub-dependent proteolysis of Hb α-subunits in β-thalassemic hemolysates. Ub molecules are conjugated to the α-chain or α-globin substrates. Without Ubal, there are few conjugates and most are only sparsely ubiquitinated, in part because of ongoing deubiquitination by isopeptidase(s). These conjugates are poorly degraded by the 26S proteasome. Deubiquitination is inhibited by low concentrations (≤1 μmol/L) of Ubal. This results in more highly ubiquitinated conjugates (either from ubiquitination of multiple substrate lysines or from elaboration of polyUb chains, or both) that are more efficiently degraded by the 26S proteasome. Also, inhibition by Ubal of an “editing” isopeptidase within the 26S proteasome promotes degradation of even poorly ubiquitinated α-subunits.42 At higher levels, Ubal also blocks another isopeptidase(s) that leads to the accumulation of unanchored polyUb chains that inhibit processing of conjugates by the 26S proteasome.
Inhibition of the degradation of Hb α-subunits and of other proteins occurred when the concentration of Ubal was substantially higher than 1 μmol/L in the unfractionated lysate systems23 (Figs 1 and 2). During ATP- and Ub-dependent proteolysis of many substrates either endogenous to the lysate or exogenously added, polyubiquitin chains are hydrolyzed from the polyUb-protein conjugates. Others43 suggested that these “unanchored” chains, which consist of Ub monomers joined together by isopeptide bonds,44 could be natural inhibitors of 26S proteasome function by binding to the site reserved for the conjugate intermediates of degradation. Normally, the unanchored chains are rapidly degraded by isopeptidase activities different from the editing activity discussed. Isopeptidase T, an abundant Ub-protein hydrolase in red blood cells, may be primarily responsible for the normal recycling of Ub from these unanchored polyUb chains.45,46 These isopeptidases are also inhibited by Ubal. In the presence of sufficiently high concentrations (>3 μmol/L), the predominant effect of Ubal on the proteolysis system is an inhibition, mediated by undegraded polyUb chains, of Ub-α-subunit conjugate processing by the 26S proteasome (Fig 5). We showed a correlation between polyUb chain accumulation and inhibition of 125I-α-globin degradation in rabbit reticulocyte lysates consistent with this hypothesis.23
That a reduction in excess Hb α-subunits in erythroid cells might be a valid therapeutic approach to β-thalassemia is suggested from differences in the pathophysiology of individuals with both α-thalassemia and β-thalassemia genes from individuals homozygous for β-thalassemia only.47 α-Thalassemia is an inherited disorder characterized by a deficiency in the synthesis of Hb α-subunits and thus is the counterpart to β-thalassemia. Individuals homozygous for β-thalassemia who also carry an α-thalassemia gene(s) have a reduced imbalance in globin chain synthesis; this results in reduced inclusion-body formation and prolonged erythroid cell survival compared with the situation in pure β-thalassemia homozygotes.48 Ubal itself may not be the ideal potential therapeutic agent for β-thalassemia, because high concentrations inhibit the degradation of α-subunits. However, it may be possible to design peptide aldehyde or other derivatives that are more selective than Ubal in inhibition of the “editing” isopeptidase(s). These inhibitors might enhance α-subunit degradation without promoting accumulation of unanchored polyUb chains. Moreover, relative to Ubal, it should be easier to deliver these low–molecular-weight derivatives to the cytoplasm of the developing erythroid cell. Other investigators12 49 showed that various peptide aldehydes could enter mammalian cells and affect ATP- and Ub-dependent proteolysis.
The therapeutic approach to β-thalassemia suggested by our investigation may be applicable to other inclusion-body disorders. For example, in α-thalassemia, the deficiency in synthesis of Hb α-subunits results in an accumulation of excess β-subunits in erythroid cells.50 Although there are some differences in the mechanisms and rates of hemolysis between α- and β-thalassemia,51 suitable isopeptidase inhibitors might similarly enhance the degradation of excess β-subunits and thereby ameliorate the anemia of α-thalassemia. Moreover, the precipitated aggregates of modified tau protein found in the brain neurons of individuals with Alzheimer's disease may contribute to pathogenesis52 in a manner analogous to the α-subunit inclusions in β-thalassemia. Ub conjugates of the modified tau protein were identified in these precipitates,53 and Ub, presumably in a conjugated form, was found in neuronal inclusion bodies in Alzheimer's and several related neurodegenerative disorders.54 These insoluble Ub conjugates may be manifestations of an abortive attempt by the Ub-dependent proteolysis system to degrade abnormal proteins. Likewise, insufficient proteolysis may contribute to the formation of eye lens cataracts55 that consist of intracellular precipitates of oxidized and otherwise damaged crystallins, the major proteins in lens cells. Ub-dependent proteolysis was observed in bovine lens epithelial cells,56 and 26S proteasome activity was detected in extracts of the differentiated fiber cells57 that can develop the cataracts. Possibly, Ubal or a related isopeptidase inhibitor could enhance the degradation of abnormal proteins such as tau and crystallins that otherwise form deleterious intracellular deposits. However, a word of caution is appropriate. The ATP- and Ub-dependent proteolytic pathway is involved in the regulation of a wide range of fundamental processes, eg, DNA repair, antigen presentation, and cell-cycle progress. It is impossible to predict whether an enhanced degradation of abnormal proteins mediated by Ubal derivatives (or other isopeptidase inhibitors) will outweigh a potentially devastating effect on the whole organism by this targeted intervention in a major metabolic pathway.
ACKNOWLEDGMENT
We thank P.C., S.D., C.G., S.O., and especially C.W. for donations of blood that made this study possible; Dr C. Patrick Burns for obtaining C.W.'s blood; and Dr E. Beutler for advice and assistance with the red blood cell enzyme activity assays.
Supported by National Institutes of Health Grant No. GM37666.
Address reprint requests to Joseph R. Shaeffer, PhD, Department of Biochemistry, University of Iowa College of Medicine, 4-403 Bowen Science Bldg, Iowa City, IA 52242-1109.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal