Abstract
A classical notion regarding the expression of murine embryonic ζ- and adult α-globin genes holds that there is a switch in globin production from the embryonic to the adult form during fetal development. Our previous in situ hybridization studies challenged this view, since both ζ- and α-globin mRNAs can be detected simultaneously in the earliest erythrocyte populations. This finding raises the possibility that ζ-globin production might be wholly or partially redundant in embryos in which the adult α-globin is also expressed. To test this possibility, we created a null mutation of the ζ-globin gene using homologous recombination in embryonic stem cells. Many outbred mice homozygous for the ζ-null mutation were able to develop normally, undermining the notion that there is an absolute need for ζ-globin and indicating that α-globin alone can serve the survival needs of the fetus. Interestingly, insertion of the PGK-Neo cassette (used to create the null mutation) into the ζ-globin gene appears to influence the expression of the nearby α-globin genes, giving rise to reduced α-globin production and to an α-thalassemia–like syndrome. There is also evidence indicating the strong influence of genetic background on the ζ-null and α1-null phenotypes, both of which are much more severe in the 129/SvEv inbred genetic background. These quantitative differences can potentially be exploited to identify genes important for erythropoiesis.
IN BOTH MICE and humans, the functional genes of the α-globin cluster are organized with an embryonic ζ gene located 5′ to two adult α-globin genes (5′ → ζ, α1, α2 → 3′).1 It has generally been accepted that the embryonic ζ gene is the first to be expressed at the onset of erythropoiesis in the yolk sac of the developing embryo. As the expression of the embryonic ζ gene begins to diminish (eventually shutting off completely), the adult α genes are activated and are expressed throughout the lifetime of the mouse. An analogous situation is held to exist in the β-globin cluster. The embryonic β-like globin genes (Y, βh1) are thought to be activated first and then, as their expression begins to diminish, the adult β-globin genes (βmajor and βminor) are activated. This developmental switch was thought to take place roughly as erythropoiesis moved from the nucleated primitive yolk sac-derived red blood cells to the definitive enucleated erythrocytes.2-6
In a previous study,7 we used in situ hybridization with specific ζ and α riboprobes to question whether ζ-mRNA could serve as a marker for the early primitive red blood cell lineage, and α-mRNA, as a marker for the definitive lineage. Surprisingly, these studies revealed co-expression of ζ- and α-globin mRNAs in the most primitive yolk sac-derived cells. Moreover, the α transcripts always exceeded those of the embryonic ζ-globin gene. Since both genes were expressed simultaneously, this indicated the possibility that they are redundant with respect to one another at the earliest stage of erythropoiesis. Further, despite the fact that ζ-globin genes have been conserved throughout mammalian evolution, they might play only a minor role in the developing embryo and might not be essential for survival. While one can imagine mechanisms by which the simultaneous expression of ζ- and α-mRNAs could nonetheless result in a switch at the protein level (for example, by differential translation8 ), the requirement for ζ-globin expression can be tested directly in the mouse by creating a null ζ-globin mutation. Moreover, such an experiment might offer a hint as to whether the ζ-globin is an essential gene or an evolutionary relic.
A number of informative and useful models of mouse thalassemias have been created using homologous recombination in embryonic stem cells.9-14 In order to assess the role that the ζ-globin gene plays in embryonic development, we have created a ζ-null strain of mice and find that such mice can indeed survive, implying that α-globin can assume the role thought to be unique to ζ-globin in early mouse development. To assess the relative importance of ζ- and α-globin during early development, we also created an α1-deficient mouse, which is null in one of the two adult globin genes. Both homozygote mutant strains survive and give evidence of a thalassemia-like syndrome in the adult. Such a phenotype would have been expected as a consequence of eliminating the α1-gene,14 but not from elimination of the embryonic ζ-gene. Moreover, we find that the severity of their disease depends very much on their genetic background. Apparently modifying genes — other than the α-globins — influence the course of this disease.
MATERIALS AND METHODS
Construction of the ζ- and α1-null alleles by homologous recombination in ES cells.Targeting vectors for homologous recombination and interruption of the ζ- and α1-globin genes were linearized at a unique Not I site and transfected into TC-1 embryonic stem (ES) cells.15 Candidates for homologous recombination were selected with G418 and FIAU, picked, and analyzed by Southern blotting16 as shown in Fig 1. Targeted clones for the ζ- and α1-mutations were microinjected into C57BL/6 blastocysts, implanted into the uteri of pseudo-pregnant Swiss Webster foster mothers, and allowed to develop to term (all mice were obtained from Taconic Farms, Germantown, NY). Male chimeras (identified by the presence of agouti coat color) were mated with NIH Black Swiss females and germline transmission was confirmed by agouti coat color in the F1 animals. The agouti offspring were tested for the presence of the interrupted ζ- and α1-alleles by Southern blot analyses as shown in Fig 1.
Targeted disruption of the ζ- and α1-globin genes. Top: Map of the mouse α-globin locus with its three genes: ζ, α1, and α2. Middle: Maps of targeting vectors containing 6 Kb and 7.5 Kb genomic sequences of the ζ- and α1-genes, respectively. In both cases, interruption by homologous recombination would result in the insertion of the PGK-Neo cassette21 close to the middle of the respective genomic fragments. Note that in both cases the inserted PGK-Neo cassette would be transcribed in the direction opposite that of the ζ- and α-genes. Homologous recombination would introduce the PGK-Neo cassette at the Xba I site at the end of exon-1 of the ζ-gene, and would replace a 295 bp BamHI fragment containing a portion of the first intron and the second exon of the α1-gene. Bottom: Shown are examples of restriction endonuclease analyses of tail DNA prepared from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) ζ- and α1-mutant mice. The position of the wild-type and mutant DNA fragments detected by the probe are indicated by labeled arrows. Filters were probed with the indicated genomic fragments (5′ ζ KO probe and 3′ α KO probe) both of which reside outside the region involved in homologous recombination. For the ζ-mutation, DNA was digested with EcoRV and probed with a 1.8 Kb R5-Xba I fragment. For the α1 mutation, DNA was digested with EcoRI and probed with 1.2 Kb BamHI-EcoRI fragment. Restriction endonuclease symbols: R5 = EcoRV; X = Xba I; E = EcoRI; B = BamHI.
Targeted disruption of the ζ- and α1-globin genes. Top: Map of the mouse α-globin locus with its three genes: ζ, α1, and α2. Middle: Maps of targeting vectors containing 6 Kb and 7.5 Kb genomic sequences of the ζ- and α1-genes, respectively. In both cases, interruption by homologous recombination would result in the insertion of the PGK-Neo cassette21 close to the middle of the respective genomic fragments. Note that in both cases the inserted PGK-Neo cassette would be transcribed in the direction opposite that of the ζ- and α-genes. Homologous recombination would introduce the PGK-Neo cassette at the Xba I site at the end of exon-1 of the ζ-gene, and would replace a 295 bp BamHI fragment containing a portion of the first intron and the second exon of the α1-gene. Bottom: Shown are examples of restriction endonuclease analyses of tail DNA prepared from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) ζ- and α1-mutant mice. The position of the wild-type and mutant DNA fragments detected by the probe are indicated by labeled arrows. Filters were probed with the indicated genomic fragments (5′ ζ KO probe and 3′ α KO probe) both of which reside outside the region involved in homologous recombination. For the ζ-mutation, DNA was digested with EcoRV and probed with a 1.8 Kb R5-Xba I fragment. For the α1 mutation, DNA was digested with EcoRI and probed with 1.2 Kb BamHI-EcoRI fragment. Restriction endonuclease symbols: R5 = EcoRV; X = Xba I; E = EcoRI; B = BamHI.
RNA and DNA analyses.Total RNA was prepared from embryos that were disaggregated by Polytron disruption in a 4 mol/L guanidine isothiocyanate, 25 mmol/L sodium citrate (pH 7.5), and 0.1 mol/L β-mercaptoethanol solution that was centrifuged through a 5.7 mol/L CsCl, 25 mmol/L Na acetate cushion according to Chirgwin et al.17 DNA was prepared from tail biopsies that were incubated overnight at 50°C in 0.5 mL of buffer containing 17 mmol/L Tris Cl at pH 7.5, 17 mmol/L EDTA, 170 mmol/L NaCl, 0.85% sodium dodecyl sulfate (SDS), and 0.2% proteinase K (usually added just before use). Following overnight incubation, 0.25 mL of saturated 6 mol/L NaCl was added to each tube; the tubes were vigorously shaken (200×), chilled on ice for 10 minutes, and spun down in a microfuge at about half speed for 10 minutes. One half milliliter of supernate was transferred to a fresh microtube to which was added 1 mL 95% ethanol. The DNA formed a stringy white precipitate after inverting the tubes several times. The precipitate was centrifuged, rinsed twice with 70% ethanol, air-dried, and dissolved in TE buffer (10 mmol/L Tris, pH 7.5, 0.1 mmol/L EDTA).
DNAs were analyzed after restriction endonuclease digestion by electrophoresis in 1% agarose, transfer to nitrocellulose, and hybridization with the appropriate probe as indicated in Fig 1. Total RNAs were analyzed by electrophoresis in 1% agarose gels containing 0.6% formaldehyde and processed as previously described.18 The hybridization probes were labeled with 32P to a specific activity of approximately 107 to 108 cpm/μg using the nick-translation reaction.19
Peripheral blood histology and hematopoietic indices.Peripheral blood was collected from tails in potassium-EDTA treated microtubes. Blood smears were prepared either manually or mechanically. Slides were stained with the Diff-Quik stain set (VWR, Bridgeport, NJ), which gives results similar to that obtained with the Wright-Giemsa stain, allowing the delineation of red cell morphology. Hematologic indices were as follows: mean corpuscular volume (MCV), mean corpuscular hemoglobin/red cell (MCH), red cell distribution width (RDW). Platelet levels and hematologic indices were determined in a clinical hematology laboratory by standard hospital methods.
Isoelectric focus analysis of mouse hemoglobins.Immobilized gradient isoelectric focusing was carried out essentially as described by Whitney et al.20 Polyacrylamide gels 0.5 mm thick were cast on Gelbond PAG film with a gradient of pH 7.2 to 7.55 created using Pharmacia Biotech AB Immobiline II pK 7.0 and 3.6. Grooves approximately 2 mm wide separated the sample lanes that are spaced 10 mm center-to-center. Gels were washed before use in multiple changes of 0.1% β-mercaptoethanol and deionized water. Blood was collected from the retroorbital sinus into heparinized microhematocrit tubes and centrifuged for 4 minutes in a Clay Adams Autocrit II hematocrit centrifuge. A 1-cm column of packed red cells (approximately 10 μL) was lysed in 100 μL of deionized water. Samples of 7 μL were applied to the sample wells and focusing was carried out on an LKB Multiphor II apparatus at 10°C overnight with maxima of 1 W, 1 mA, and 5,000 V set using an LKB Macrodrive 5 power. The unstained hemoglobin bands were photographed with Kodak KR-135 film or spectrophotometric quantification was performed using a Shimadzu CS9000 flying spot densitometer at 430 nm.
RESULTS
Disruption of ζ- and α1-globin genes by homologous recombination.The α-like globin gene cluster was isolated from mouse strain 129/SvEv genomic DNA cloned in a λ phage library (Fig 1). In order that we might make appropriate comparisons, this DNA was used to make two constructs, one interrupting the embryonic ζ-gene, the other, the adult α1-gene. The embryonic ζ-gene, the 5′ most gene of the cluster, was interrupted by inserting a PGK-Neo cassette into its first exon. The adult α1-globin gene was interrupted by replacing a small 295 bp BamHI fragment in its second exon with the PGK-Neo cassette. In both cases, PGK-Neo was placed in the opposite orientation relative to globin gene transcription (Fig 1).
It was expected that both constructs would create null mutations. Since there is only one ζ gene per chromosome, a null mutation could be easily verified by the absence of intact mRNA in the embryonic precursors of red blood cells isolated from homozygous ζ-null embryos. With respect to the α1 mutation, the expectation was different. There are two effectively identical α-globin genes in the mouse, α1 and α2, and disruption of α1 might allow undiminished or even up-regulated expression of α2. It has been reported that there is a compensatory upregulation of the 3′ α gene when the 5′ α gene is mutated in humans.22
Both ζ and α1 targeting constructs were transfected into TC1 embryonic stem cells and Southern blot analyses detected a relatively high proportion of homologous recombination in the G418 and FIAU double-resistant TC1 clones. More specifically, 25% of the ζ clones and 18% of the α1 clones contained the correct targeting event. The targeted ES clones were then injected into C57BL/6 blastocysts, often giving rise to highly chimeric mice with the capacity for germline transmission. Having multiple targeted clones for each construct made it possible to generate two mutant mice for each construct derived from two independent ES clones. Reassuringly, the two ζ-null mice demonstrated essentially the same phenotype, as did the two α1-null mice.
Originally the mice were created on a mixed genetic background by mating the male chimeras with outbred Black Swiss females. Later, we also placed our null alleles on an inbred 129/SvEv background by mating male chimeras with 129/SvEv females. Interestingly, both ζ and α1 mutants exhibited very different homozygous phenotypes on these two genetic backgrounds (see below).
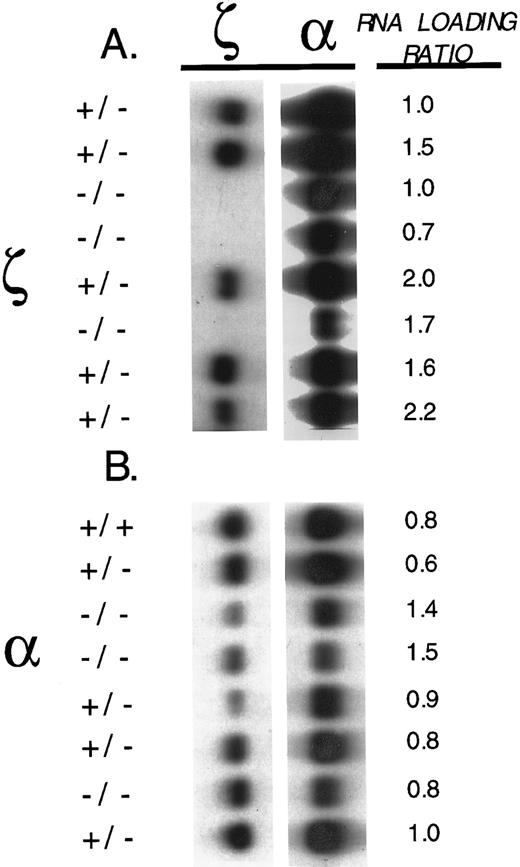
ζ- and α-globin mRNA expression in embryos.Ten and one-half–day post-coitus (pc) embryonic litters of crosses between heterozygous ζ-null females and homozygous ζ-null males were genotyped by Southern analysis and several well-developed homozygous ζ-null embryos were identified. To verify that we had created a null mutation, we used Northern blot analyses to detect ζ and α mRNA in genotyped members of the embryonic litter. The blots were hybridized with ζ- and α-specific probes and the results of the analysis of a representative litter are shown in Fig 2A. As can be seen, embryos heterozygous for the ζ mutation express ζ mRNA, while those homozygous for this mutation do not. The analyses also showed that the homozygous ζ−/− null embryos expressed reduced amounts of α-globin mRNA (Fig 2A). We shall return to this latter observation below.
ζ- and α-globin mRNAs in ζ- and α1-heterozygote and null embryos. (A) Total RNA analyses of 10-day pc littermate embryos that are heterozygous (+/−) and homozygous (−/−) for the ζ-null mutation. The blot was hybridized sequentially with three different probes: ζ- 1 Kb genomic Xba I-EcoRI fragment containing exon-1 plus a portion of the 5′ untranslated region of the ζ-globin gene), α1- 600 bp genomic BamHI-EcoRI fragment containing exon-3 and a portion of the 3′ untranslated region of the α1-globin gene), and a murine actin probe used to quantitate RNA loading. (B) Total RNA analyses of 12-day pc littermate embryos that are wild type (+/+), heterozygous (+/−), and homozygous (−/−) for the α1-null mutation. The filter was hybridized sequentially with the three different probes as described above in A. The relative amount of mRNA loaded on the gel was determined by measuring the relative density of the actin mRNA band detected with the actin probe in each sample and is indicated by the relative “load factor” shown in the figure.
ζ- and α-globin mRNAs in ζ- and α1-heterozygote and null embryos. (A) Total RNA analyses of 10-day pc littermate embryos that are heterozygous (+/−) and homozygous (−/−) for the ζ-null mutation. The blot was hybridized sequentially with three different probes: ζ- 1 Kb genomic Xba I-EcoRI fragment containing exon-1 plus a portion of the 5′ untranslated region of the ζ-globin gene), α1- 600 bp genomic BamHI-EcoRI fragment containing exon-3 and a portion of the 3′ untranslated region of the α1-globin gene), and a murine actin probe used to quantitate RNA loading. (B) Total RNA analyses of 12-day pc littermate embryos that are wild type (+/+), heterozygous (+/−), and homozygous (−/−) for the α1-null mutation. The filter was hybridized sequentially with the three different probes as described above in A. The relative amount of mRNA loaded on the gel was determined by measuring the relative density of the actin mRNA band detected with the actin probe in each sample and is indicated by the relative “load factor” shown in the figure.
Twelve-day pc embryos from matings between heterozygous α1+/− mice were also analyzed for ζ and α mRNAs. As shown in Fig 2B, homozygous α1−/− embryos showed reduced amounts of α-globin mRNA in comparison with wild-type and heterozygote embryos, with the remaining mRNA arising from the intact α2-globin gene. In the same figure, it can also be noted that there is some variation in the amounts of ζ-mRNA detected in these embryos. Note that this variation does not correlate with the mutant α1 allele. We shall return to this point below.
Establishing the ζ- and α1-globin phenotypes.A number of viable and reproductively competent ζ-null adult mice were derived from our heterozygote matings, indicating that the adult α genes could serve the survival needs of the developing embryo in the absence of ζ-globin production. As data from heterozygous crosses were analyzed, however, it became apparent that the expected Mendelian ratio of possible genotypes was distorted against ζ−/− homozygotes. The results of genotyping weanlings from several litters produced in an outbred genetic background is indicated in Table 1A.
Genotypes of Live Weanling Mice Produced by Crosses Between Mice Heterozygous for ζ− or α1− Null Alleles
| Cross . | Genetic Background . | Genotype of Offspring Observed (expected) . | ||
|---|---|---|---|---|
| . | . | +/+ . | +/− . | −/− . |
| A.ζ+/− × ζ+/− | Mixed (129/Bl Swiss) | |||
| Strain c1-13 | 43 (32) | 73 (64) | 11 (32) | |
| Strain c1-24 | 35 (26) | 63 (52) | 8 (26) | |
| B.ζ+/− × ζ+/− | Inbred 129 | |||
| Strain c1-13 | 45 (31) | 79 (62) | 0 (31) | |
| C.α1+/− × α1+/− | Mixed (129/Bl Swiss) | |||
| Strain c1-38 | 19 (25) | 62 (50) | 20 (25) | |
| D.α1+/− × α1+/− | Inbred 129 | |||
| Strain c1-38 | 6 (6) | 13 (12) | 5 (6) | |
| Cross . | Genetic Background . | Genotype of Offspring Observed (expected) . | ||
|---|---|---|---|---|
| . | . | +/+ . | +/− . | −/− . |
| A.ζ+/− × ζ+/− | Mixed (129/Bl Swiss) | |||
| Strain c1-13 | 43 (32) | 73 (64) | 11 (32) | |
| Strain c1-24 | 35 (26) | 63 (52) | 8 (26) | |
| B.ζ+/− × ζ+/− | Inbred 129 | |||
| Strain c1-13 | 45 (31) | 79 (62) | 0 (31) | |
| C.α1+/− × α1+/− | Mixed (129/Bl Swiss) | |||
| Strain c1-38 | 19 (25) | 62 (50) | 20 (25) | |
| D.α1+/− × α1+/− | Inbred 129 | |||
| Strain c1-38 | 6 (6) | 13 (12) | 5 (6) | |
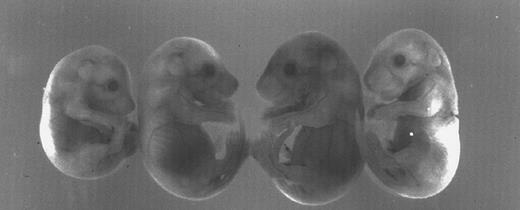
There is also variable size and viability among the homozygous newborns, some of which are initially quite small, whereas others are of normal size. The homozygous adults are fertile but crosses between them yield small litters of three to six pups. Here again, some of the ζ-null newborns look perfectly normal, while others are significantly runted and anemic. A typical example of this variable expressivity is shown in Fig 3 in which a small litter of ζ-null 12-day pc live embryos display considerable variation in size and pallor (reabsorbed and dead embryos are not shown). The smaller litter size seen in crosses between ζ-null homozygotes and the reduced number of homozygous pups seen in litters derived from heterozygous ζ+/− matings point to poor survival during development.
Variation in size and pallor of homozygous ζ−/− null embryos produced in an outbred genetic background. The four 12-day pc embryos are the littermate progeny of a mating between ζ−/− parents of the mixed 129/Black Swiss background. Note the variation in size among the littermates. Not easily discerned in the black and white photograph is the extreme pallor of the two embryos at the ends of the row.
Variation in size and pallor of homozygous ζ−/− null embryos produced in an outbred genetic background. The four 12-day pc embryos are the littermate progeny of a mating between ζ−/− parents of the mixed 129/Black Swiss background. Note the variation in size among the littermates. Not easily discerned in the black and white photograph is the extreme pallor of the two embryos at the ends of the row.
In the comparable case of the α1-null mice, we do not see the degree of attrition observed for the ζ-null mice, although homozygous crosses yield somewhat smaller litters. The expected number of homozygous weanlings seen in a cross between α1+/− heterozygotes does not vary greatly from that anticipated (Table 1C).
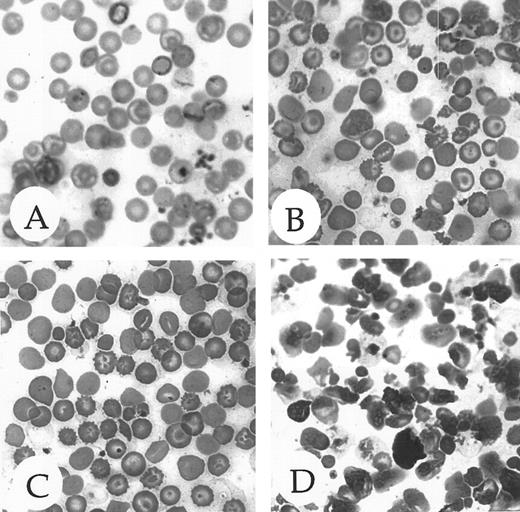
The pallor and size variation among late stage ζ-null embryos indicated that the embryos were anemic. Since mice at this stage of embryonic development are almost completely dependent on α-globin, not ζ-globin, this observation suggested that the deficiency might not be restricted to loss of ζ-globin alone. Histologic examination of the peripheral blood of adult ζ-globin null mice (Fig 4, compare A and B) revealed a striking, thalassemia-like picture with marked anisocytosis, poikilocytosis, and polychromasia and many target and burr cells. Similar, but marginally less severe, changes are seen in α1-null mice (Fig 4, compare A and C). The surprising morphologic changes in the peripheral blood of the ζ-null mice were confirmed on determination of hematologic indices (Table 2). The reduced hemoglobin, mean corpuscular volume, and mean corpuscular hemoglobin are consistent with thalassemia, possibly as a result of reduced α-globin production at the mutant locus (see below). As noted previously by Chang et al,14 the comparable indices in the α1-null mice (Table 3A) are consistent with α-thalassemia. Platelet counts are elevated in both types of thalassemic animals (not shown for α1-nulls). Moreover, the compound heterozygote, carrying a ζ-null on one chromosome and an α1-null on the other (ζ−α1α2/ζα1−α2) develops a clear thalassemic blood picture (Table 2). As would also be expected, the heterozygotes of both ζ- and α-null mutations display an intermediate phenotype with respect to their hematologic indices (Tables 2 and 3).
Peripheral blood morphology of homozygous ζ- and α1-null adult mice. (A) Blood from a mouse with wild-type globin genes on an outbred 129/Black Swiss genetic background demonstrating normal erythrocyte morphology. (B) Blood from a mouse with a ζ−/− null genotype on an outbred 129/Black Swiss genetic background demonstrating abnormal red cell morphology. (C) Blood from a mouse with a α1−/− null genotype on an outbred 129/Black Swiss genetic background demonstrating mildly abnormal red cell morphology. (D) Blood from a mouse with an α1−/− null genotype on an inbred 129 genetic background displaying severe anisopoikilocytosis and highly abnormal red blood cell morphology. A more detailed description of the red cell morphology is given in the text.
Peripheral blood morphology of homozygous ζ- and α1-null adult mice. (A) Blood from a mouse with wild-type globin genes on an outbred 129/Black Swiss genetic background demonstrating normal erythrocyte morphology. (B) Blood from a mouse with a ζ−/− null genotype on an outbred 129/Black Swiss genetic background demonstrating abnormal red cell morphology. (C) Blood from a mouse with a α1−/− null genotype on an outbred 129/Black Swiss genetic background demonstrating mildly abnormal red cell morphology. (D) Blood from a mouse with an α1−/− null genotype on an inbred 129 genetic background displaying severe anisopoikilocytosis and highly abnormal red blood cell morphology. A more detailed description of the red cell morphology is given in the text.
Hematologic Parameters in ζ− Null and ζ−/α1− Compound Heterozygote Mice
| Mice 129/BL Swiss . | Genotype . | Hgb g/dL . | MCV fL . | MCH pg . | Platelets × 106/mL . |
|---|---|---|---|---|---|
| Wild-type (N = 7) | ζα1α2/ζα1α2 | 15.7 (0.5) | 52.3 (0.9) | 17.4 (0.9) | 1219 (115) |
| ζ Heterozygote (N = 5) | ζ−α1α2/ζα1α2 | 13.5 (1.0) | 47.0 (3) | 15.1 (1.4) | 1116 (224) |
| ζ Homozygote (N = 5) | ζ−α1α2/ζ−α1α2 | 10.1 (0.7) | 41.8 (1) | 13.2 (0.8) | 3820 (712) |
| Compound α−/ζ− heterozygote (N = 3) | ζ−α1α2/ζα1−α2 | 10.3 (1.0) | 41.6 (1) | 14.9 (0.2) | 4933 (832) |
| Mice 129/BL Swiss . | Genotype . | Hgb g/dL . | MCV fL . | MCH pg . | Platelets × 106/mL . |
|---|---|---|---|---|---|
| Wild-type (N = 7) | ζα1α2/ζα1α2 | 15.7 (0.5) | 52.3 (0.9) | 17.4 (0.9) | 1219 (115) |
| ζ Heterozygote (N = 5) | ζ−α1α2/ζα1α2 | 13.5 (1.0) | 47.0 (3) | 15.1 (1.4) | 1116 (224) |
| ζ Homozygote (N = 5) | ζ−α1α2/ζ−α1α2 | 10.1 (0.7) | 41.8 (1) | 13.2 (0.8) | 3820 (712) |
| Compound α−/ζ− heterozygote (N = 3) | ζ−α1α2/ζα1−α2 | 10.3 (1.0) | 41.6 (1) | 14.9 (0.2) | 4933 (832) |
Abbreviations: Hb, hemoglobin concentration (g/dL); MCV, mean corpuscular volume ( μ3/red cell); MCH, mean corpuscular hemoglobin (Hb pg/red cell); N, number of mice.
A Comparison of Hematologic Indices in Outbred 129/BL Swiss and Inbred 129 Mice Bearing Hetero- and Homozygous α1− Null Alleles
| . | A.129/BL Swiss Mixed Background . | ||||
|---|---|---|---|---|---|
| . | Genotype . | Hb g/dL . | MCV fL . | MCH pg . | RDW % . |
| Wild-type (N = 7) | ζα1α2/ζα1α2 | 15.7 (0.5) | 52.3 (0.9) | 17.4 (0.9) | 12.6 (1.6) |
| α1− Heterozygote (N = 5) | ζα1−α2/ζα1α2 | 13.8 (2.0) | 42.2 (7.0) | 14.6 (0.5) | 14.2 (1.4) |
| α1− Homozygote (N = 9) | ζα1−α2/ζα1−α2 | 12.7 (0.4) | 41.6 (2.5) | 13.3 (0.9) | 18.1 (1.4) |
| B.129 Inbred Background | |||||
| Genotype | Hb g/dL | MCV fL | MCH pg | RDW % | |
| . | A.129/BL Swiss Mixed Background . | ||||
|---|---|---|---|---|---|
| . | Genotype . | Hb g/dL . | MCV fL . | MCH pg . | RDW % . |
| Wild-type (N = 7) | ζα1α2/ζα1α2 | 15.7 (0.5) | 52.3 (0.9) | 17.4 (0.9) | 12.6 (1.6) |
| α1− Heterozygote (N = 5) | ζα1−α2/ζα1α2 | 13.8 (2.0) | 42.2 (7.0) | 14.6 (0.5) | 14.2 (1.4) |
| α1− Homozygote (N = 9) | ζα1−α2/ζα1−α2 | 12.7 (0.4) | 41.6 (2.5) | 13.3 (0.9) | 18.1 (1.4) |
| B.129 Inbred Background | |||||
| Genotype | Hb g/dL | MCV fL | MCH pg | RDW % | |
| Wild-type (N = 7) | ζα1α2/ζα1α2 | 16.0 (0.2) | 47.4 (1.0) | 16.5 (0.8) | 13.2 (0.2) (N = 3) |
| α1− Heterozygote (N = 7) | ζα1−α2/ζα1α2 | 15.0 (0.3) | 41.4 (0.8) | 14.0 (0.2) | 18.2 (0.5) (N = 4) |
| α1− Homozygote (N = 7) | ζα1−α2/ζα1−α2 | 7.9 (0.4) | 38.5 (0.5) | 12.2 (0.7) | 33.8 (10.0) |
| Wild-type (N = 7) | ζα1α2/ζα1α2 | 16.0 (0.2) | 47.4 (1.0) | 16.5 (0.8) | 13.2 (0.2) (N = 3) |
| α1− Heterozygote (N = 7) | ζα1−α2/ζα1α2 | 15.0 (0.3) | 41.4 (0.8) | 14.0 (0.2) | 18.2 (0.5) (N = 4) |
| α1− Homozygote (N = 7) | ζα1−α2/ζα1−α2 | 7.9 (0.4) | 38.5 (0.5) | 12.2 (0.7) | 33.8 (10.0) |
Abbreviation: RDW, red cell distribution width.
Adult α gene expression is reduced in ζ-null mice.Several of the observations we noted above point to a broader effect of the ζ-null mutation than might otherwise have been expected. First, as shown in Fig 2A, 10-day pc embryos carrying the ζ-null mutation as expected display a complete loss of ζ-globin mRNA, but unexpectedly display a clear diminution of α-globin mRNA as well. Second, adult ζ-null mice clearly develop an α-thalassemia–like syndrome, a syndrome that persists long after the embryonic ζ-globin gene is normally inactivated. Together, these observations point to an α-thalassemia, possibly resulting from the influence of the inserted PGK-Neo cassette on the nearby α-genes.
In view of the α-thalassemia–like phenotype, it was important to assess the extent of α-chain reduction in adult mutant mice and to determine whether the α-deficiency arises from the chromosome bearing the insertionally disrupted ζ-globin gene. For this purpose, we used genetic analyses in conjunction with an isoelectric focusing system20 wherein the separation of blood hemoglobins (α2β2) depends on the allelic origin of α chain incorporated into the hemoglobin molecule. We selected a genetic system in which the α-globin products of the 129/SvEv chromosome (the mutant chromosome) could be distinguished from the α products of a normal BALB/c chromosome. In this way we could assess the α-alleles from each chromosome, the one in cis and the one in trans to the insertionally interrupted ζ-gene.
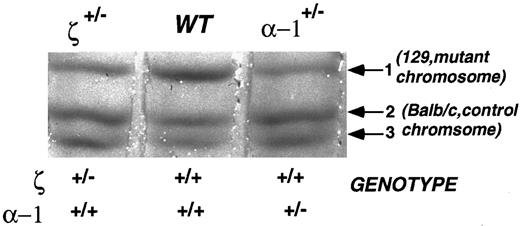
Accordingly, we crossed our ζ- and α1-null mutants with BALB/c mice and used the F1 heterozygotes for the analyses (Fig 5). The F1 heterozygotes have a 129/SvEv allele expressing an α-globin chain designated as chain-1 (α1 and α2 are indistinguishable in 129/SvEv mice) and a BALB/c allele expressing α-globins designated chain-2 and chain-3 corresponding to α1 and α2, respectively. ζ-null heterozygotes, α-null heterozygotes, and normal littermates are all expected to express chain-2 and chain-3 (the products of the normal BALB/c chromosome) at a similar level and this serves as an internal control. The product of the mutation-bearing 129/SvEv chromosome is reflected via the production of α chain-1. In the α1-null heterozygote, chain-1 reflects the output of the remaining α2 gene. In ζ-null heterozygotes, chain-1 reflects expression from both intact neighboring α1 and α2 genes. Since in strain 129/SvEv both α1 and α2 give rise to the same chain-1, it is impossible to distinguish between α1 and α2 production. Nonetheless, as shown in Fig 5, chain-1 (the product of the chromosome bearing the insertion mutation) is dramatically reduced in both ζ and α1 insertion mutants, indicating a clear in cis effect for the ζ mutation and, as expected, for the α1 mutation as well. In the ζ case, the α1 chain reduction is due to a cis effect of the ζ-gene insertion. In the α case, it is due to the mutational insertion in the α1 gene. The BALB/c chromosome shows no change in chain-2 or -3 production indicating that there is no effect of the insertion in trans. These impressions are confirmed by quantifying chain-1 (mutant chromosome) hemoglobin expression using densitometry as shown in Table 4. In both insertion mutants, there is a reduction in both the percent of total chain-1–bearing hemoglobin (Table 4, column A) and the proportion of chain 1 that arises from the 129/SvEv chromosome (Table 4, column B).
Immobilized gradient isoelectric focusing analysis of mouse hemoglobins. This analysis allows the detection of the products of each parental chromosome separately, the BALB/c wild-type chromosome and the 129/SvEv chromosome bearing the inserted ζ- or α1-globin genes in heterozygous mice. Each band represents a mouse hemoglobin carrying a different α-globin chain. The 129/SvEv chromosome bearing the PGK-Neo insertion in the ζ- or α1-gene specifies α-chain–1 (Hbaa). The normal BALB/c chromosome borne by each of these heterozygous mutant mice carries the allele for Hbab and specifies α-chains–2 and –3. Thus, the reduced amount of hemoglobin with chain-1 in the ζ-heterozygote reflects a repressive effect on α-globin expression in cis to the insertion of the PGK-Neo cassette.
Immobilized gradient isoelectric focusing analysis of mouse hemoglobins. This analysis allows the detection of the products of each parental chromosome separately, the BALB/c wild-type chromosome and the 129/SvEv chromosome bearing the inserted ζ- or α1-globin genes in heterozygous mice. Each band represents a mouse hemoglobin carrying a different α-globin chain. The 129/SvEv chromosome bearing the PGK-Neo insertion in the ζ- or α1-gene specifies α-chain–1 (Hbaa). The normal BALB/c chromosome borne by each of these heterozygous mutant mice carries the allele for Hbab and specifies α-chains–2 and –3. Thus, the reduced amount of hemoglobin with chain-1 in the ζ-heterozygote reflects a repressive effect on α-globin expression in cis to the insertion of the PGK-Neo cassette.
Densitometry of Hemoglobins of Heterozygous Thalassemic and Control Mice Analyzed by Immobilized Gradient Isoelectric Focusing
| Sample . | A.Percent Hemoglobin . | B.Relative Amount of Hemoglobin . |
|---|---|---|
| . | with Chain-1 . | with Chain-1 . |
| Wild type | 48.3 ± 1.2 | 100 |
| ζ−/+ | 20.5 ± 2.8 | 42.4 ± 5.8 |
| α1−/+ | 17.9 ± 1.2 | 37.1 ± 2.5 |
| Sample . | A.Percent Hemoglobin . | B.Relative Amount of Hemoglobin . |
|---|---|---|
| . | with Chain-1 . | with Chain-1 . |
| Wild type | 48.3 ± 1.2 | 100 |
| ζ−/+ | 20.5 ± 2.8 | 42.4 ± 5.8 |
| α1−/+ | 17.9 ± 1.2 | 37.1 ± 2.5 |
Samples from three individual mice of each genotype were scanned with a Shimadzu CS9000 densitometer and data analyzed using the Shimadzu CS Turbo software. All mice carry one normal Balb/c chromosome 11, which encodes α-globin chains-2 and -3. The mutated 129/SvEv chromosome encodes α-globin chain-1. The relative amount of hemoglobin with α-chain–1 (encoded by the mutant chromosome) is significantly reduced from normal in the insertional mutants, ζ−/+ and α1+/−. The data are presented (+/− SD). The difference between the mutants themselves is not significant (t-test). Thus, the presence of the inserted PGK-Neo cassette is accompanied by a reduction in expression of the α-gene in cis, both as a percent of the total hemoglobin present normally (column A) and relative to the normal product of the 129/SvEv chromosome (chain 1) (column B ). Expression of the α-genes encoded in trans are unaffected.
Evidence for modifying genes.Above we noted the variable expressivity of the ζ-null phenotype illustrated by the variation in size of 12-day pc embryonic littermates (Fig 3). While intrauterine variation in embryonic development is not uncommon, it is worth noting in this instance that this litter is the product of a mating between two outbred parents (of mixed 129/SvEv and B1 Swiss outbred stock). Thus, it is possible that variation in the strength of the thalassemic phenotype is a consequence of modifying genes present and transmitted to varying degrees in the outbred parental background. Because we established our ES cell lines from the mouse strain 129/SvEv, we were able to assess the behavior of the ζ- and α-null mutations in this inbred genetic background and compare it with behavior in the outbred 129/B1 Swiss background. Such an experiment is shown in Table 1 (compare A and B) in which no live homozygous ζ−/− null weanlings were recovered among 124 mice analyzed in the inbred 129/SvEv background, whereas eight of 106 were recovered in the outbred background. In contrast, live homozygous α1−/− null weanlings were recovered in roughly equivalent proportions in both the inbred and outbred backgrounds (Table 1, C and D). Thus, the ζ-null mutation (recall that it is associated with an α-deficiency) is lethal in the 129/SvEv background, but not invariably in the outbred background. Using weanling survival as an endpoint, the α1−/− null appears to survive in both genetic backgrounds.
Although some of α1−/− null mice survive in the 129/SvEv background, they nonetheless suffer a more severe thalassemic phenotype. (Indeed, it has not been possible to establish a colony of α1−/− mice with this background.) The severe phenotype in the 129/SvEv background is reflected in the morphology of the peripheral blood (Fig 4, compare C to D) in which very marked anisocytosis, poikilocytosis with extensive rouloux, and nucleated red blood cell precursors are seen in the 129/SvEv background (Fig 4D). Though α1−/− mice viable in the 129/SvEv background are quite hard to obtain, one such mouse had a recticulocytosis of 33.7% compared with 4.7% in a control, the α1−/− outbred mouse. The nucleated red blood cell precursors seen in the peripheral blood and the intense reticulocytosis strongly points to increased hematopoiesis in the face of increased red cell destruction as the underlying anemic mechanism, a mechanism typical of thalassemia. This genetic background effect is also reflected in the more severe hematologic indices evident in the 129/SvEv background as compared with the outbred background (Table 3, A and B). While MCV and MCH do not vary greatly between inbred and outbred α1−/− mice, there are striking reductions in hemoglobin levels in the inbred strain (Table 3, compare A and B) together with a sharply increased red cell distribution width in the inbred background.
DISCUSSION
The embryonic ζ-globin gene is not absolutely required for embryonic survival.The survival of viable ζ-null mice addresses one of the major questions we had hoped to answer, namely, whether adult α globin genes can functionally provide for the survival of the embryo from the onset of erythropoiesis in the primitive cells of the yolk sac. Taken together with previous in situ hybridization data that indicated that both ζ- and α-globin mRNAs were simultaneously expressed in the primitive yolk sac-derived red blood cells,7 this finding indicates that functional adult α-globin chains are synthesized throughout the earliest period of erythropoiesis. Thus, initial co-expression of α- and ζ-globin with subsequent extinction of ζ-globin is the correct order of events in early erythropoiesis.
The viability of ζ-null mice allows us to raise a further question regarding the physiologic redundancy of the ζ-globin genes. Embryonic ζ-like globin genes are found in many, if not all, mammalian α-globin loci suggesting that they confer some survival advantage on the organism. While the survival of ζ-null mice clearly identifies a genetic redundancy with adult α-globin genes, it does not rule out the possibility of marginal advantages that might be conferred by the availability of the embryonic ζ genes.
Creation of the ζ-globin gene insertion induces α-thalassemia: Near neighbor effects.In addition to the inactivation of the ζ-globin gene by insertional mutagenesis, creation of this mutation results in the down-regulation of the nearby adult α-globin genes and the consequent induction of α-thalassemia. It is quite likely that this is a consequence of the insertion of the selectable Neor cassette, which is driven by the phosphoglycerate kinase I promoter. Similar unexpected inhibitory effects on neighboring genes have been observed in other murine mutations created by inserting this cassette (see review by Olson et al23 ). Although unpredictable, it seems reasonable that multigenic and highly regulated loci are especially vulnerable to this effect. Indeed, such effects have been observed on introducing insertional mutations into the Hox cluster24 the myogenic regulatory genes (MRF ),23 the immunoglobulins,25,26 and the β-globin locus.9,12,13 27
The work of Fiering et al12 and Hug et al13 on the β-globin locus is especially relevant to our study since both β- and α-globins are similarly regulated despite being located on different chromosomes. The α-globin locus and the better-studied β-globin locus are controlled by a distant 5′ sequence known as the locus control region (LCR) (see review by Epner et al28 ). The LCR acts as a classical enhancer, but also influences the locus' replication and its chromatin structure. Both Fiering et al12 and Hug et al13 found that the PGK-Neo targeting cassette left in the LCR resulted in a significant reduction in β-globin expression, which was relieved by removing the cassette. It seems likely that by introducing the PGK-Neo cassette into the ζ-gene, we have created a similar effect leading to the down-regulation of the neighboring α-genes, most likely by compromising the action of the α-LCR. It is worth noting that the same inserted cassette behaves differently depending on whether it is inserted into the ζ- or the α1-globin gene. When the cassette is inserted into the more 5′ ζ-globin gene, it produces a profound effect on the neighboring 3′ α-genes; when it is inserted into the α1-globin gene, it seems to have little effect on the expression of its 5′ ζ and 3′ α2 neighbors. Clearly, position and context influence this interference.
The potential role of modifying genes in thalassemia.The dramatic differences in the severity of the thalassemic phenotypes observed when the ζ- and α1-null genes are homozygous in inbred 129/SvEv and outbred genetic backgrounds can best be explained by postulating the existence of polymorphic modifying genes. Such genes would be unlinked to the ζ- or α-globin loci and thus could influence virtually any aspect of erythroid function. A similar genetic situation has been reported in humans; individuals carrying the same mutant α-globin allele show a significant variability in the severity of their disease implying participation of modifying genes.29 Fortunately, growing information regarding the mouse genome and its linkage map make it possible to map and subsequently identify genes in mice that govern quantitative genetic traits such as the severity of the thalassemias, which we observe. Since it appears that polymorphic forms of these as-yet-unknown modifying genes can ameliorate the severity of thalassemia in mice and humans, their identification may lead to new therapeutic targets and, hence, novel treatments for this disease.
ACKNOWLEDGMENT
We are very grateful to Anne Harrington and Ann Kou for their expert technical assistance. We are especially grateful to Terri Borderick for her editorial assistance. We are also grateful to Laufey Amundadottir, Carlos Bruganera, Ari Elson, Tim Lane, Marc Rothenberg, David Seldin, Radek Skoda, and Yoaqi Wang for their very helpful discussions.
Address reprint requests to Aya Leder, Department of Genetics, Harvard Medical School, Howard Hughes Medical Institute, 200 Longwood Ave, Boston, MA 02115.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal